Insult to Injury: Cross-Sectional Analysis of Preoperative Psychosocial Vulnerabilities in Adult Patients Undergoing Major Elective Cancer Surgery
Simple Summary
Abstract
1. Introduction
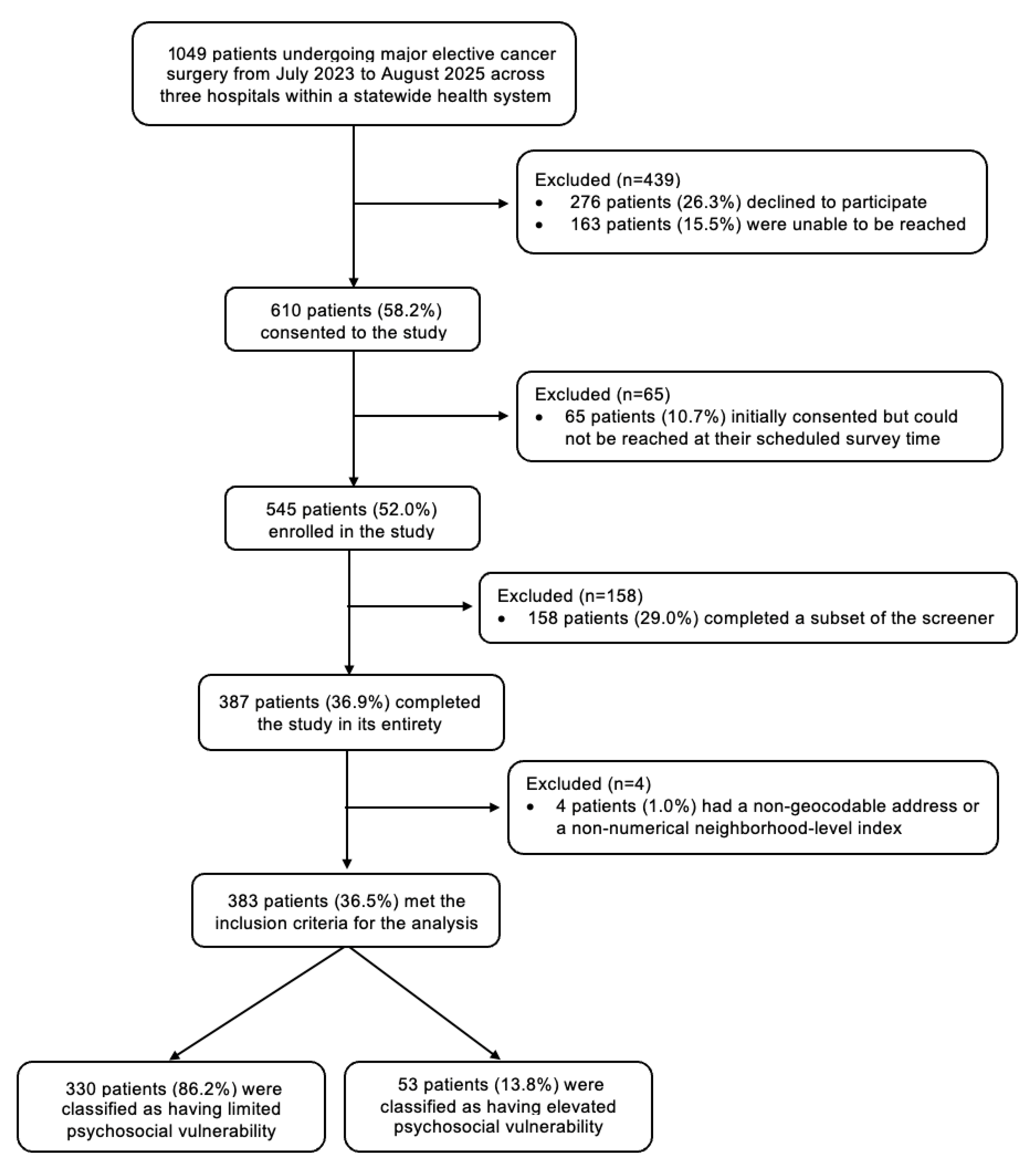
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Inclusion and Exclusion Criteria
2.3. Preoperative Psychosocial Screener
2.4. Sociodemographic and Clinical Covariates of Interest
2.5. Statistical Analyses
3. Results
3.1. Demographics and Overall Preoperative Psychosocial Vulnerability Score
3.2. Psychosocial Vulnerability Across Primary Surgical Services
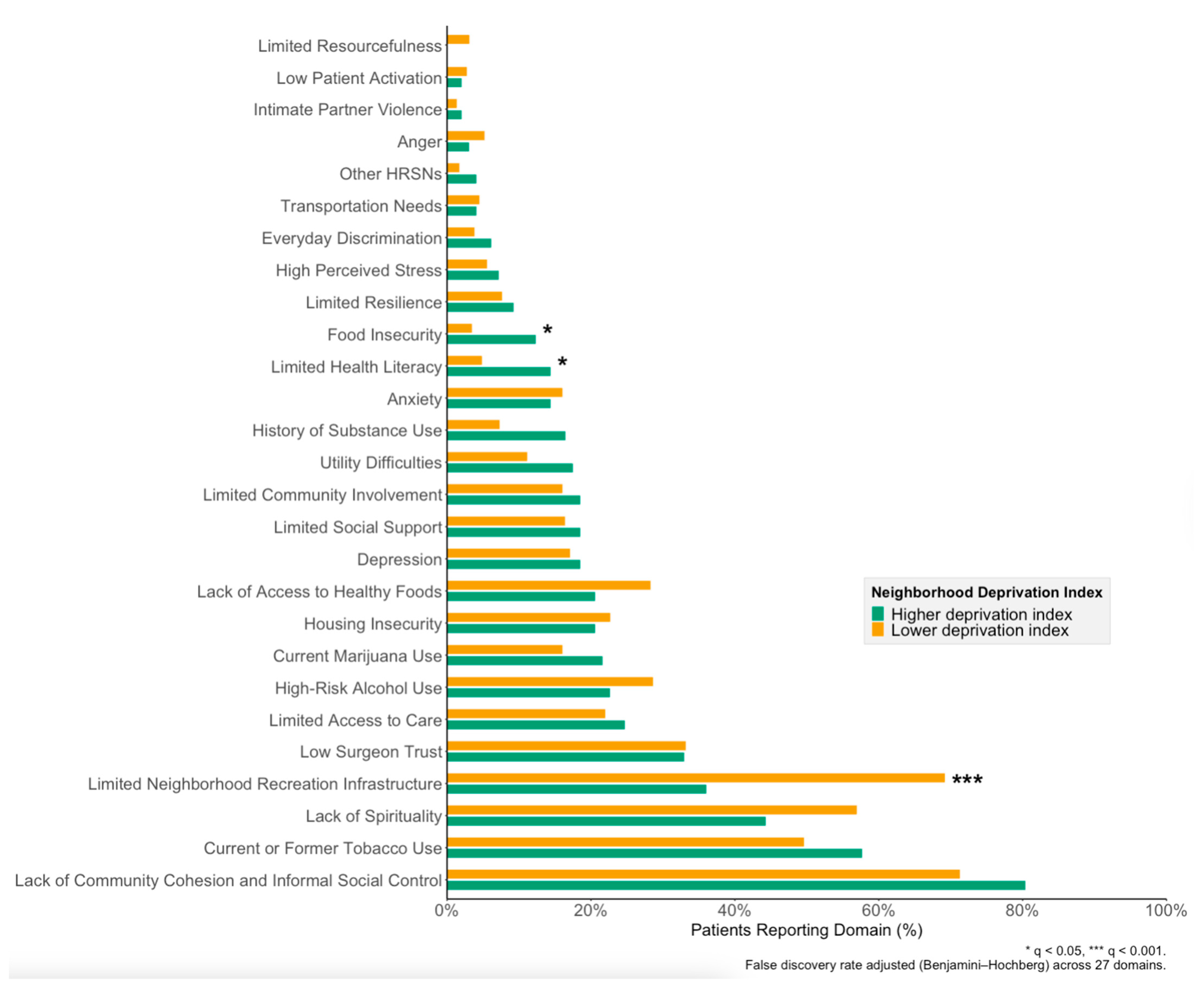
3.3. Relationship Between Patient- and Neighborhood-Level Vulnerability
3.4. Psychosocial Vulnerability by Self-Identified Gender, Race, and Ethnicity
3.5. Psychosocial Vulnerability by Socioeconomic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRSN | Health-related social need |
| SEDOH-88 | Socioecological Determinants of Health-88 |
| ADI | Area Deprivation Index |
| SVI | Social Vulnerability Index |
| FPL | Federal Poverty Line |
| EMR | Electronic medical record |
| REDCap | Research Electronic Data Capture |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| BMI | Body mass index |
| COPD | Chronic obstructive pulmonary disease |
| SUD | Substance use disorder |
| IQR | Interquartile range |
| U.S. | United States |
References
- Guerra-Londono, C.E.; Uribe-Marquez, S.; Shah, R.; Gottumukkala, V. The increasing global burden of cancer: Implications for anaesthesia and peri-operative medicine. Anaesthesia 2025, 80, 3–11. [Google Scholar] [CrossRef]
- Downey, C.L.; Bainbridge, J.; Jayne, D.G.; Meads, D.M. Impact of in-hospital postoperative complications on quality of life up to 12 months after major abdominal surgery. Br. J. Surg. 2023, 110, 1206–1212. [Google Scholar] [CrossRef]
