Cytokines and Chemokines in Cancer Cachexia and Its Long-Term Impact on COVID-19
Abstract
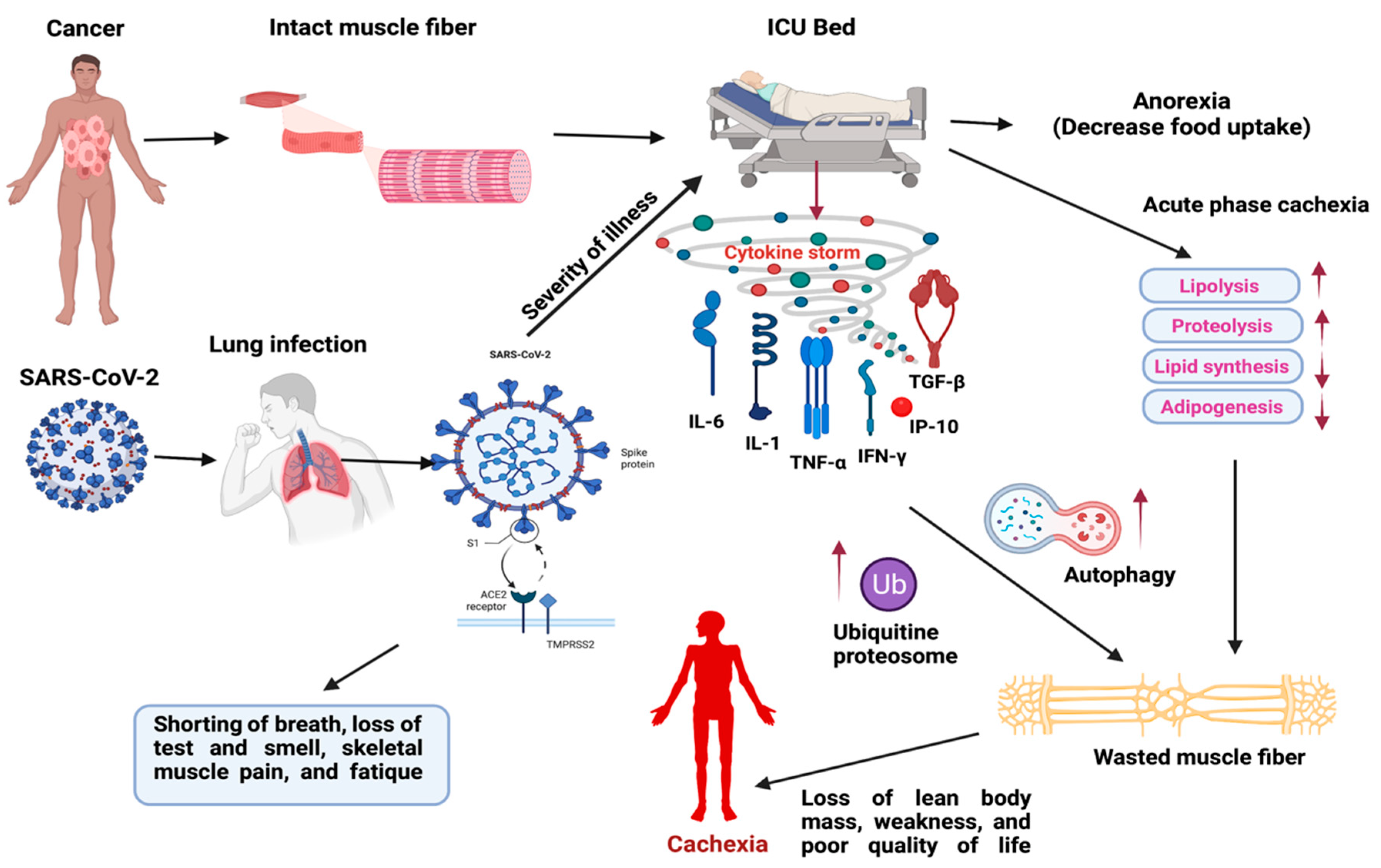
:1. Introduction
2. Local and Systemic Cytokines Associated with Cachexia
2.1. Chemokines in Cachexia
2.2. Cytokines in Cachexia
2.2.1. Interleukins
2.2.2. Tumor Necrosis Factors
2.2.3. Transforming Growth Factor
2.3. SARS-CoV-2 (COVID-19) Induced Cytokine Storm in Muscle Wasting and Their Clinical Management
3. Clinical Management of Cancer Cachexia
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | severe acute respiratory syndrome- corona virus-2 |
| SARS | severe acute respiratory syndrome |
| ACE2 | angiotensin-converting enzyme 2 |
| IL | interleukins |
| IF | interferons |
| TNF | tumor necrosis factors |
| TGF | transforming growth factors |
| CSF | colony-stimulating factors |
| LPL | lipoprotein lipase |
| ATGL | adipocyte triglyceride lipase |
| CRP | C- reactive protein |
| ICU | intensive care unit |
| CIF | cachexia- inducing factors |
| CRC | colorectal cancer |
| TAMs | tumor-associated macrophages |
| MCP1 | monocyte chemoattractant protein 1 |
| ACTRIIB | activin receptor type IIB |
| NFB | nuclear factor B |
| CHO | Chinese hamster ovary |
| CRH | corticotrophin-releasing hormone |
| TNFR1 | TNF-α-receptor protein type I |
| ECM | extracellular matrix |
| RyR | ryanodine receptor5 |
| LSMM | low skeletal muscle mass |
| SMM | skeletal muscle mass |
| CT | computed tomography |
| MERS | middle east respiratory syndrome |
| Th1 | T helper 1 |
| ARDS | acute respiratory distress syndrome |
| CRP | C-reactive protein |
| FDA | Food and Drug Administration |
| NSCLC | non-small cell lung carcinoma |
| MIC1 | macrophage inhibitory cytokine 1 |
| SNP | single-nucleotide polymorphisms |
References
- Freire, P.P.; Fernandez, G.J.; de Moraes, D.; Cury, S.S.; Dal Pai-Silva, M.; Dos Reis, P.P.; Rogatto, S.R.; Carvalho, R.F. The expression landscape of cachexia-inducing factors in human cancers. J. Cachexia Sarcopenia Muscle 2020, 11, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.B. Does COVID-19-related cachexia mimic cancer-related cachexia? Examining mechanisms, clinical biomarkers, and potential targets for clinical management. J. Cachexia Sarcopenia Muscle 2021, 12, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Azcón-Bieto, J. The metabolic environment of cancer. Mol. Cell Biochem. 1988, 81, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Med. Res. Rev. 1999, 19, 223–248. [Google Scholar] [CrossRef]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Fukawa, T.; Yan-Jiang, B.C.; Min-Wen, J.C.; Jun-Hao, E.T.; Huang, D.; Qian, C.N.; Ong, P.; Li, Z.; Chen, S.; Mak, S.Y.; et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat. Med. 2016, 22, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Simbirtsev, A.S.; Kozlov, I.G. Cytokine System. In Mechanical Stretch and Cytokines. Mechanosensitivity in Cells and Tissues; Kamkin, A., Kiseleva, I., Eds.; Springer: Dotrecht, The Netherlands, 2012; Volume 5, pp. 1–33. [Google Scholar] [CrossRef]
- Maccio, A.; Sanna, E.; Neri, M.; Oppi, S.; Madeddu, C. Cachexia as Evidence of the Mechanisms of Resistance and Tolerance during the Evolution of Cancer Disease. Int. J. Mol. Sci. 2021, 22, 2890. [Google Scholar] [CrossRef]
- Baazim, H.; Antonio-Herrera, L.; Bergthaler, A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol. 2021, 1–13. [Google Scholar] [CrossRef]
