Outcome of High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma after Different Numbers of Salvage Regimens
Abstract
1. Introduction
2. Patients and Methods
2.1. Setting and Design
2.2. HL Diagnosis and Definitions
2.3. Salvage Therapy, Autologous Stem Cell Harvest and HDT
2.4. Engraftment
2.5. Routine Surveillance and Infection Definitions
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Engraftment
3.3. ASCT Toxicity
3.4. Outcome after HDT/ASCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Tresckow, B.; Engert, A. The role of autologous transplantation in Hodgkin lymphoma. Curr. Hematol. Malig. Rep. 2011, 6, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Linch, D.C.; Winfield, D.; Goldstone, A.H.; Moir, D.; Hancock, B.; McMillan, A.; Chopra, R.; Milligan, D.; Hudson, G.V. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: Results of a BNLI randomised trial. Lancet 1993, 341, 1051–1054. [Google Scholar] [CrossRef]
- Schmitz, N.; Pfistner, B.; Sextro, M.; Sieber, M.; Carella, A.M.; Haenel, M.; Boissevain, F.; Zschaber, R.; Müller, P.; Kirchner, H.; et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet 2002, 359, 2065–2071. [Google Scholar] [PubMed]
- Morschhauser, F.; Brice, P.; Fermé, C.; Diviné, M.; Salles, G.; Bouabdallah, R.; Sebban, C.; Voillat, L.; Casasnovas, O.; Stamatoullas, A.; et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: Results of the prospective multicenter H96 trial by the GELA/SFGM study group. J. Clin. Oncol. 2008, 26, 5980–5987. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.A.; Ceberio, I.; Armand, P.; Burns, L.J.; Chen, R.; Cole, P.D.; Evens, A.M.; Laport, G.G.; Moskowitz, C.H.; Popat, U.; et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Constans, M.; Iriondo, A.; Arranz, R.; Caballero, M.D.; Vidal, M.J.; Petit, J.; López, A.; Lahuerta, J.J.; Carreras, E.; et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann. Oncol. 2005, 16, 625–633. [Google Scholar] [CrossRef]
- Lazarus, H.; Loberiza, F.; Zhang, M.; Armitage, J.O.; Ballen, K.K.; Bashey, A.; Bolwell, B.J.; Burns, L.J.; Freytes, C.O.; Gale, R.P.; et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: A report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplant. 2001, 27, 387–396. [Google Scholar] [CrossRef]
- Santoro, A.; Mazza, R.; Pulsoni, A.; Re, A.; Bonfichi, M.; Zilioli, V.R.; Zanni, M.; Merli, F.; Anastasia, A.; Luminari, S.; et al. Five-year results of the BEGEV salvage regimen in relapsed/refractory classical Hodgkin lymphoma. Blood Adv. 2020, 4, 136–140. [Google Scholar] [CrossRef]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; de Vos, S.; et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar] [CrossRef]