- Healy, M.A.; Mullard, A.J.; Campbell, D.A., Jr.; Dimick, J.B. Hospital and Payer Costs Associated with Surgical Complications. JAMA Surg. 2016, 151, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Yin, H.; Wong, S.L. Postoperative Complications and Long-Term Survival After Complex Cancer Resection. Ann. Surg. Oncol. 2017, 24, 638–644. [Google Scholar] [CrossRef]
- Leeds, I.L.; Moore, M.S.; Schultz, K.; Canner, J.K.; Pantel, H.J.; Mongiu, A.K.; Reddy, V.; Schneider, E. More problems, more money: Identifying and predicting high-cost rescue after colorectal surgery. Surg. Open. Sci. 2023, 16, 148–154. [Google Scholar] [CrossRef]
- Schultz, K.S.; Moore, M.S.; Pantel, H.J.; Mongiu, A.K.; Reddy, V.B.; Schneider, E.B.; Leeds, I.L. For whom the bell tolls: Assessing the incremental costs associated with failure to rescue after elective colorectal surgery. J. Gastrointest. Surg. 2024, 28, 1812–1818. [Google Scholar] [CrossRef]
- Keller, D.S.; Curtis, N.; Burt, H.A.; Ammirati, C.A.; Collings, A.T.; Polk, H.C., Jr.; Carrano, F.M.; Antoniou, S.A.; Hanna, N.; Piotet, L.M.; et al. EAES/SAGES evidence-based recommendations and expert consensus on optimization of perioperative care in older adults. Surg. Endosc. 2024, 38, 4104–4126. [Google Scholar] [CrossRef]
- Leeds, I.L.; Canner, J.K.; Gani, F.; Meyers, P.M.; Haut, E.R.; Efron, J.E.; Johnston, F.M. Increased Healthcare Utilization for Medical Comorbidities Prior to Surgery Improves Postoperative Outcomes. Ann. Surg. 2020, 271, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Butow, P.; Laidsaar-Powell, R.; Konings, S.; Lim, C.Y.S.; Koczwara, B. Return to work after a cancer diagnosis: A meta-review of reviews and a meta-synthesis of recent qualitative studies. J. Cancer Surviv. 2020, 14, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Brandenbarg, D.; Maass, S.; Geerse, O.P.; Stegmann, M.E.; Handberg, C.; Schroevers, M.J.; Duijts, S.F.A. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long-term cancer survivors: Implications for primary care. Eur. J. Cancer Care 2019, 28, e13086. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Dominici, L.S.; Gelber, S.; Poorvu, P.D.; Ruddy, K.J.; Wong, J.S.; Tamimi, R.M.; Schapira, L.; Come, S.; Peppercorn, J.M.; et al. Association of Breast Cancer Surgery With Quality of Life and Psychosocial Well-being in Young Breast Cancer Survivors. JAMA Surg. 2020, 155, 1035–1042. [Google Scholar] [CrossRef]
- Tsimopoulou, I.; Pasquali, S.; Howard, R.; Desai, A.; Gourevitch, D.; Tolosa, I.; Vohra, R. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Ann. Surg. Oncol. 2015, 22, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Leeds, I.L.; Meyers, P.M.; Enumah, Z.O.; He, J.; Burkhart, R.A.; Haut, E.R.; Efron, J.E.; Johnston, F.M. Psychosocial Risks are Independently Associated with Cancer Surgery Outcomes in Medically Comorbid Patients. Ann. Surg. Oncol. 2019, 26, 936–944. [Google Scholar] [CrossRef]
- Schultz, K.S.; Moore, M.S.; Park, E.Y.; Mastrorilli, J.M.; Schneider, E.B.; Pantel, H.J.; Boffa, D.J.; Reddy, V.B.; Leeds, I.L. Patient-Reported Health-Related Social Needs Obtained at the Bedside and Outcomes After Elective Major Surgery. Surgery 2025, in press. [Google Scholar]
- Meyers, P.M.; Leeds, I.L.; Enumah, Z.O.; Burkhart, R.A.; He, J.; Haut, E.R.; Efron, J.E.; Johnston, F.M. Missed psychosocial risk factors during routine preoperative evaluations are associated with increased complications after elective cancer surgery. Surgery 2019, 166, 177–183. [Google Scholar] [CrossRef]
- Schultz, K.S.; Richburg, C.E.; Park, E.Y.; Leeds, I.L. Identifying and optimizing psychosocial frailty in surgical practice. Semin. Colon Rectal Surg. 2024, 35, 101061. [Google Scholar] [CrossRef]
- Area Deprivation Index, v4.0.1; University of Wisconsin School of Medicine Public Health: Madison, WI, USA; Available online: https://www.neighborhoodatlas.medicine.wisc.edu/ (accessed on 11 February 2025).
