A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes
Abstract
1. Introduction
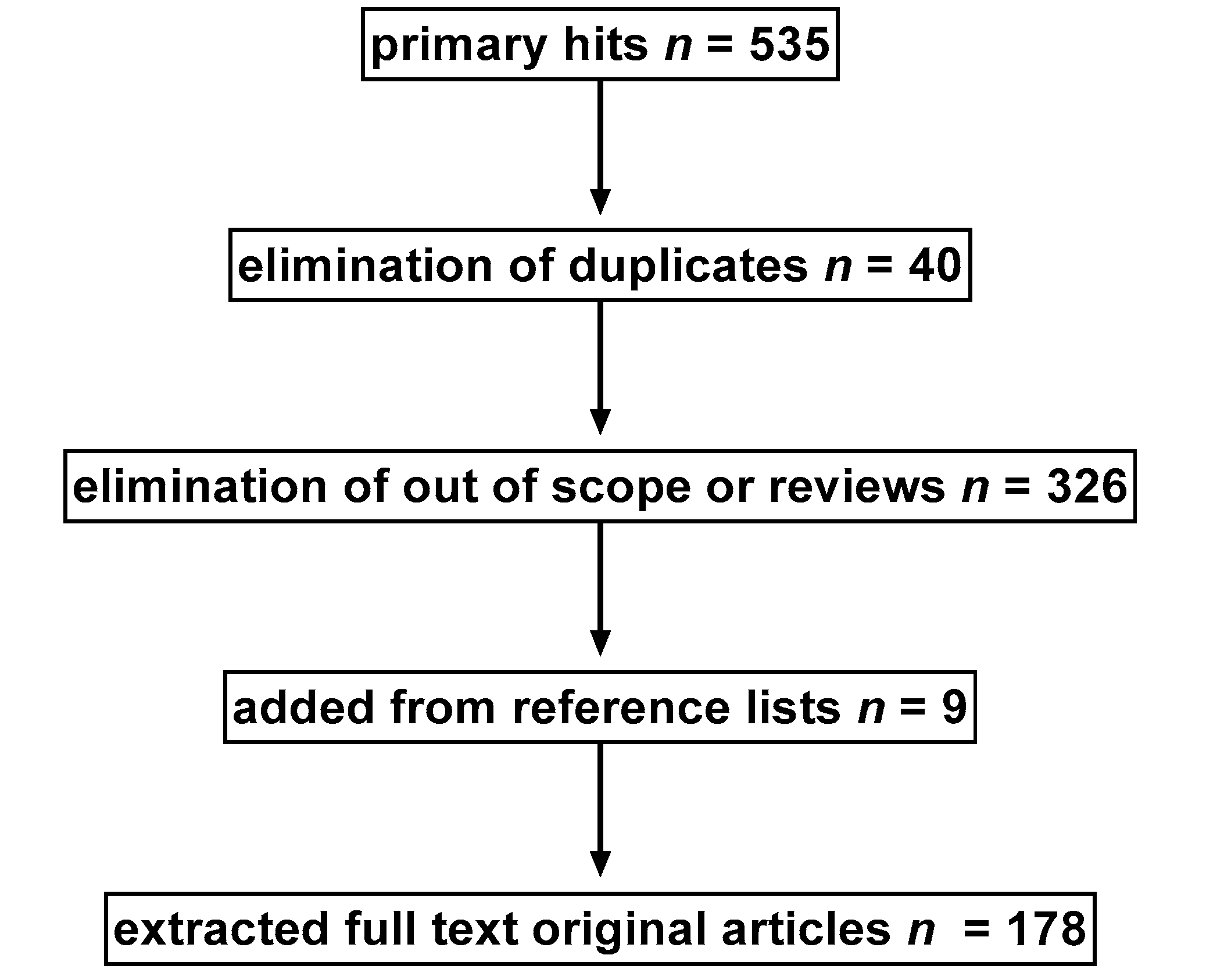
2. Search Strategy
3. Methodological Aspects of Studying IA
4. Compounds Exhibiting IA at AR Subtypes in Cellular Models
4.1. α1-AR
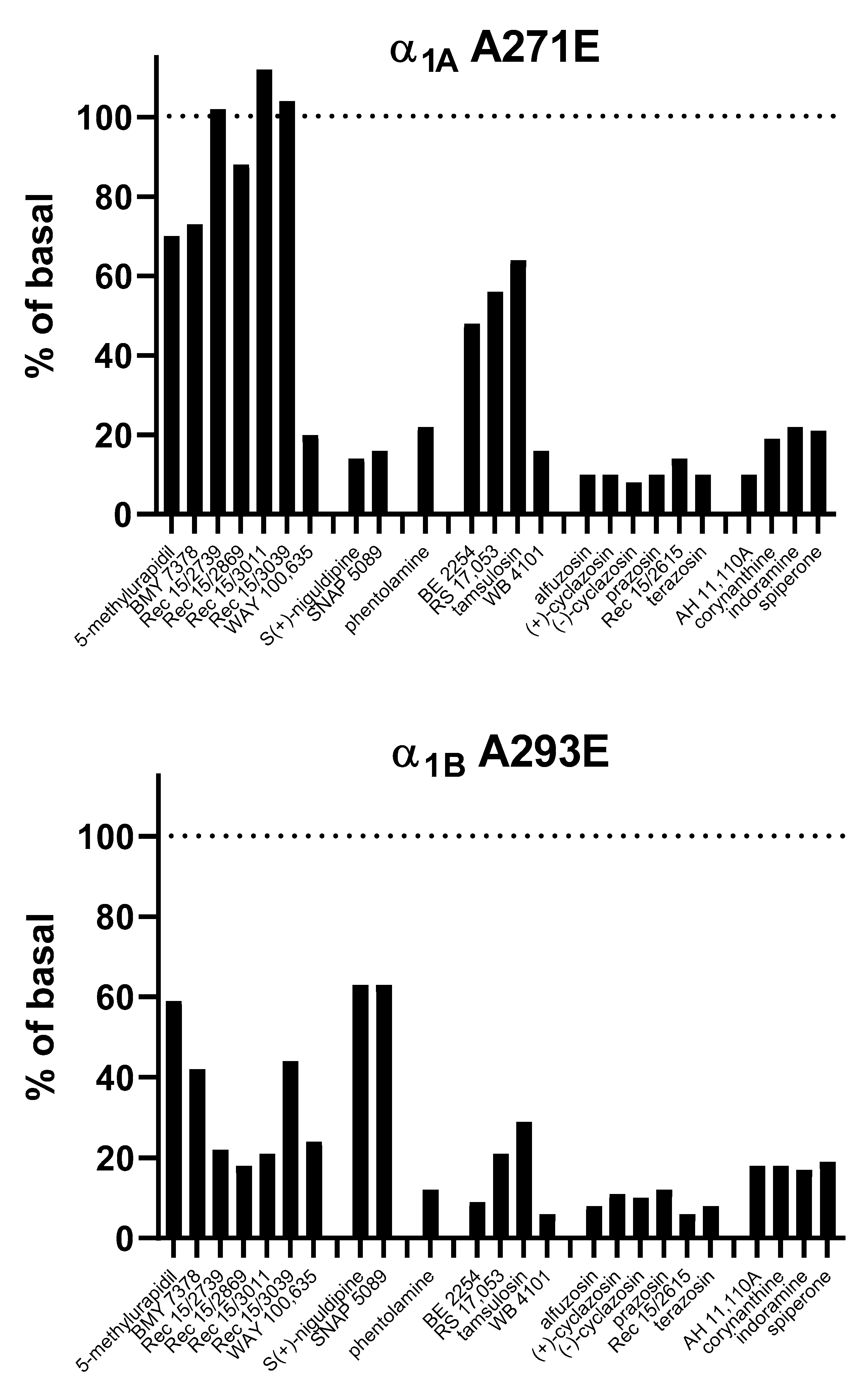
4.1.1. α1A-AR
4.1.2. α1B-AR
4.1.3. α1D-AR
4.2. α2-AR
4.2.1. α2A-AR
4.2.2. α2B-AR
4.2.3. α2C-AR
4.3. β-AR
4.3.1. β1-AR
4.3.2. β2-AR
5. Effects of Compounds with IA Data for Tissue and In Vivo Function
5.1. α1-AR
5.2. α2-AR
5.3. β-AR
5.3.1. Heart
5.3.2. Lung
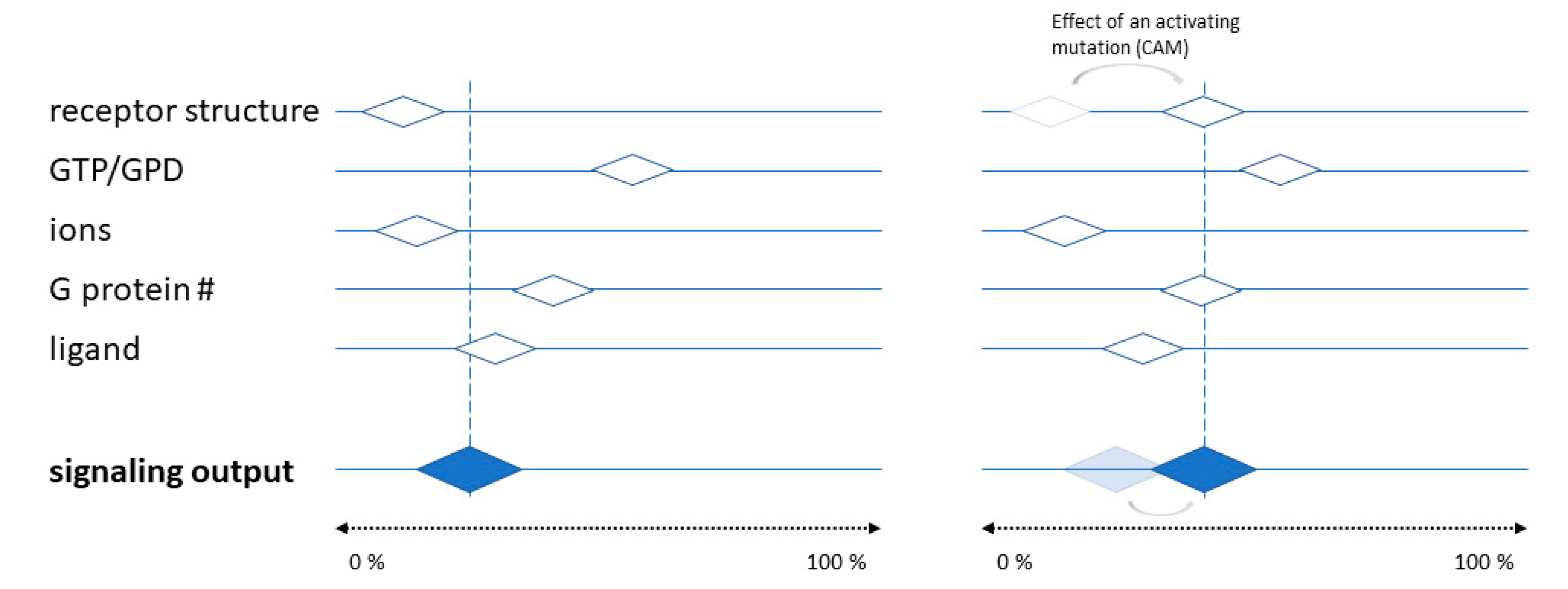
6. Molecular Mechanisms and Structural Basis of Inverse Agonism at AR
7. Conclusions and Implications for Drug Development
Author Contributions
Funding
Conflicts of Interest
References
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R., Jr.; Trendelenburg, U. International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar] [PubMed]
- Hieble, J.P.; Bylund, D.B.; Clarke, D.E.; Eikenburg, D.C.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Ruffolo, R.R., Jr. International Union of Pharmacology X. Recommendation for nomenclature of α1-adrenoceptors: Consensus update. Pharmacol. Rev. 1995, 47, 267–270. [Google Scholar] [PubMed]
- Michel, M.C.; Kenny, B.A.; Schwinn, D.A. Classification of α1-adrenoceptor subtypes. Naunyn Schmiedeberg’s Arch. Pharmacol. 1995, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cotecchia, S.; Exum, S.; Caron, M.G.; Lefkowitz, R.J. Regions of the α1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc. Natl. Acad. Sci. USA 1990, 87, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Cotecchia, S. Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2005, 26, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Schütz, W.; Freissmuth, M. Reverse intrinsic activity of antagonists on G protein-coupled receptors. Trends Pharmacol. Sci. 1992, 13, 376–380. [Google Scholar] [CrossRef]
- Milligan, G.; Bond, R.A.; Lee, M. Inverse agonism: Pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol. Sci. 1995, 16, 10–13. [Google Scholar] [CrossRef]
- de Ligt, R.A.F.; Kourounakis, A.P.; Ijzerman, A.P. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br. J. Pharmacol. 2000, 130, 1–12. [Google Scholar] [CrossRef]
- Michel, M.C.; Foster, C.; Brunner, H.R.; Liu, L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev. 2013, 65, 809–848. [Google Scholar] [CrossRef]
- Nelson, C.P.; Nahorski, S.R.; Chaliss, R.A.J. Constitutive activity and inverse agonism at the M2 muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 2006, 316, 279–288. [Google Scholar] [CrossRef]
- Monczor, F.; Fernandez, N.; Fitzsimons, C.P.; Shayo, C.; Davio, C. Antihistaminergics and inverse agonism: Potential therapeutic applications. Eur. J. Pharmacol. 2013, 715, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Strange, P.G. Antipsychotic drug action: Antagonism, inverse agonism or partial agonism. Trends Pharmacol. Sci. 2008, 29, 314–321. [Google Scholar] [CrossRef]
- Kenakin, T. Efficacy as a vector: The relative prevalence and paucity of inverse agonism. Mol. Pharmacol. 2004, 65, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Galandrin, S.; Oligny-Longpré, G.; Bonin, H.; Ogawa, K.; Galés, C.; Bouvier, M. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the ß1-adrenergic receptor. Mol. Pharmacol. 2008, 74, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.G.; Hall, I.P.; Hill, S.J. Agonist and inverse agonist actions of ß-blockers at the human ß2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 2003, 64, 1357–1369. [Google Scholar] [CrossRef]
- Zhu, J.; Taniguchi, T.; Takauji, R.; Suzuki, F.; Tanaka, T.; Muramatsu, I. Inverse agonism and neutral antagonism at a constitutively active alpha-1a adrenoceptor. Br. J. Pharmacol. 2000, 131, 546–552. [Google Scholar]
- Carrillo, J.J.; Stevens, P.A.; Milligan, G. Measurement of agonist-dependent and -independent signal initiation of α1b-adrenoceptor mutants by direct analysis of guanine nucleotide exchange on the G protein Gα11. J. Pharmacol. Exp. Ther. 2002, 302, 1080–1088. [Google Scholar] [CrossRef]
- Pauwels, P.J.; Tardif, S.; Wurch, T.; Colpaert, C.F. Facilitation of constitutive α2A-adrenoceptor activity by both single amino acid mutation (Thr373Lys) and Gαo protein coexpression: Evidence for inverse agonism. J. Pharmacol. Exp. Ther. 2000, 292, 654–663. [Google Scholar]
- Rossier, O.; Abuin, L.; Fanelli, F.; Leonardi, A.; Cotecchia, S. Inverse agonism and neutral antagonism at α1a- and α1b-adrenergic receptor subtypes. Mol. Pharmacol. 1999, 56, 858–866. [Google Scholar] [CrossRef]
- Ammer, H.; Schulz, R. Chronic morphine treatment increases stimulatory beta-2 adrenoceptor signaling in A431 cells stably expressing the Mu opioid receptor. J. Pharmacol. Exp. Ther. 1997, 280, 512–520. [Google Scholar]