- Gopal, A.K.; Chen, R.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Chi, X.; et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 2015, 125, 1236–1243. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Martin, P.; Tsai, N.; Kim, Y.; Chen, B.T.; Popplewell, L.; Siddiqi, T.; Thomas, S.H.; Mott, M.; et al. Results of a multicenter phase II trial of X vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol. Blood Marrow Transpl. 2015, 21, 2136–2140. [Google Scholar] [CrossRef]
- Chen, R.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Huebner, D.; et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016, 128, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.H.; Moskowitz, A.J.; Bartlett, N.L.; Vose, J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, A.S.; Christian, B.A.; Ansell, S.M.; Moskowitz, C.H.; et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood 2021, 138, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Sica, R.A.; Tamaresis, J.S.; Lu, Y.; Chang, C.; Lowsky, R.; Frank, M.J.; Johnston, L.J.; Miklos, D.B.; Muffly, L.S.; et al. Improved outcomes for relapsed/refractory Hodgkin lymphoma after autologous transplantation in the era of novel agents. Blood 2023, 141, 2727–2737. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Moskowitz, A.J. Where do the new drugs fit in for relapsed/refractory Hodgkin lymphoma? Curr. Hematol. Malig. Rep. 2017, 12, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Merryman, R.W.; Castagna, L.; Giordano, L.; Ho, V.T.; Corradini, P.; Guidetti, A.; Casadei, B.; Bond, D.A.; Jaglowski, S.; Spinner, M.A.; et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia 2021, 35, 2672–2683. [Google Scholar] [CrossRef]
- Shah, G.L.; Moskowitz, C.H. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood 2018, 131, 1689–1697. [Google Scholar] [CrossRef]
- Casadei, B.; Argnani, L.; Morigi, A.; Lolli, G.; Broccoli, A.; Pellegrini, C.; Nanni, L.; Stefoni, V.; Coppola, P.E.; Carella, M.; et al. Potential survival benefit for patients receiving autologous hematopoietic stem cell transplantation after checkpoint inhibitors for relapsed/refractory Hodgkin lymphoma: A real-life experience. Hematol. Oncol. 2020, 38, 737–741. [Google Scholar] [CrossRef]
- Merryman, R.W.; Redd, R.A.; Nishihori, T.; Chavez, J.; Nieto, Y.; Darrah, J.M.; Rao, U.; Byrne, M.T.; Bond, D.A.; Maddocks, K.J.; et al. Autologous stem cell transplantation after anti-PD-1 therapy for multiply relapsed or refractory Hodgkin lymphoma. Blood Adv. 2021, 5, 1648–1659. [Google Scholar] [CrossRef]
- Wang, H.W.; Balakrishna, J.P.; Pittaluga, S.; Jaffe, E.S. Diagnosis of Hodgkin lymphoma in the modern era. Br. J. Haematol. 2019, 184, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Van Den Neste, E.; Casasnovas, O.; André, M.; Touati, M.; Senecal, D.; Edeline, V.; Stamatoullas, A.; Fornecker, L.; Deau, B.; Gastinne, T.; et al. Classical Hodgkin’s lymphoma: The Lymphoma Study Association guidelines for relapsed and refractory adult patients eligible for transplant. Haematologica 2013, 98, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Gooley, T.A.; Leisenring, W.; Crowley, J.; Storer, B.E. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat. Med. 1999, 18, 695–706. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Matasar, M.J.; Zelenetz, A.D.; Nimer, S.D.; Gerecitano, J.; Hamlin, P.; Horwitz, S.; Moskowitz, A.J.; Noy, A.; Palomba, L.; et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 2012, 119, 1665–1670. [Google Scholar] [CrossRef]
- Brice, P.; Divine, M.; Simon, D.; Coiffier, B.; Leblond, V.; Simon, M.; Voilat, L.; Devidas, A.; Morschhauser, F.; Rohrlich, P.; et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin’s disease (HD). SFGM/GELA Study Group. Ann. Oncol. 1999, 10, 1485–1488. [Google Scholar] [CrossRef]
- Bento, L.; Boumendil, A.; Finel, H.; Khvedelidze, I.; Blaise, D.; Fegueux, N.; Castagna, L.; Forcade, E.; Chevallier, P.; Mordini, N.; et al. Tandem autologous-reduced intensity allogeneic stem cell transplantation in high-risk relapsed Hodgkin lymphoma: A retrospective study of the Lymphoma Working Party-EBMT. Bone Marrow Transpl. 2021, 56, 655–663. [Google Scholar] [CrossRef]
- Mariotti, J.; Bramanti, S.; Devillier, R.; Furst, S.; El Cheikh, J.; Sarina, B.; Granata, A.; Faucher, C.; Harbi, S.; Morabito, L.; et al. Tandem autologous-haploidentical transplantation is a feasible and effective program for refractory Hodgkin lymphoma. Bone Marrow Transpl. 2018, 53, 366–370. [Google Scholar] [CrossRef]
- Armand, P.; Zinzani, P.L.L.; Lee, H.J.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; Tomita, A.; et al. Five-year follow-up of KEYNOTE-087: Pembrolizumab monotherapy in relapsed/refractory classical Hodgkin lymphoma (R/R cHL). Blood 2021, 38 (Suppl. S1), 1366. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Thomas, S.H.; Tsai, N.C.; Farol, L.; Nademanee, A.; Forman, S.J.; Gopal, A.K. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood 2012, 119, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Ebadi, M.; Cashen, A.F. Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: A systematic review and meta-analysis. Bone Marrow Transpl. 2016, 5, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Carreau, N.A.; Armand, P.; Merryman, R.W.; Advani, R.H.; Spinner, M.A.; Herrera, A.F.; Ramchandren, R.; Hamid, M.S.; Assouline, S.; Santiago, R.; et al. Checkpoint blockade treatment sensitises relapsed/refractory non-Hodgkin lymphoma to subsequent therapy. Br. J. Haematol. 2020, 191, 44–51. [Google Scholar] [CrossRef]
- Calabretta, E.; Guidetti, A.; Ricci, F.; Di Trani, M.; Monfrini, C.; Magagnoli, M.; Bramanti, S.; Maspero, D.; Morello, L.; Merli, M.; et al. Chemotherapy after PD-1 inhibitors in relapsed/refractory Hodgkin lymphoma: Outcomes and clonal evolution dynamics. Br. J. Haematol. 2022, 198, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.H.; Spinner, M.A.; David, K.A.; Bachanova, V.; Goyal, G.; Azzi, J.; Dorritie, K.; Kenkre, V.P.; Chang, C.; Arai, S.; et al. Novel Salvage Regimens Lead to Better Response and Survival in Relapsed Refractory Classic Hodgkin Lymphoma after Autologous Stem Cell Transplant. Blood 2021, 138 (Suppl. S1), 878. [Google Scholar] [CrossRef]