- Beutler, B.; Mahoney, J.; Le Trang, N.; Pekala, P.; Cerami, A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J. Exp. Med. 1985, 161, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, X.; Heckmann, B.L.; Lu, X.; Liu, J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-α (TNF-α)-induced lipolysis in adipocytes. J. Biol. Chem. 2011, 286, 40477–40485. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Singh, S.; Singh, R. Targeting novel coronavirus SARS-CoV-2 spike protein with phytoconstituents of Momordica charantia. J. Ovarian Res. 2021, 14, 126. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; Pujadas, E.; Wajnberg, A.; Sebra, R.; Patel, G.; Firpo-Betancourt, A.; Fowkes, M.; Sordillo, E.; Paniz-Mondolfi, A.; Gregory, J.; et al. COVID-19: Staging of a New Disease. Cancer Cell 2020, 38, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.H.; Lin, H.M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Macciò, A.; Oppi, S.; Madeddu, C. COVID-19 and cytokine storm syndrome: Can what we know about interleukin-6 in ovarian cancer be applied? J. Ovarian Res. 2021, 14, 28. [Google Scholar] [CrossRef]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachexia Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. Semin. Musculoskelet Radiol. 2011, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.K.; Hsieh, S.T.; Chang, Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan 2005, 14, 113–119. [Google Scholar] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Gerber, M.H.; Underwood, P.W.; Judge, S.M.; Delitto, D.; Delitto, A.E.; Nosacka, R.L.; DiVita, B.B.; Thomas, R.M.; Permuth, J.B.; Hughes, S.J.; et al. Local and Systemic Cytokine Profiling for Pancreatic Ductal Adenocarcinoma to Study Cancer Cachexia in an Era of Precision Medicine. Int. J. Mol. Sci. 2018, 19, 3836. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.; Loetscher, P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001, 2, 123–128. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Tong, Q.; Feng, X. Potential regulatory effects of CXCR2 and CPEB1 on cardiac cachexia. Trop. J. Pharm. Res. 2020, 20, 709–714. [Google Scholar] [CrossRef]
- Scheede-Bergdahl, C.; Watt, H.L.; Trutschnigg, B.; Kilgour, R.D.; Haggarty, A.; Lucar, E.; Vigano, A. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin. Nutr. 2012, 31, 85–88. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, B.; Yang, Z. The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression. Cell Physiol. Biochem. 2018, 45, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.M.; Tan, Y.C.; Beretta, O.; Jiang, D.; Yeap, W.H.; Tai, J.J.; Wong, W.C.; Yang, H.; Schwarz, H.; Lim, K.H.; et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur. J. Immunol. 2012, 42, 89–100. [Google Scholar] [CrossRef]
- Kasprzak, A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef]
- Talbert, E.E.; Lewis, H.L.; Farren, M.R.; Ramsey, M.L.; Chakedis, J.M.; Rajasekera, P.; Haverick, E.; Sarna, A.; Bloomston, M.; Pawlik, T.M.; et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wang, C.J.; Chao, Y.J.; Chen, H.Y.; Wang, H.C.; Tung, H.L.; Lin, J.T.; Shan, Y.S. Elevated Serum Interleukin-8 Level Correlates with Cancer-Related Cachexia and Sarcopenia: An Indicator for Pancreatic Cancer Outcomes. J. Clin. Med. 2018, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Dianliang, Z.; Hongmei, Z.; Xinxiang, W.; Yanbing, Z.; Xiaobo, L. Association of interleukin-8 gene polymorphism with cachexia from patients with gastric cancer. J. Interferon Cytokine Res. 2010, 30, 9–14. [Google Scholar] [CrossRef]