- Pauwels, P.J.; Rauly, I.; Wurch, T. Dissimilar pharmacological responses by a new series of imidazoline derivatives at precoupled and ligand-activated α2A-adrenoceptor states: Evidence for effector pathway-dependent differential antagonism. J. Pharmacol. Exp. Ther. 2003, 305, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.M.; Lan, K.L.; Moore, D.J.; Neubig, R.R. Inverse agonist activity at the α2A-adrenergic receptor. Mol. Pharmacol. 2001, 59, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Cotecchia, S.; Milligan, G. Up-regulation of the levels of expression and function of a constitutively active mutant of the hamster α1B-adrenoceptor by ligands that act as inverse agonists. Biochem. J. 1997, 325, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Morris, D.P.; Smith, M.P.; Schwinn, D.A. Lipid rafts constrain basal α1A-adrenergic receptor signaling by maintaining receptor in an inactive conformation. Cell. Signal. 2009, 21, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, J.D.; Milligan, G. Inverse agonist-induced up-regulation of the human ß2-adrenoceptor in transfected neuroblastoma x glioma hybrid cells. Mol. Pharmacol. 1996, 50, 1479–1486. [Google Scholar] [PubMed]
- Peng, H.; Bond, R.A.; Knoll, B.J. The effects of acute and chronic nadolol treatment on ß2AR signaling in HEK293 cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 2011, 383, 209–216. [Google Scholar] [CrossRef]
- Ramsay, D.; Bevan, N.; Rees, S.; Milligan, G. Detection of receptor ligands by monitoring selective stabilization of a Renilla luciferase-tagged, constitutively active mutant, G-protein-coupled receptor. Br. J. Pharmacol. 2001, 133, 315–323. [Google Scholar] [CrossRef]
- Samama, P.; Pei, G.; Costa, T.; Cotecchia, S.; Lefkowitz, R.J. Negative antagonists promote an inactive conformation of the ß2-adrenergic receptor. Mol. Pharmacol. 1994, 45, 390–394. [Google Scholar]
- Nobles, M.; Benians, A.; Tinker, A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc. Natl. Acad. Sci. USA 2005, 102, 18706–18711. [Google Scholar] [CrossRef]
- Rochais, F.; Vilardaga, J.P.; Nikolaev, V.O.; Bünemann, M.; Lohse, M.J.; Engelhardt, S. Real-time optical recording of ß1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J. Clin. Investig. 2007, 117, 229–235. [Google Scholar] [CrossRef]
- Malik, R.U.; Ritt, M.; DeVree, B.T.; Neubig, R.R.; Sunahara, R.K.; Sivaramakrishnan, S. Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells. J. Biol. Chem. 2013, 288, 17167–17178. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Wenzel-Seifert, K.; Lee, T.W.; Gether, U.; Sanders-Bush, E.; Kobilka, B.K. Different effects of Gsα splice variants on ß2-adrenoreceptor-mediated signaling. The ß2-adrenoceptor coupled to the long splice variant of Gsα has properties of a constitutively active receptor. J. Biol. Chem. 1998, 273, 5109–55116. [Google Scholar] [CrossRef] [PubMed]
- Hothersall, J.D.; Black, J.; Caddick, S.; Vinter, J.G.; Tinker, A.; Baker, J.R. The design, synthesis and pharmacological characterization of novel β2-adrenoceptor antagonists. Br. J. Pharmacol. 2011, 164, 317–331. [Google Scholar] [CrossRef]
- McCune, D.F.; Edelmann, S.E.; Olges, J.R.; Post, G.R.; Waldrop, B.A.; Waugh, D.J.J.; Perez, D.M.; Piascik, M.T. Regulation of cellular localization and signaling properties of the α1B- and α1D-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 2000, 57, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sainz, A.J.; Torres-Padilla, M.E. Modulation of basal intracellular calcium by inverse agonists and phorbol myristate acetate in rat-1 fibroblasts stably expressing α1d-adrenoceptors. FEBS Lett. 1999, 443, 277–281. [Google Scholar] [CrossRef]
- Mijares, A.; Lebesgue, D.; Argibay, J.; Hoebeke, J. Anti-peptide antibodies sensitive to the ‘active’ state of the β2adrenergic receptor. FEBS Lett. 1996, 399, 188–191. [Google Scholar] [CrossRef]
- Duffy, S.M.; Cruse, G.; Lawley, W.J.; Bradding, P. β2-Adrenoceptor regulation of the K+ channel iKCa1 in human mast cells. FASEB J. 2005, 19, 1006–1008. [Google Scholar] [CrossRef]
- Saeki, S.; Kunitomo, H.; Narita, Y.; Mimura, H.; Nishi, T.; Sasaki, K. A reporter assay for G-protein-coupled receptors using a B-cell line suitable for stable episomal expression. Anal. Biochem. 2010, 400, 163–172. [Google Scholar] [CrossRef]
- Noguera, M.A.; Ivorra, M.D.; D’Ocon, P. Functional evidence of inverse agonism in vascular smooth muscle. Br. J. Pharmacol. 1996, 119, 158–164. [Google Scholar] [CrossRef]
- Ragnarsson, L.; Wang, C.-I.A.; Andersson, Å.; Fajarningsih, D.; Monks, T.; Brust, A.; Rosengren, K.J.; Lewis, R.J. Conopeptide ρ-TIA defines a new allosteric site on the extracellular surface of the α1B-adrenoceptor. J. Biol. Chem. 2013, 288, 1814–1827. [Google Scholar] [CrossRef]
- Ahn, S.; Kahsai, A.W.; Pani, B.; Wang, Q.-T.; Zhao, S.; Wall, A.L.; Strachan, R.T.; Staus, D.P.; Wingler, L.M.; Sun, L.D.; et al. Allosteric “beta-blocker” isolated from a DNA-encoded small molecule library. Proc. Natl. Acad. Sci. USA 2017, 114, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, H.E.; Latif, M.L.; Hill, S.J. Non-competitive antagonism of ß2-agonist-mediated cyclic AMP accumulation by ICI 118,551 in BC3H1 cells endogenously expressing constitutively active ß2-adrenoceptors. Br. J. Pharmacol. 2000, 131, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jansson, C.C.; Kukkonen, J.P.; Näsman, J.; Huifang, G.; Wurster, S.; Virtanen, R.; Savola, J.M.; Cockcroft, V.; Akerman, K.E.O. Protean agonism at α2A-adrenoceptors. Mol. Pharmacol. 1998, 53, 963–968. [Google Scholar] [PubMed]