- Federico, M.; Fortpied, C.; Stepanishyna, Y.; Gotti, M.; van der Maazen, R.; Cristinelli, C.; Re, A.; Plattel, W.; Lazarovici, J.; Merli, F.; et al. Long-Term Follow-Up of the Response-Adapted Intergroup EORTC/LYSA/FIL H10 Trial for Localized Hodgkin Lymphoma. J. Clin. Oncol. 2023, 42, 19–25. [Google Scholar] [CrossRef]
- el Cheikh, J.; Massoud, R.; Abudalle, I.; Haffar, B.; Mahfouz, R.; Kharfan-Dabaja, M.A.; Jisr, T.; Mougharbel, A.; Ibrahim, A.; Bazarbachi, A. Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transpl. 2017, 52, 1074–1077. [Google Scholar] [CrossRef]
- Armand, P.; Zinzani, P.L.; Collins, G.P.; Cohen, J.B.; Halwani, A.S.; Carlo-Stella, C.; Millenson, M.M.; Provencio, M.; Domenech, E.D.; Roth, L.G.; et al. Outcomes of allogeneic hematopoietic stem cell transplantation (HSCT) after treatment with nivolumab for relapsed/refractory Hodgkin lymphoma. Blood 2016, 128, 3502. [Google Scholar] [CrossRef]
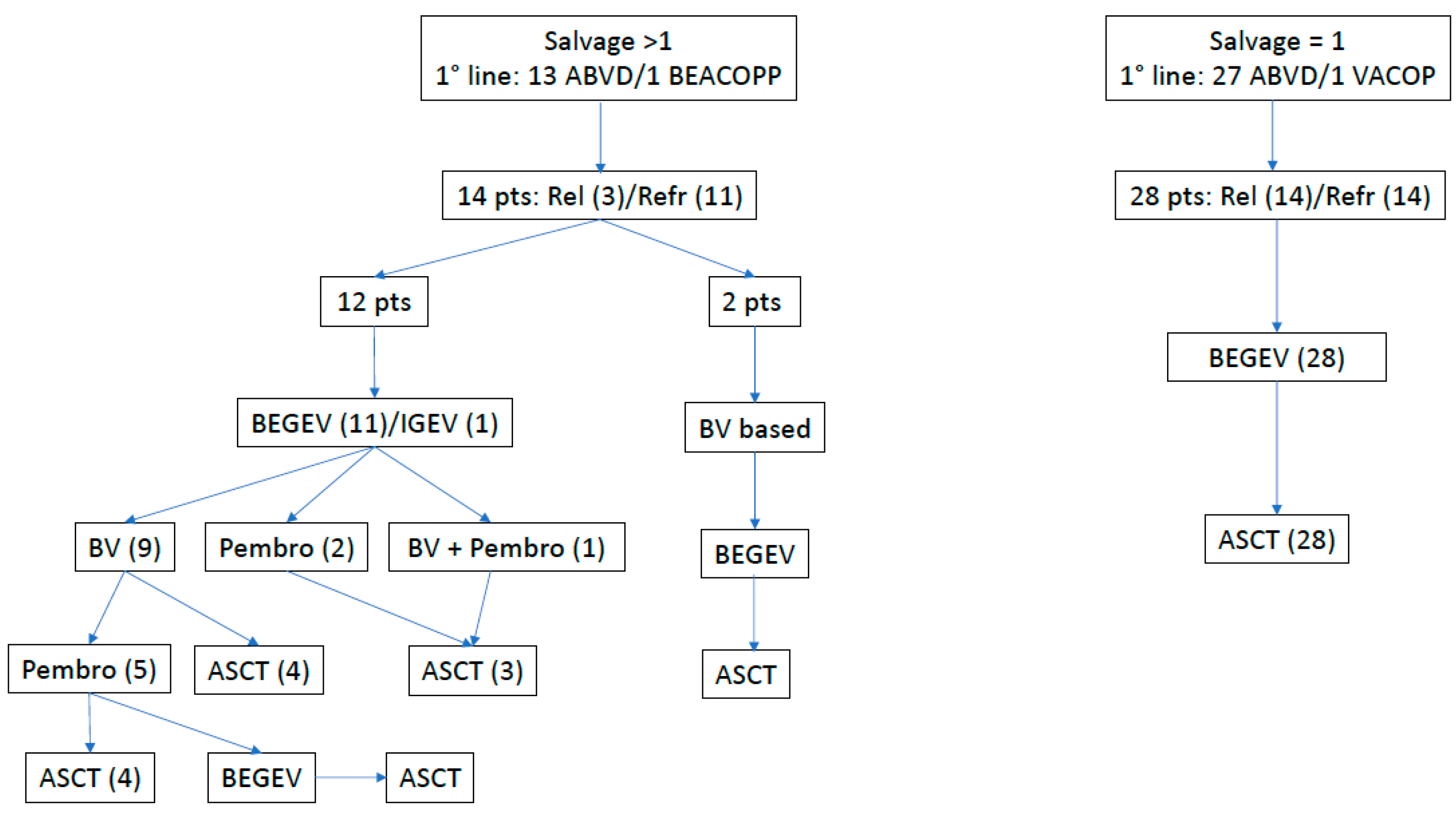


| All, n (%) | 1 Salvage (BEGEV Only) n (%) | >1 Salvage (New Drugs) n (%) | p-Value | |
|---|---|---|---|---|
| N patients | 42 (100) | 28(100) | 14 (100) | - |
| Follow-up (months), median (range) | 24 (3–58) | 24 (3–56) | 23 (3–58) | 0.512 |
| Age (years), median (range) | 35 (18–71) | 37 (20–64) | 32 (18–71) | 0.163 |
| Sex, F/M | 21/21 | 14/14 | 7/7 | 1.000 |
| Stage at diagnosis: | 0.100 | |||
| ● I–II | 21 (51%) | 11 (39%) | 10 (71%) | |
| ● III–IV | 20 (49%) | 16 (61%) | 4 (29%) | |
| Extranodal site at diagnosis: | 0.520 | |||
| ● No | 23 (56%) | 14 (52%) | 9 (64%) | |
| ● Yes | 18 (44%) | 13 (48%) | 5 (36%) | |
| Disease status pre-auto-transplantation: | 0.333 | |||
| ● CR | 41 (98%) | 28 (100%) | 13 (93%) | |
| ● PR | 1 (2%) | 0 | 1 (7%) | |
| Relapsed/refractory: | 0.294 | |||
| ● Refractory | 25 (60%) | 14 (50%) | 11 (79%) | |
| ● Relapsed ≤ 12 months | 11 (26%) | 9 (32%) | 2 (14%) | |
| ● Relapsed > 12 months | 6 (14%) | 5 (18%) | 1 (7%) | |
| Stage at relapse/refractory: | 0.277 | |||
| ● I–II | 30 (71%) | 18 (64%) | 12 (86%) | |
| ● III–IV | 12 (29%) | 10 (36%) | 2 (14%) | |
| Extranodal site at relapse/refractory: | 1.000 | |||
| ● No | 32 (76%) | 21 (75%) | 11 (79%) | |
| ● Yes | 10 (24%) | 7 (25%) | 3 (21%) | |
| n alvage lines before auto-transplantation: | <0.001 | |||
| ● 1 | 28 (67%) | 28 (100%) | - | |
| ● 2 | 9 (21%) | - | 9 (64%) | |
| ● 3 | 4 (10%) | - | 4 (29%) | |
| ● 4 | 1 (2%) | - | 1 (7%) |
| 1 Salvage (BEGEV Only) n (%) | >1 Salvage (New Drugs) n (%) | p-Value | |
|---|---|---|---|
| Mucositis: | 0.526 | ||
| ● Grade 1 | 5 (18%) | 4 (29%) | |
| ● Grade 2 | 3 (11%) | 3 (21%) | |