- Xia, W.; Chen, W.; Zhang, Z.; Wu, D.; Wu, P.; Chen, Z.; Li, C.; Huang, J. Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: A meta-analysis. PLoS ONE 2015, 10, e0123484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.L., Jr.; Henriques, F.S.; Neves, R.X.; Olivan, M.R.; Matos-Neto, E.M.; Alcântara, P.S.; Maximiano, L.F.; Otoch, J.P.; Alves, M.J.; Seelaender, M. Cachexia-associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 2016, 7, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef]
- de Matos-Neto, E.M.; Lima, J.D.; de Pereira, W.O.; Figuerêdo, R.G.; Riccardi, D.M.; Radloff, K.; das Neves, R.X.; Camargo, R.G.; Maximiano, L.F.; Tokeshi, F.; et al. Systemic Inflammation in Cachexia—Is Tumor Cytokine Expression Profile the Culprit? Front. Immunol. 2015, 6, 629. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.P.; Kleiman, A.; Moriggl, R.; Spanbroek, R.; Neumann, A.; Illing, A.; Clausen, B.E.; Stride, B.; Förster, I.; Habenicht, A.J.; et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Investig. 2007, 117, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Matthys, P.; Billiau, A. Cytokines and cachexia. Nutrition 1997, 13, 763–770. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Klein, G.L.; Petschow, B.W.; Shaw, A.L.; Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: A new therapeutic target. Curr. Opin. Support. Palliat. Care 2013, 7, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.L.; Walton, K.L.; Winbanks, C.E.; Murphy, K.T.; Thomson, R.E.; Makanji, Y.; Qian, H.; Lynch, G.S.; Harrison, C.A.; Gregorevic, P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014, 28, 1711–1723. [Google Scholar] [CrossRef]
- Braun, T.P.; Grossberg, A.J.; Krasnow, S.M.; Levasseur, P.R.; Szumowski, M.; Zhu, X.X.; Maxson, J.E.; Knoll, J.G.; Barnes, A.P.; Marks, D.L. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 2013, 27, 3572–3582. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Yoshida, T.; Horinouchi, H.; Yagishita, S.; Matsumoto, Y.; Shinno, Y.; Okuma, Y.; Goto, Y.; Yamamoto, N.; Takahashi, K.; et al. Prognostic significance of cachexia in advanced non-small cell lung cancer patients treated with pembrolizumab. Cancer Immunol. Immunother. 2022, 71, 387–398. [Google Scholar] [CrossRef]
- Gerhardt, C.C.; Romero, I.A.; Cancello, R.; Camoin, L.; Strosberg, A.D. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol. Cell Endocrinol. 2001, 175, 81–92. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Uezono, Y.; Minami, K.; Yamaguchi, T.; Niijima, A.; Yada, T.; Maejima, Y.; Sedbazar, U.; Sakai, T.; et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl. Psychiatry 2011, 1, e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macciò, A.; Madeddu, C. Blocking inflammation to improve immunotherapy of advanced cancer. Immunology 2020, 159, 357–364. [Google Scholar] [CrossRef] [Green Version]
- van Norren, K.; Dwarkasing, J.T.; Witkamp, R.F. The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 396–401. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, J.J.; McMillan, D.C.; Laird, B.J.A. Targeting IL-1α in cancer cachexia: A narrative review. Curr. Opin. Support. Palliat. Care 2018, 12, 453–459. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narsale, A.A.; Carson, J.A. Role of interleukin-6 in cachexia: Therapeutic implications. Curr. Opin. Support. Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [Green Version]