- Peter, J.-C.; Eftekhari, P.; Billiald, P.; Wallukat, G.; Hoebeke, J. scFv single chain antibody variable fragment as inverse agonist of the β2-adrenergic receptor. J. Biol. Chem. 2003, 278, 36740–36747. [Google Scholar] [CrossRef] [PubMed]
- Scheer, A.; Fanelli, F.; Costa, T.; de Benedetti, P.G.; Cotecchia, S. Constitutitvely active mutants of the α1B-adrenergic receptor, Role of highly conserved polar amino acids in receptor activation. EMBO J. 1996, 15, 3566–3578. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Olli-Lähdesmäki, T.; Kallio, J.; Scheinin, M. α2B-Adrenoceptor levels govern agonist and inverse agonist responses in PC12 cells. Biochem. Biophys. Res. Commun. 2003, 308, 12–18. [Google Scholar] [CrossRef]
- Engelhardt, S.; Grimmer, Y.; Fan, F.H.; Lohse, M.J. Constitutive activity of the human ß1-adrenergic receptor in ß1-receptor transgenic mice. Mol. Pharmacol. 2001, 60, 712–717. [Google Scholar]
- Chidiac, P.; Hebert, T.E.; Valiquette, M.; Dennis, M.; Bouvier, M. Inverse agonist activity of ß-adrenergic antagonists. Mol. Pharmacol. 1994, 45, 490–499. [Google Scholar]
- Small, K.M.; Forbes, S.L.; Rahman, F.F.; Liggett, S.B. Fusion of β2-adrenergic receptor to Gαs in mammalian cells: Identification of a specific signal transduction species not characteristic of constitutive activation or precoupling. Biochemistry 2000, 39, 2815–2821. [Google Scholar] [CrossRef]
- Wurch, T.; Colpaert, F.C.; Pauwels, P.J. G-protein activation by putative antagonists at mutant Thr373Lys α2A adrenergic receptors. Br. J. Pharmacol. 1999, 126, 939–948. [Google Scholar] [CrossRef]
- Stevens, P.A.; Milligan, G. Efficacy of inverse agonists in cells overexpressing a constitutively active ß2-adrenoceptor and type II adenylyl cyclase. Br. J. Pharmacol. 1998, 123, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R. Monovalent anions differentially modulate coupling of the β2-adrenoceptor to Gsα splice variants. J. Pharmacol. Exp. Ther. 2001, 298, 840–847. [Google Scholar] [PubMed]
- Ammer, H.; Schulz, R. Opioid tolerance/dependence in neuroblastoma×glioma (NG108-15) hybrid cells is associated with a reduction in spontaneous stimulatory receptor activity. FEBS Lett. 2000, 485, 157–162. [Google Scholar] [CrossRef]
- Rathz, D.A.; Gregory, K.N.; Fang, Y.; Brown, K.M.; Liggett, S.B. Hierarchy of polymorphic variation and desensitization permutations relative to β1- and β2-adrenergic receptor signaling. J. Biol. Chem. 2003, 278, 10784–10789. [Google Scholar] [CrossRef]
- Levin, M.C.; Marullo, S.; Muntaner, O.; Andersson, B.; Magnusson, Y. The myocardium-protective Gly-49 variant of the ß1-adrenergic receptor exhibits constitutive activity and increased desensitization and down-regulation. J. Biol. Chem. 2002, 277, 30429–30435. [Google Scholar] [CrossRef]
- Seifert, R.; Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: Cause of disease and common property of wild-type receptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2002, 366, 381–416. [Google Scholar] [CrossRef]
- Hein, P.; Goepel, M.; Cotecchia, S.; Michel, M.C. A quantitative analysis of antagonism and inverse agonism at wild-type and constitutively active hamster α1B-adrenoceptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2001, 363, 34–39. [Google Scholar] [CrossRef]
- Varma, D.R.; Shen, H.; Deng, X.F.; Peri, K.G.; Chemtob, S.; Mulay, S. Inverse agonist activities of ß-adrenoceptor antagonists in rat myocardium. Br. J. Pharmacol. 1999, 127, 895–902. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef]
- Ozkan, M.H.; Uma, S. β-Adrenergic receptor blocker ICI 118,551 selectively increases intermediate-conductance calcium-activated potassium channel (IKCa)-mediated relaxations in rat main mesenteric artery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 570–576. [Google Scholar] [CrossRef]
- Cotecchia, S. Constitutive activity and inverse agonism at the α1adrenoceptors. Biochem. Pharmacol. 2007, 73, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Stanasila, L.; Perez, J.-B.; Vogel, H.; Cotecchia, S. Oligomerization of the α1a- and α1b-adrenergic receptor subtypes: Potential implications for internalization. J. Biol. Chem. 2003, 278, 40239–40251. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.A.; Bevan, N.; Rees, S.; Milligan, G. Resolution of inverse agonist-induced up-regulation from constitutive activitiy mutants of the α1b-adrenoceptor. Mol. Pharmacol. 2000, 58, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.P.; Price, R.R.; Smith, M.P.; Lei, B.; Schwinn, D.A. Cellular trafficking of human α1a-adrenergic receptors is continuous and primarily agonist-independent. Mol. Pharmacol. 2004, 66, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Kolarovszki-Sipiczki, Z.; Gáspár, R.; Ducza, E.; Páldy, E.; Benyhe, S.; Borsodi, A.; Falkay, G. Effect of α1-adrenoceptor subtype-selective inverse agonists on non-pregnant and late-pregnant cervical resistance in vitro in the rat. Clin. Exp. Pharmacol. Physiol. 2007, 34, 42–47. [Google Scholar] [CrossRef]