| ● Grade 3 | 19 (68%) | 7 (50%) | |
| ● None | 1 (3%) | 0 | |
| Diarrhea: | 0.963 | ||
| ● Grade 1 | 5 (18%) | 2(15%) | |
| ● Grade 2 | 5 (18%) | 3 (21%) | |
| ● Grade 3 | 3 (10%) | 1 (7%) | |
| ● None | 15 (54%) | 8 (57%) | |
| Infections: | 0.939 | ||
| ● Fever of unknown origin | 19 (54%) | 11 (58%) | |
| ● Pneumonia | 5 (14%) | 2 (14%) | |
| ● Bacteremia | 4 (10%) | 3 (21%) | |
| ● Septic shock | 1 (4%) | 1 (7%) | |
| ● Virus reactivation | 4 (10%) | 1 (7%) | |
| ● Campylobacter enteritis | 1 (4%) | 1 (7%) | |
| ● Other severe infections | 1 (4%) | – |
| 3-Year OS (%) | p-Value | 3-Year PFS (%) | p-Value | 2-Year CIR (%) | p-Value | |
|---|---|---|---|---|---|---|
| Stage at diagnosis: | 0.746 | 0.526 | 0.522 | |||
| ● I–II | 94 | 79 | 21 | |||
| ● III–IV | 88 | 75 | 25 | |||
| ExN at diagnosis: | 0.731 | 0.715 | 0.714 | |||
| ● No | 91 | 84 | 16 | |||
| ● Yes | 89 | 72 | 28 | |||
| Stage at relapse/refractory: | 0.569 | 0.216 | 0.223 | |||
| ● I–II | 89 | 84 | 16 | |||
| ● III–IV | 100 | 40 | 60 | |||
| ExN at relapse/refractory: | 0.459 | 0.484 | 0.473 | |||
| ● No | 88 | 78 | 22 | |||
| ● Yes | 100 | 75 | 25 | |||
| Type of salvage: | 0.731 | 0.822 | 0.822 | |||
| ● 1 salvage (BEGEV) | 91 | 74 | 26 | |||
| ● >1 salvage (new drugs) | 89 | 83 | 18 | |||
| Relapse/Refractory: | 0.800 | 0.635 | 0.627 | |||
| ● Refractory | 100 | 83 | 17 | |||
| ● <12 months | 83 | 88 | 13 | |||
| ● >12 months | 93 | 72 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariotti, J.; Ricci, F.; Giordano, L.; Taurino, D.; Sarina, B.; De Philippis, C.; Mannina, D.; Carlo-Stella, C.; Bramanti, S.; Santoro, A. Outcome of High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma after Different Numbers of Salvage Regimens. Cells 2024, 13, 118. https://doi.org/10.3390/cells13020118
Mariotti J, Ricci F, Giordano L, Taurino D, Sarina B, De Philippis C, Mannina D, Carlo-Stella C, Bramanti S, Santoro A. Outcome of High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma after Different Numbers of Salvage Regimens. Cells. 2024; 13(2):118. https://doi.org/10.3390/cells13020118
Chicago/Turabian StyleMariotti, Jacopo, Francesca Ricci, Laura Giordano, Daniela Taurino, Barbara Sarina, Chiara De Philippis, Daniele Mannina, Carmelo Carlo-Stella, Stefania Bramanti, and Armando Santoro. 2024. "Outcome of High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma after Different Numbers of Salvage Regimens" Cells 13, no. 2: 118. https://doi.org/10.3390/cells13020118
APA StyleMariotti, J., Ricci, F., Giordano, L., Taurino, D., Sarina, B., De Philippis, C., Mannina, D., Carlo-Stella, C., Bramanti, S., & Santoro, A. (2024). Outcome of High-Dose Chemotherapy Followed by Autologous Stem Cell Transplantation in Relapsed/Refractory Hodgkin Lymphoma after Different Numbers of Salvage Regimens. Cells, 13(2), 118. https://doi.org/10.3390/cells13020118