- White, J.P.; Puppa, M.J.; Gao, S.; Sato, S.; Welle, S.L.; Carson, J.A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: A role for AMPK. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1042–E1052. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef]
- Oliff, A.; Defeo-Jones, D.; Boyer, M.; Martinez, D.; Kiefer, D.; Vuocolo, G.; Wolfe, A.; Socher, S.H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 1987, 50, 555–563. [Google Scholar] [CrossRef]
- Costelli, P.; Carbó, N.; Tessitore, L.; Bagby, G.J.; Lopez-Soriano, F.J.; Argilés, J.M.; Baccino, F.M. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J. Clin. Investig. 1993, 92, 2783–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, Y.; Moldawer, L.L.; Marano, M.; Wei, H.; Barber, A.; Manogue, K.; Tracey, K.J.; Kuo, G.; Fischman, D.A.; Cerami, A.; et al. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am. J. Physiol. 1989, 256, R659–R665. [Google Scholar] [CrossRef] [PubMed]
- Llovera, M.; García-Martínez, C.; López-Soriano, J.; Agell, N.; López-Soriano, F.J.; Garcia, I.; Argilés, J.M. Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett. 1998, 130, 19–27. [Google Scholar] [CrossRef]
- Tijerina, A.J. The biochemical basis of metabolism in cancer cachexia. Dimens. Crit. Care Nurs. 2004, 23, 237–243. [Google Scholar] [CrossRef]
- Borst, S.E. The role of TNF-alpha in insulin resistance. Endocrine 2004, 23, 177–182. [Google Scholar] [CrossRef]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Van Quickelberghe, E.; De Sutter, D.; van Loo, G.; Eyckerman, S.; Gevaert, K. A protein-protein interaction map of the TNF-induced NF-κB signal transduction pathway. Sci. Data 2018, 5, 180289. [Google Scholar] [CrossRef] [Green Version]
- Mondrinos, M.J.; Alisafaei, F.; Yi, A.Y.; Ahmadzadeh, H.; Lee, I.; Blundell, C.; Seo, J.; Osborn, M.; Jeon, T.J.; Kim, S.M.; et al. Surface-directed engineering of tissue anisotropy in microphysiological models of musculoskeletal tissue. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Lima, J.; Simoes, E.; de Castro, G.; Morais, M.; de Matos-Neto, E.M.; Alves, M.J.; Pinto, N.I.; Figueredo, R.G.; Zorn, T.M.T.; Felipe-Silva, A.S.; et al. Tumour-derived transforming growth factor-β signalling contributes to fibrosis in patients with cancer cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 1045–1059. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C. A TGF-β pathway associated with cancer cachexia. Nat. Med. 2015, 21, 1248–1249. [Google Scholar] [CrossRef] [Green Version]
- Kordes, M.; Larsson, L.; Engstrand, L.; Löhr, J.M. Pancreatic cancer cachexia: Three dimensions of a complex syndrome. Br. J. Cancer 2021, 124, 1623–1636. [Google Scholar] [CrossRef]
- Penna, F.; Baccino, F.M.; Costelli, P. Coming back: Autophagy in cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 241–246. [Google Scholar] [CrossRef]