- Pauwels, P.J.; Tardif, S. Enhanced stability of wild-type and constitutively active α2A-adrenoceptors by ligands with agonist, silent and inverse agonist properties. Naunyn Schmiedeberg’s Arch. Pharmacol. 2002, 166, 134–141. [Google Scholar] [CrossRef]
- Ge, H.; Scheinin, M.; Kallio, J. Constitutive precoupling to Gi and increased agonist potency in the α2B-adrenoceptor. Biochem. Biophys. Res. Commun. 2003, 306, 959–965. [Google Scholar] [CrossRef]
- Vilardaga, J.-P.; Steinmeyer, R.; Harms, G.S.; Lohse, M.J. Molecular basis of inverse agonism in a G protein–coupled receptor. Nat. Chem. Biol. 2005, 1, 25–28. [Google Scholar] [CrossRef]
- Tian, W.N.; Duzic, E.; Lanier, S.M.; Deth, R.C. Determinants of α2-sdrenergic receptor activation of G proteins: Evidence for a precoupled receptor/G protein state. Mol. Pharmacol. 1994, 45, 524–531. [Google Scholar]
- Tian, W.-N.; Miller, D.D.; Deth, R.C. Bidirectional allosteric effects of agonists and GTP at α2A/D-adrenoceptors. J. Pharmacol. Exp. Ther. 2000, 292, 664–671. [Google Scholar]
- Zhu, Q.; Qi, L.J.; Shi, A.; Abou-Samra, A.; Deth, R.C. Protein kinase C regulates α2A/D-adrenoceptor constitutive activity. Pharmacology 2004, 71, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Polanco, M.J.; López-Giménez, J.F.; González-Martín, C.; Alguacil, L.F. Yohimbine does not affect opioid receptor activation but prevents adenylate cyclase regulation by morphine in NG108-15 cells. Life Sci. 2011, 89, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Cayla, C.; Schaak, S.; Roquelaine, C.; Gales, C.; Quinchon, F.; Paris, H. Homologous regulation of the α2C-adrenoceptor subtype in human hepatocarcinoma, HepG2. Br. J. Pharmacol. 1999, 126, 69–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murrin, L.C.; Gerety, M.E.; Happe, H.K.; Bylund, D.B. Inverse agonism at α2-adrenoceptors in native tissue. Eur. J. Pharmacol. 2000, 398, 185–191. [Google Scholar] [CrossRef]
- Erdbrügger, W.; Raulf, M.; Otto, T.; Michel, M.C. Does [3H]2-methoxy-idazoxan (RX 821002) detect more alpha-2-adrenoceptor agonist high-affinity sites than [3H]rauwolscine? A comparison of nine tissues and cell lines. J. Pharmacol. Exp. Ther. 1995, 273, 1287–1294. [Google Scholar] [PubMed]
- Michel, M.C.; Brass, L.F.; Williams, A.; Bokoch, G.M.; LaMorte, V.J.; Motulsky, H.J. α2-Adrenergic receptor stimulation mobilizes intracellular Ca2+ in human erythroleukemia cells. J. Biol. Chem. 1989, 264, 4986–4991. [Google Scholar]
- Dixon, R.A.F.; Kobilka, B.K.; Strader, D.J.; Benovic, J.L.; Dohlman, H.G.; Frielle, T.; Bolanowski, M.A.; Bennett, C.D.; Rands, E.; Diehl, R.E.; et al. Cloning of the gene and cDNA for mammalian ß-adrenergic receptor and homology with rhodopsin. Nature 1986, 321, 75–79. [Google Scholar] [CrossRef]
- Sato, T.; Baker, J.; Warne, T.; Brown, G.A.; Leslie, A.G.W.; Congreve, M.; Tate, C.G. Pharmacological analysis and structure determination of 7-methylcyanopindolol–bound β1-adrenergic receptor. Mol. Pharmacol. 2015, 88, 1024–1034. [Google Scholar] [CrossRef]
- Warne, T.; Serrano-Vega, M.J.; Baker, J.G.; Moukhametzianov, R.; Edwards, P.C.; Henderson, R.; Leslie, A.G.W.; Tate, C.G.; Schertler, G.F.X. Structure of a ß1-adrenergic G-protein-coupled receptor. Nature 2008, 454, 486–491. [Google Scholar] [CrossRef]
- Delos Santos, N.M.; Gardner, L.A.; White, S.W.; Bahouth, S.W. Characterization of the residues in Helix 8 of the Human β1-adrenergic receptor that are involved in coupling the receptor to G proteins. J. Biol. Chem. 2006, 281, 12896–12907. [Google Scholar] [CrossRef]
- Janssens, K.; Boussemaere, M.; Wagner, S.; Kopka, K.; Denef, C. β1-Adrenoceptors in Rat Anterior Pituitary May Be Constitutively Active. Inverse Agonism of CGP 20712A on Basal 3′,5′-Cyclic Adenosine 5′-Monophosphate Levels. Endocrinology 2008, 149, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Chidiac, P.; Nouet, S.; Bouvier, M. Agonist-induced modulation of inverse agonist efficacy at the ß2-adrenergic receptor. Mol. Pharmacol. 1996, 50, 662–669. [Google Scholar] [PubMed]
- Nakahara, T.; Maruko, T.; Sakamoto, K.; Ishii, K. Influence of receptor number on the cAMP response to forskolin in Chinese hamster ovary cells transfected with human b2-adrenoceptor. Biol. Pharmacol. Bull. 2004, 27, 239–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaya, A.I.; Uǧur, Ö.; Öner, S.S.; Bastepe, M.; Onaran, H.O. Coupling of β2-adrenoceptors to XLαs and Gαs: A new insight into ligand-induced G protein activation. J. Pharmacol. Exp. Ther. 2009, 329, 350–359. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.J.; Bevan, N.; Rees, S.; Milligan, G. Visualizing differences in ligand regulation of wild-type and constitutively active mutant ß2-adrenoceptor-green fluorescent protein fusion proteins. Mol. Pharmacol. 1999, 56, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, D.J.; Milligan, G. Up-regulation of a constitutively active form of the ß2-adrenoceptor by sustained treatment with inverse agonists but not antagonists. FEBS Lett. 1996, 339, 108–112. [Google Scholar] [CrossRef]