- Centers for Disease Control and Prevention; Agency for Toxic Substances and Disease Registry; Geospatial Research, Analysis, and Services Program. CDC/ATSDR Social Vulnerability Index 2020 Database USA. Available online: https://www.atsdr.cdc.gov/place-health/php/svi/svi-data-documentation-download.html?CDC_AAref_Val=https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html (accessed on 13 February 2025).
- Flanagan, B.E.; Hallisey, E.J.; Adams, E.; Lavery, A. Measuring community vulnerability to natural and anthropogenic hazards: The Centers for Disease Control and Prevention’s Social Vulnerability Index. J. Environ. Health 2018, 80, 34. [Google Scholar]
- Smith, B.; Smith, B.P.; Hollis, R.H.; Jones, B.A.; Shao, C.; Katta, M.; Wood, L.; Bateman, L.B.; Oates, G.R.; Chu, D.I. Development of a comprehensive survey to assess key socioecological determinants of health. Surgery 2024, 175, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Beckett, M.K.; Martino, S.C.; Agniel, D.; Mathews, M.; Hudson Scholle, S.; James, C.; Wilson-Frederick, S.; Orr, N.; Darabidian, B.; Elliott, M.N. Distinguishing neighborhood and individual social risk factors in health care. Health Serv. Res. 2022, 57, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.S. Ecological Correlations and the Behavior of Individuals. Am. Sociol. Rev. 1950, 15, 351–357. [Google Scholar] [CrossRef]
- Kim, L.Y.; Nishime, N.; Ansah-Twum, J.; Grauer, J.N.; Wiznia, D.H. Disparities in Social Determinants of Health Associated with Decreased Likelihood of Receiving Total Joint Arthroplasty. J. Am. Acad. Orthop. Surg. 2025, in press. [Google Scholar] [CrossRef]
- Theiss, L.M.; Wood, T.; McLeod, M.C.; Shao, C.; Santos Marques, I.D.; Bajpai, S.; Lopez, E.; Duong, A.M.; Hollis, R.; Morris, M.S.; et al. The association of health literacy and postoperative complications after colorectal surgery: A cohort study. Am. J. Surg. 2022, 223, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, A.N.; Berkowitz, S.A.; Ranganathan, K. Strategies to Mitigate Food Insecurity in Patients Undergoing Surgery. JAMA Surg. 2024, 159, 1101–1102. [Google Scholar] [CrossRef]
- Duncan, G.J.; Magnuson, K.; Kunin-Batson, A.S.; Yoshikawa, H.; Fox, N.A.; Halpern-Meekin, S.; Ainsworth, N.J.; Black, S.R.; Nelson, J.M.; Nelson, T.D.; et al. Cash Transfers and Their Effect on Maternal and Young Children’s Health: A Randomized Clinical Trial. JAMA Pediatr. 2025, 179, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Podugu, P.; Ho, V.P.; Crandall, M.L. Addressing Food Insecurity Among US Surgical Patients. JAMA Surg. 2025, 160, 853–854. [Google Scholar] [CrossRef]
- English, N.C.; Jones, B.A.; Chu, D.I. Addressing Low Health Literacy in Surgical Populations. Clin. Colon Rectal Surg. 2025, 38, 26–33. [Google Scholar] [CrossRef]
- Haggerty, J.L.; Levesque, J.F. Validation of a new measure of availability and accommodation of health care that is valid for rural and urban contexts. Health Expect. 2017, 20, 321–334. [Google Scholar] [CrossRef]
- Lamarche, P.A.; Pineault, R.; Haggerty, J.; Hamel, M.; Levesque, J.F.; Gauthier, J. The experience of primary health care users: A rural-urban paradox. Can. J. Rural. Med. 2010, 15, 61–66. [Google Scholar]
- English, N.C.; Smith, B.P.; Jones, B.A.; Oslock, W.; Hollis, R.H.; Wood, L.; Rubyan, M.; Kennedy, G.; Kaushik, M.; Gibson, Q.X.; et al. Novel Characterization of Socioecological Determinants of Health in Rural Alabama. J. Surg. Res. 2024, 301, 468–481. [Google Scholar] [CrossRef]