- Liu, Z.; Sin, K.W.T.; Ding, H.; Doan, H.A.; Gao, S.; Miao, H.; Wei, Y.; Wang, Y.; Zhang, G.; Li, Y.P. p38β MAPK mediates ULK1-dependent induction of autophagy in skeletal muscle of tumor-bearing mice. Cell Stress 2018, 2, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Zhang, G.; Zhang, Z.; Zhu, J.Z.; Zuo, Y.; Frost, J.A.; Li, M.; Li, Y.P. Cancer-Induced Muscle Wasting Requires p38β MAPK Activation of p300. Cancer Res. 2021, 81, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, B.; Li, Y.P. C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J. 2011, 30, 4323–4335. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.L.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.M.; Murray, A.J.; et al. Skeletal muscle alterations in patients with acute COVID-19 and post-acute sequelae of COVID-19. J. Cachexia Sarcopenia Muscle 2022. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buszko, M.; Nita-Lazar, A.; Park, J.H.; Schwartzberg, P.L.; Verthelyi, D.; Young, H.A.; Rosenberg, A.S. Lessons learned: New insights on the role of cytokines in COVID-19. Nat. Immunol. 2021, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Kitase, Y.; Mitra, S.; Erne, C.E.; Novinger, L.J.; Zimmers, T.A.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Radigan, K.A.; Nicholson, T.T.; Welch, L.C.; Chi, M.; Amarelle, L.; Angulo, M.; Shigemura, M.; Shigemura, A.; Runyan, C.E.; Morales-Nebreda, L.; et al. Influenza A Virus Infection Induces Muscle Wasting via IL-6 Regulation of the E3 Ubiquitin Ligase Atrogin-1. J. Immunol. 2019, 202, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.J.; Wienke, A.; Surov, A. Computed tomography-defined body composition as prognostic markers for unfavourable outcomes and in-hospital mortality in coronavirus disease 2019. J. Cachexia Sarcopenia Muscle 2022, 13, 159–168. [Google Scholar] [CrossRef]
- Kizilarslanoglu, M.C.; Kuyumcu, M.E.; Yesil, Y.; Halil, M. Sarcopenia in critically ill patients. J. Anesth. 2016, 30, 884–890. [Google Scholar] [CrossRef]
- Krams, I.A.; Jõers, P.; Luoto, S.; Trakimas, G.; Lietuvietis, V.; Krams, R.; Kaminska, I.; Rantala, M.J.; Krama, T. The Obesity Paradox Predicts the Second Wave of COVID-19 to Be Severe in Western Countries. Int. J. Environ. Res. Public Health 2021, 18, 1029. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R.; Martini, W.Z. Changes in intermediary metabolism in severe surgical illness. World J. Surg. 2000, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Jacob Filho, W.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira Júnior, G.N.; Swinton, P.; Gualano, B.; et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: A prospective observational study. J. Cachexia Sarcopenia Muscle 2021, 12, 1871–1878. [Google Scholar] [CrossRef]
- Cheval, B.; Sieber, S.; Maltagliati, S.; Millet, G.P.; Formánek, T.; Chalabaev, A.; Cullati, S.; Boisgontier, M.P. Muscle strength is associated with COVID-19 hospitalization in adults 50 years of age or older. J. Cachexia Sarcopenia Muscle 2021, 12, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, T.; Ichikawa, Y.; Kitazume, E.; Mizukoshi, A.; Tohara, T.; Takahashi, N.; Tamura, F.; Matsutani, M.; Onishi, J.; Makino, E. COVID-19 Infection-Related Weight Loss Decreases Eating/Swallowing Function in Schizophrenic Patients. Nutrients 2021, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Li, S.B.; Wang, H.Y.; Zhang, S.; Gao, H.N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef]
- De Waele, E.; Mattens, S.; Honoré, P.M.; Spapen, H.; De Grève, J.; Pen, J.J. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite 2015, 91, 298–301. [Google Scholar] [CrossRef]