- Scarselli, M.; Annibale, P.; Radenovic, A. Cell type-specific β2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity. J. Biol. Chem. 2012, 287, 16768–16780. [Google Scholar] [CrossRef]
- Azzi, M.; Pineyro, G.; Pontier, S.; Parent, S.; Ansanay, H.; Bouvier, M. Allosteric effects of G protein overexpression on the binding of ß-adrenergic ligands with distinct inverse efficacies. Mol. Pharmacol. 2001, 60, 999–1007. [Google Scholar] [CrossRef]
- Chakir, K.; Xiang, Y.; Yang, D.; Zhang, S.J.; Cheng, H.; Kobilka, B.K.; Xiao, R.P. The third intracellular loop and the carboxyl terminus of ß2-adrenergic receptor confer spontaneous activity of the receptor. Mol. Pharmacol. 2003, 64, 1048–1058. [Google Scholar] [CrossRef]
- Gregg, C.J.; Steppan, J.; Gonzalez, D.R.; Champion, H.C.; Phan, A.C.; Nyhan, D.; Shoukas, A.A.; Hare, J.M.; Barouch, L.A.; Berkowitz, D.E. β2-Adrenergic receptor-coupled phosphoinositide 3-kinase constrains cAMP-dependent increases in cardiac inotropy through phosphodiesterase 4 activation. Anesth. Analg. 2010, 111, 870–877. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Zhang, C.; Lyons, J.A.; Holl, R.; Aragao, D.; Arlow, D.H.; Rasmussen, S.G.F.; Choi, H.J.; DeVree, B.T.; Sunahara, R.K.; et al. Structure and function of an irreversible agonist-ß2-adrenoceptor complex. Nature 2011, 469, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Boychuk, C.R.; Bateman, R.J.; Philbin, K.E.; Mendelowitz, D. α1-Adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 2011, 193, 154–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rank, M.M.; Murray, K.C.; Stephens, M.J.; D'Amico, J.; Gorassini, M.A.; Bennett, D.J. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J. Neurophysiol. 2011, 105, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Morrison, A.E.; Lipworth, B.J. Effects of the inverse alpha-agonist doxazosin in allergic rhinitis. Clin. Exp. Allergy 2016, 46, 696–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Micucci, M.; Chiarini, A.; Budriesi, R. Neutral/negative α1-AR antagonists and calcium channel blockers at comparison in functional tests on guinea-pig smooth muscle and myocardium. Pharmacol. Rep. 2019, 71, 128–132. [Google Scholar] [CrossRef]
- Doze, V.A.; Handel, E.M.; Jensen, K.A.; Darsie, B.; Luger, E.J.; Haselton, J.R.; Talbot, J.N.; Rorabaugh, B.R. α1A- and α1B-adrenergic receptors differentially modulate antidepressant-like behavior in the mouse. Brain Res. 2009, 1285, 148–157. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Kam, W.R.; Sullivan, D.A. Effect of brimonidine, an α2 adrenergic agonist, on human meibomian gland epithelial cells. Exp. Eye Res. 2018, 170, 20–28. [Google Scholar] [CrossRef]
- Bruzzone, A.; Perez, C.; Castillo, L.F.; Sarappa, M.G.; Rojas, P.; Lanari, C.; Lüthy, I.A. α2-Adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br. J. Pharmacol. 2008, 155, 494–504. [Google Scholar] [CrossRef]
- Janhunen, S.K.; van der Zwaal, E.M.; la Fleur, S.E.; Adan, R.A.H. Inverse agonism at α2A adrenoceptors augments the hypophagic effect of sibutramine in rats. Obesity 2011, 19, 1979–1986. [Google Scholar] [CrossRef]
- Janhunen, S.K.; la Fleur, S.E.; Adan, R.A.H. Blocking alpha2A adrenoceptors, but not dopamine receptors, augments bupropion-induced hypophagia in rats. Obesity 2013, 21, E700–E708. [Google Scholar] [CrossRef]
- Akaishi, Y.; Hattori, Y.; Kanno, M.; Sakuma, I.; Kitabatake, A. Agonist-independent tonic inhibitory influence of Gi on adenylate cyclase activity in rabbit ventricular myocardium and its removal by pertussis toxin: A role of empty receptor-mediated Gi activation. J. Mol. Cell. Cardiol. 1997, 29, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Liggett, S.B.; Mialet-Perez, J.; Thaneemit-Chen, S.; Weber, S.A.; Greene, S.M.; Hodne, D.; Nelson, B.; Morrison, J.; Domanski, M.J.; Wagoner, L.E.; et al. A polymorphism within a conserved β1-adrenergic receptor motif alters cardiac function and β-blocker response in human heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 11288–11293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Yang, D.; Zhu, W.Z.; Zhang, S.J.; Wang, D.J.; Rohrer, D.K.; Devic, E.; Kobilka, B.; Lakatta, E.G.; Cheng, H.; et al. Spontaneous activation of ß2- but not ß1-adrenoceptors expressed in cardiac myocytes from ß1ß2 double knockout mice. Mol. Pharmacol. 2000, 58, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Sun, H.; Koch, W.J.; Rau, T.; Eschenhagen, T.; Ravens, U.; Heubach, J.F.; Adamson, D.L.; Harding, S.E. Specific b2AR blocker ICI 118,551 actively decreases contraction through a Gi-coupled form of the b2AR in myocytes from failing human heart. Circulation 2002, 105, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.A.; Leff, P.; Johnson, T.D.; Milano, C.A.; Rockman, H.A.; McMinn, T.R.; Apparsundaram, S.; Hyek, M.F.; Kenakin, T.P.; Allen, L.F.; et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the ß2-adrenoceptor. Nature 1995, 374, 272–276. [Google Scholar] [CrossRef]
- Gong, H.; Adamson, D.L.; Ranu, H.K.; Koch, W.J.; Heubach, J.F.; Ravens, U.; Zolk, O.; Harding, S.E. The effect of Gi-protein inactivation on basal, and ß1- and ß2AR-stimulated contraction of myocytes from transgenic mice overexpressing the ß2-adrenoceptor. Br. J. Pharmacol. 2000, 131, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.M.; Heubach, J.F.; Ravens, U. The hyperpolarization-activated current If in ventricular myocytes of non-transgenic and ß2-adrenoceptor overexpressing mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2001, 364, 131–139. [Google Scholar] [CrossRef]
- Heubach, J.F.; Graf, E.M.; Molenaar, P.; Jäger, A.; Schröder, F.; Herzig, S.; Harding, S.E.; Ravens, U. Murine ventricular L-type Ca2+ current is enhanced by zinterol via ß1-adrenoceptors, and is reduced in TG4 mice overexpressing the human ß2-adrenoceptor. Br. J. Pharmacol. 2001, 133, 73–82. [Google Scholar] [CrossRef]
- Heubach, J.F.; Blaschke, M.; Harding, S.E.; Ravens, U.; Kaumann, A.J. Cardiostimulant and cardiodepressant effects through overexpressed human ß2-adrenoceptors in murine heart: Regional differences and functional role of ß1-adrenoceptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 380–390. [Google Scholar] [CrossRef]
- Liu, X.; Callaerts-Vegh, Z.; Evans, K.L.; Bond, R.A. Chronic infusion of ß-adrenoceptor antagonist and inverse agonists decreases elevated protein kinase A activity in transgenic mice with cardiac-specific overexpression of human ß2-adrenoceptor. J. Cardiovasc. Pharmacol. 2002, 40, 448–455. [Google Scholar] [CrossRef]
- Nagaraja, S.; Iyer, S.; Liu, X.; Eichberg, J.; Bond, R.A. Treatment with inverse agonists enhances baseline atrial contractility in transgenic mice with chronic beta2-adrenoceptor activation. Br. J. Pharmacol. 1999, 127, 1099–1104. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Cheng, H.; Zhou, Y.-Y.; Wang, D.-J.; Zhu, W.; Ziman, B.; Spurgoen, H.; Lefkowitz, R.J.; Lakatta, E.G.; Koch, W.J.; et al. Inhibition of apontaneous β2-adrenergic activation rescues β1-adrenergic contractile response in cardiomyocytes overexpressing β2-adrenoceptor. J. Biol. Chem. 2000, 275, 21773–21779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Y.; Song, L.-S.; Lakatta, E.G.; Xiao, R.-P.; Cheng, H. Constitutive β2-adrenergic signalling enhances sarcoplasmic reticulum Ca2+ cycling to augment contraction in mouse heart. J. Physiol. 1999, 521, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Cheng, H.; Song, L.S.; Wang, D.; Lakatta, E.G.; Xio, R.P. Spontaneous ß2-adrenergic signaling fails to modulate L-type Ca2+ current in mouse ventricular myocytes. Mol. Pharmacol. 1999, 56, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Callaerts-Vegh, Z.; Evans, K.L.J.; Shipley, G.L.; Davies, P.J.A.; Cuba, D.L.; Gurji, H.A.; Giles, H.; Bond, R.A. Effects of different beta adrenoceptor ligands in mice with permanent occlusion of the left anterior descending coronary artery. Br. J. Pharmacol. 2003, 138, 1505–1516. [Google Scholar] [CrossRef]
- Escobar, A.L.; Fernández-Gómez, R.; Peter, J.-C.; Mobini, R.; Hoebeke, J.; Mijares, A. IgGs and Mabs against the β2-adrenoreceptor block A-V conduction in mouse hearts: A possible role in the pathogenesis of ventricular arrhythmias. J. Mol. Cell. Cardiol. 2006, 40, 829–837. [Google Scholar] [CrossRef]
- Maack, C.; Cremers, B.; Flesch, M.; Höper, A.; Südkamp, M.; Böhm, M. Different intrinsic activities of bucindolol, carvedilol and metoprolol in human failing myocardium. Br. J. Pharmacol. 2000, 130, 1131–1139. [Google Scholar] [CrossRef]
- Maack, C.; Tyroller, S.; Schnabel, P.; Cremers, B.; Dabew, E.; Südkamp, M.; Böhm, M. Characterization of ß1-selectivity, adrenoceptor-Gs-protein interaction and inverse agonism of nebivolol in human myocardium. Br. J. Pharmacol. 2001, 132, 1817–1826. [Google Scholar] [CrossRef]
- Rozier, K.; Bondarenko, V.E. Mathematical modeling physiological effects of the overexpression of β2-adrenoceptors in mouse ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H643–H658. [Google Scholar] [CrossRef]
- Varma, D.R. Ligand-independent negative chronotropic responses of rat and mouse right atria to beta-adrenoceptor antagonists. Can. J. Physiol. Pharmacol. 1999, 77, 943–949. [Google Scholar] [CrossRef]
- Lin, R.; Peng, H.; Nguyen, L.P.; Dudekula, N.B.; Shardonofsky, F.; Knoll, B.J.; Parra, S.; Bond, R.A. Changes in β2-adrenoceptor and other signaling proteins produced by chronic administration of ‘β-blockers’ in a murine asthma model. Pulm. Pharmacol. Ther. 2008, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Lin, R.; Parra, S.; Omoluabi, O.; Hanania, N.A.; Tuvim, M.J.; Knoll, B.J.; Dickey, B.F.; Bond, R.A. ß2-Adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc. Natl. Acad. Sci. USA 2009, 106, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Singh, B.; Okulate, A.A.; Alfaro, V.Y.; Tuvim, M.J.; Dickey, B.F.; Bond, R.A. Complementary anti-inflammatory effects of a ß-blocker and a corticosteroid in an asthma model. Naunyn Schmiedeberg’s Arch. Pharmacol. 2012, 385, 203–210. [Google Scholar] [CrossRef] [PubMed]