- Virgens, I.P.A.; Santana, N.M.; Lima, S.; Fayh, A.P.T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 2021, 126, 552–560. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- Munshi, R.; Hussein, M.H.; Toraih, E.A.; Elshazli, R.M.; Jardak, C.; Sultana, N.; Youssef, M.R.; Omar, M.; Attia, A.S.; Fawzy, M.S.; et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021, 93, 733–740. [Google Scholar] [CrossRef]
- Eden, T.; McAuliffe, S. Critical care nutrition and COVID-19: A cause of malnutrition not to be underestimated. BMJ Nutr. Prev. Health 2021, 4, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 79. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoInt therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Prado, B.L.; Qian, Y. Anti-cytokines in the treatment of cancer cachexia. Ann. Palliat. Med. 2019, 8, 67–79. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Hanna, D.L.; Zhang, W.; Baba, H.; Lenz, H.J. Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clin. Cancer Res. 2016, 22, 3999–4004. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Yang, L.; Yu, J.; Lu, M.; Zhang, X.; Li, J.; Zhou, J.; Wang, X.; Gong, J.; Gao, J.; et al. Change of body weight and macrophage inhibitory cytokine-1 during chemotherapy in advanced gastric cancer: What is their clinical significance? PLoS ONE 2014, 9, e88553. [Google Scholar] [CrossRef]
- Tsai, V.W.; Lin, S.; Brown, D.A.; Salis, A.; Breit, S.N. Anorexia-cachexia and obesity treatment may be two sides of the same coin: Role of the TGF-b superfamily cytokine MIC-1/GDF15. Int. J. Obes. 2016, 40, 193–197. [Google Scholar] [CrossRef] [Green Version]
- van Horssen, R.; Ten Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M., Jr.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: Results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef]
- Wiedenmann, B.; Malfertheiner, P.; Friess, H.; Ritch, P.; Arseneau, J.; Mantovani, G.; Caprioni, F.; Van Cutsem, E.; Richel, D.; DeWitte, M.; et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J. Support. Oncol. 2008, 6, 18–25. [Google Scholar] [PubMed]
- Johnston, A.J.; Murphy, K.T.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G.; et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, G.; Macciò, A.; Madeddu, C.; Serpe, R.; Massa, E.; Dessì, M.; Panzone, F.; Contu, P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010, 15, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, T.; Geva, R.; Richards, D.; Madhusudan, S.; Lin, B.K.; Wang, H.T.; Walgren, R.A.; Stemmer, S.M. LY2495655, an antimyostatin antibody, in pancreatic cancer: A randomized, phase 2 trial. J. Cachexia Sarcopenia Muscle 2018, 9, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 2018, 18, 1174. [Google Scholar] [CrossRef] [Green Version]
- Bayliss, T.J.; Smith, J.T.; Schuster, M.; Dragnev, K.H.; Rigas, J.R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin. Biol. Ther. 2011, 11, 1663–1668. [Google Scholar] [CrossRef]
- Hickish, T.; Andre, T.; Wyrwicz, L.; Saunders, M.; Sarosiek, T.; Kocsis, J.; Nemecek, R.; Rogowski, W.; Lesniewski-Kmak, K.; Petruzelka, L.; et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017, 18, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.N.; Trebble, T.M.; Ellis, R.D.; Duncan, H.D.; Johns, T.; Goggin, P.M. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut 2005, 54, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Z.; Güzel, N.A.; Kafa, N.; Köktürk, N. Respiratory muscle strength in volleyball players suffered from COVID-19. Ir. J. Med. Sci. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Patients | Intervention | Status | Identifier Numbers | Initiation Date | Outcomes |