- De Vries, B.; Meurs, H.; Roffel, A.F.; Elzinga, C.R.S.; Hoiting, B.H.; de Vries, M.M.L.; Zaagsma, J. β-Agonist-induced constitutive β2-adrenergic receptor activity in bovine tracheal smooth muscle. Br. J. Pharmacol. 2000, 131, 915–920. [Google Scholar] [CrossRef]
- de Vries, B.; Roffel, A.F.; Zaagsma, J.; Meurs, H. Effect of fenoterol-induced constitutive β2-adrenoceptor activity on contractile receptor function in airway smooth muscle. Eur. J. Pharmacol. 2001, 431, 353–359. [Google Scholar] [CrossRef]
- Peitzman, E.R.; Zaidman, N.A.; Maniak, P.J.; O'Grady, S.M. Carvedilol binding to β2-adrenergic receptors inhibits CFTR-dependent anion secretion in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L50–L58. [Google Scholar] [CrossRef]
- Baramki, D.; Koester, J.; Anderson, A.J.; Borish, L. Modulation of T-cell function by (R)- and (S)-isomers of albuterol: Anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomer. J. Allergy Clin. Immunol. 2002, 109, 449–454. [Google Scholar] [CrossRef]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human ß2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Rasmussen, S.G.F.; Choi, H.-J.; Rosenbaum, D.M.; Kobilka, T.S.; Thian, F.S.; Edwards, P.C.; Burghammer, M.; Ratnala, V.R.P.; Sanishvili, R.; Fischetti, R.F.; et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 2007, 450, 383–387. [Google Scholar] [CrossRef]
- Vanni, S.; Neri, M.; Tavernelli, I.; Rothlisberger, U. Predicting novel binding modes of agonists to β adrenergic receptors using all-atom molecular dynamics simulations. PLoS Comput. Biol. 2011, 7, e1001053. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Hall, S.E.; Vaidehi, N. Agonist-induced conformational changes in bovine rhodopsin: Insight into activation of G-protein-coupled receptors. J. Mol. Biol. 2008, 382, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Gether, U.; Lin, S.; Kobilka, B.K. Flurescent labeling of purified ß2 adrenergic receptor. Evidence for ligand-specific conformational changes. J. Biol. Chem. 1995, 270, 28268–28275. J. Biol. Chem. 1995, 270, 28268–28275. [Google Scholar] [PubMed]
- Goetz, A.; Lanig, H.; Gmeiner, P.; Clark, T. Molecular dynamics simulations of the effect of the G-protein and diffusible ligands on the β2-adrenergic receptor. J. Mol. Biol. 2011, 414, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Katritch, V.; Reynolds, K.A.; Cherezov, V.; Hanson, M.A.; Roth, C.B.; Yeager, M.; Abagyan, R. Analysis of full and partial agonists binding to β2-adrenergic receptor suggests a role of transmembrane helix V in agonist-specific conformational changes. J. Mol. Recognit. 2009, 22, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.F.; Choi, H.J.; Fung, J.J.; Pardon, E.; Casarosa, P.; Chae, P.S.; DeVree, B.T.; Rosenbaum, D.M.; Thian, F.S.; Kobilka, T.S. Structure of a nanobody-stabilized active state of the ß2-adrenoceptor. Nature 2011, 469, 175–180. [Google Scholar] [CrossRef]
- Kofuku, Y.; Ueda, T.; Okude, J.; Shiraishi, Y.; Kondo, K.; Maeda, M.; Tsujishita, H.; Shimada, I. Efficacy of the β2-adrenergic receptor is determined by conformational equilibrium in the transmembrane region. Nat. Commun. 2012, 3, 1045. [Google Scholar] [CrossRef]
- Manglik, A.; Kim, T.; Masureel, M.; Altenbach, C.; Yang, Z.; Hilger, D.; Lerch, M.; Kobilka, T.; Thian, F.; Hubbell, W.; et al. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 2015, 161, 1101–1111. [Google Scholar] [CrossRef]
- Kooistra, A.J.; Leurs, R.; de Esch, I.J.P.; de Graaf, C. Structure-based prediction of G-protein-coupled receptor ligand function: A β-adrenoceptor case study. J. Chem. Inf. Modeling 2015, 55, 1045–1061. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Katritch, V.; Abagyan, R. Identifying conformational changes of the b2 adrenoceptor that enable accurate prediction of ligand/receptor interactions and screening for GPCR modulators. J. Comput. Aided Mol. Des. 2009, 23, 273–288. [Google Scholar] [CrossRef][Green Version]
- Weiss, D.R.; Ahn, S.; Sassano, M.F.; Kleist, A.; Zhu, X.; Strachan, R.; Roth, B.L.; Lefkowitz, R.J.; Shoichet, B.K. Conformation guides molecular efficacy in docking screens of activated β-2 adrenergic G protein coupled receptor. ACS Chem. Biol. 2013, 8, 1018–1026. [Google Scholar] [CrossRef]