|---|---|---|---|---|---|
| Cachectic or non- cahectic Egyptian patients with lung, pancreas, or colon cancer | Pharmacogenetic testing for TNF-α | Unknown | NCT04131478 | October 2019 | TNF-α -1031T/C and 308 G/A as a cachexia risk factor and SOCS1, TAB2, and FOXP3 biochemical markers |
| Pancreatic cancer | Xilonix (IL-α antagonist) + Onivyde and 5-FU Drug | I | NCT03207724 | July 2017 | Increased LBM, WS, QOL |
| Non-Small Cell Lung Cancer | Anamorelin HCl | III | NCT01395914 | July 2011 | Improved BW and symptom burden |
| Cachexia and anorexia in patients with advanced cancer | Etanercept (TNF-α inhibitor) | III | NCT00046904 | January 2003 | No inhibition of cancer anorexia/weight loss syndrome [115] |
| Pancreatic cancer | Infliximab (TNF-α inhibitor) + Gemcitabine | II | NCT00060502 | May 2003 | No difference in LBM |
| Lung or Pancrease | BYM338 (mAb against activin receptor type2B) | II | NCT01433263 | August 2011 | Increased thigh muscle volume |
| Non-small cell lung cancer | GTx-024 (Enobosarm) | III | NCT01355484 | May 2011 | Improvement in LBM |
| Non-small cell lung cancer | ALD518 (anti-I-L6 mAb) | II | NCT00866970 | March 2009 | Raised Hb, improved cachexia [121] |
| Pancreatic cancer | LY2495655 (anti-myostatin- mAb) | II | NCT01505530 | February 2012 | PFS, OS [119] |
| Colorectal cancer | MABp1 (IL-1α inhibitor) | III | NCT02138422 | May 2014 | Increased LBM, less pain, fatigue, or anorexia [122] |
| Advanced cancer | OHR/AVR118 (immunomodulator that targets TNF-α and IL-6) | II | NCT01206335 | September 2010 | Improved WG, decreased fatigue, improved appetite, and strength |
| Advanced cancer | Cannabis capsules | I | NCT02359123 | February 2015 | Decreased TNF-α level, stable weight, the weight increase of ≥10%, improved appetite, reduced pain and fatigue |
| Advanced tumors | Thalidomide (anti-TNF-α) | II | NCT00027638 | January 2003 | Attenuates loss of weight and LBM [123] |
| Skeletal muscle wasting in SARS-CoV-2 infected patients | Muscle Biopsy | Completed | NCT04698798 | January 2021 | Myalgia, elevated C-reactive protein, and cytokine |
| Respiratory muscle strength in volleyball players suffered from COVID-19 | Respiratory function | Completed | NCT04789512 | March 2021 | COVID-19 players had considerably lower percent maximum inspiratory pressure and measured maximal expiratory pressure values than non-COVID-19 players. [124] |
| Relaxation exercise in patients with COVID-19 | Relaxation technique | Completed | NCT04998708 | August 2021 | Positive effects on immune functions, progressive muscle relaxation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.K.; Singh, R. Cytokines and Chemokines in Cancer Cachexia and Its Long-Term Impact on COVID-19. Cells 2022, 11, 579. https://doi.org/10.3390/cells11030579
Singh SK, Singh R. Cytokines and Chemokines in Cancer Cachexia and Its Long-Term Impact on COVID-19. Cells. 2022; 11(3):579. https://doi.org/10.3390/cells11030579
Chicago/Turabian StyleSingh, Santosh Kumar, and Rajesh Singh. 2022. "Cytokines and Chemokines in Cancer Cachexia and Its Long-Term Impact on COVID-19" Cells 11, no. 3: 579. https://doi.org/10.3390/cells11030579