- Kolb, P.; Rosenbaum, D.M.; Irwin, J.J.; Fung, J.J.; Kobilka, B.K.; Shoichet, B.K. Structure-based discovery of β2-adrenergic receptor ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Bokoch, M.P.; Zou, Y.; Rasmussen, S.G.F.; Liu, C.W.; Nygaard, R.; Rosenbaum, D.M.; Fung, J.J.; Choi, H.J.; Thian, F.S.; Kobilka, T.S.; et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 2010, 463, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Pérez-Sánchez, H.; Zhang, Y.; Shao, Y.; Shi, D.; Liu, H.; Yao, X. Ligand induced change of β2 adrenergic receptor from active to inactive conformation and its implication for the closed/open state of the water channel: Insight from molecular dynamics simulation, free energy calculation and Markov state model analysis. Phys. Chem. Chem. Phys. 2014, 16, 15874–15885. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zhang, Y.; Ban, Y.; Liu, H.; Yao, X. Computational study on the different ligands induced conformation change of β2 adrenergic receptor-Gs protein complex. PLoS ONE 2013, 8, e68138. [Google Scholar] [CrossRef] [PubMed]
- Lakkaraju, S.K.; Lemkul, J.A.; Huang, J.; MacKerell, A.D., Jr. DIRECT-ID: An automated method to identify and quantify conformational variations—Application to β2-adrenergic GPCR. J. Comput. Chem. 2016, 37, 416–425. [Google Scholar] [CrossRef]
- West, G.M.; Chien, E.Y.T.; Katritch, V.; Gatchalian, J.; Chalmers, M.J.; Stevens, R.C.; Griffin, P.R. Ligand-dependent perturbation of the conformational ensemble for the GPCR β2 adrenergic receptor revealed by HDX. Structure 2011, 19, 1424–1432. [Google Scholar] [CrossRef]
- Yao, X.J.; Ruiz, G.V.; Whorton, M.R.; Rasmussen, S.G.F.; DeVree, B.T.; Deupi, X.; Sunahara, R.K.; Kobilka, B. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc. Natl. Acad. Sci. USA 2009, 106, 9501–9506. [Google Scholar] [CrossRef]
- Granier, S.; Kim, S.; Shafer, A.M.; Ratnala, V.R.P.; Fung, J.J.; Zare, R.N.; Kobilka, B. Structure and conformational changes in the C-terminal domain of the ß2-adrenoceptor. Insights from fluorescence resonance energy transfer studies. J. Biol. Chem. 2007, 282, 13895–13905. [Google Scholar]
- Hebert, T.E.; Moffett, S.; Morello, J.-P.; Loisel, T.P.; Bichet, D.G.; Barret, C.; Bouvier, M. A peptide derived from a β2-adrenergic receptor transmembrane domain Inhibits both receptor dimerization and activation. J. Biol. Chem. 1996, 271, 16384–16392. [Google Scholar] [CrossRef]
- DeVree, B.T.; Mahoney, J.P.; Vélez-Ruiz, G.A.; Rasmussen, S.G.F.; Kuszak, A.J.; Edwald, E.; Fung, J.-J.; Manglik, A.; Masureel, M.; Du, Y.; et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature 2016, 535, 182–186. [Google Scholar] [CrossRef]
- Lachance, M.; Ethier, N.; Wolbring, G.; Schnetkamp, P.P.M.; Hébert, T.E. Stable association of G proteins with β2AR is independent of the state of receptor activation. Cell. Signal. 1999, 11, 523–533. [Google Scholar] [CrossRef]
- Insel, P.A.; Tang, C.M.; Hahntow, I.; Michel, M.C. Impact of GPCRs in clinical medicine: Genetic variants and drug targets. Biochim. Biophys. Acta 2007, 1768, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Port, J.D.; Bristow, M.R. ß-Adrenergic receptors, transgenic mice, and pharmacological model systems. Mol. Pharmacol. 2001, 60, 629–631. [Google Scholar] [PubMed]
- Hanania, N.A.; Singh, S.; El-Wali, R.; Flashner, M.; Franklin, A.E.; Garner, W.J.; Dickey, B.F.; Parra, S.; Ruoss, S.; Shardonofsky, F.R.; et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: An open-label pilot study. Pulm. Pharmacol. Ther. 2008, 21, 134–141. [Google Scholar] [CrossRef]
- Thanawala, V.J.; Valdez, D.J.; Joshi, R.; Forkuo, G.S.; Parra, S.; Knoll, B.J.; Bouvier, M.; Leff, P.; Bond, R.A. ß-Blockers have differential effects on the murina asthma phenotype. Br. J. Pharmacol. 2015, 172, 4833–4846. [Google Scholar] [CrossRef]
- Dickey, B.F.; Walker, J.K.L.; Hanania, N.A.; Bond, R.A. β-Adrenoceptor inverse agonists in asthma. Curr. Opin. Pharmacol. 2010, 10, 254–259. [Google Scholar] [CrossRef]
- Penn, R.B. Agonizing over agonism: Should asthmatics turn their β-receptors on or off? Proc. Natl. Acad. Sci. USA 2009, 106, 2095–2096. [Google Scholar] [CrossRef]
- Parra, S.; Bond, R.A. Inverse agonism: From curiosity to accepted dogma, but is it clinically relevant? Curr. Opin. Pharmacol. 2007, 7, 146–150. [Google Scholar] [CrossRef]
- Michel, M.C.; Seifert, R.; Bond, R.A. Dynamic bias and its implications for GPCR drug discovery. Nat. Rev. Drug Discov. 2014, 13, 869–870. [Google Scholar] [CrossRef]
- Michel, M.C.; Charlton, S.J. Biased agonism in drug discovery—Is it too soon to choose a path? Mol. Pharmacol. 2018, 93, 259–265. [Google Scholar] [CrossRef]
- Kenakin, T. Is the quest for signaling bias worth the effort? Mol. Pharmacol. 2018, 93, 266–269. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, M.C.; Michel-Reher, M.B.; Hein, P. A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes. Cells 2020, 9, 1923. https://doi.org/10.3390/cells9091923
Michel MC, Michel-Reher MB, Hein P. A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes. Cells. 2020; 9(9):1923. https://doi.org/10.3390/cells9091923
Chicago/Turabian StyleMichel, Martin C., Martina B. Michel-Reher, and Peter Hein. 2020. "A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes" Cells 9, no. 9: 1923. https://doi.org/10.3390/cells9091923
APA StyleMichel, M. C., Michel-Reher, M. B., & Hein, P. (2020). A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes. Cells, 9(9), 1923. https://doi.org/10.3390/cells9091923