- Estabrooks, P.A.; Lee, R.E.; Gyurcsik, N.C. Resources for physical activity participation: Does availability and accessibility differ by neighborhood socioeconomic status? Ann. Behav. Med. 2003, 25, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Fecht, D.; Jones, A.; Hill, T.; Lindfield, T.; Thomson, R.; Hansell, A.L.; Shukla, R. Inequalities in rural communities: Adapting national deprivation indices for rural settings. J. Public Health 2018, 40, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.S.; Linhares, S.M.; Godfrey, E.L.; Dhanda, U.; Hellmann, Z.J.; Van Dusen, H.W.; Chu, D.I.; Leeds, I.L. Neighborhood- and patient-level socioecological determinants of health assessed before major surgery. JAMA Netw. Open 2025, in press. [Google Scholar]
- Nelson, K.S.; Nguyen, T.D. Community assets and relative rurality index: A multi-dimensional measure of rurality. J. Rural. Stud. 2023, 97, 322–333. [Google Scholar] [CrossRef]
- Haider, A.H.; Scott, V.K.; Rehman, K.A.; Velopulos, C.; Bentley, J.M.; Cornwell, E.E., III; Al-Refaie, W. Racial disparities in surgical care and outcomes in the United States: A comprehensive review of patient, provider, and systemic factors. J. Am. Coll. Surg. 2013, 216, 482–492e412. [Google Scholar] [CrossRef]
- Hamid, S.A.; Lee, D.H.; Herrin, J.; Yu, J.B.; Pollack, C.E.; Dean, L.T.; Gaddy, J.J.; Oladele, C.R.; Feder, S.L.; Canavan, M.E.; et al. Mediators of Racial Inequities in Non-Small Cell Lung Cancer Care. Cancer Med. 2025, 14, e70757. [Google Scholar] [CrossRef]
- Leeds, I.L.; Drabo, E.F.; Lehmann, L.S.; Safar, B.; Johnston, F.M. On All Accounts: Cost-Effectiveness Analysis of Limited Preoperative Optimization Efforts Before Colon Cancer Surgery. Dis. Colon Rectum 2021, 64, 744–753. [Google Scholar] [CrossRef]
- Howard, R.; Delaney, L.; Kilbourne, A.M.; Kidwell, K.M.; Smith, S.; Englesbe, M.; Dimick, J.; Telem, D. Development and Implementation of Preoperative Optimization for High-Risk Patients With Abdominal Wall Hernia. JAMA Netw. Open 2021, 4, e216836. [Google Scholar] [CrossRef]
- Lussiez, A.; Hallway, A.; Lui, M.; Perez-Escolano, J.; Sukhon, D.; Palazzolo, W.; Elhady, H.; Englesbe, M.; Howard, R. Evaluation of an Intervention to Address Smoking and Food Insecurity at Preoperative Surgical Clinic Appointments. JAMA Netw. Open 2022, 5, e2238677. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Rieck, H.; Roy, S.; Farjo, R.; Preston, Y.; Elhady, H.; Englesbe, M.; Brummett, C.; Waljee, J.; Bicket, M.C. Patient-related acceptability of implementing preoperative screening for at-risk opioid and substance use. Pain Med. 2023, 24, 1014–1016. [Google Scholar] [CrossRef]
- Schneiderman, N.; McIntosh, R.C.; Antoni, M.H. Psychosocial risk and management of physical diseases. J. Behav. Med. 2019, 42, 16–33. [Google Scholar] [CrossRef]
- Howard, R.; Englesbe, M. Leveraging the perioperative period to improve population health. Perioper. Med. 2023, 12, 21. [Google Scholar] [CrossRef]
- Bamdad, M.C.; Englesbe, M.J. Surgery and Population Health-Redesigning Surgical Quality for Greater Impact. JAMA Surg. 2020, 155, 799–800. [Google Scholar] [CrossRef]
- Petridis, A.P.; Koh, C.; Solomon, M.; Karunaratne, S.; Alexander, K.; Hirst, N.; Pillinger, N.; Denehy, L.; Riedel, B.; Gillis, C.; et al. An Online Preoperative Screening Tool to Optimize Care for Patients Undergoing Cancer Surgery: A Mixed-Method Study Protocol. Cancers 2025, 17, 861. [Google Scholar] [CrossRef]
- Lussiez, A.; Englesbe, M.; Howard, R. Surgery and Population Health: Closing the Gap Between Margin and Mission. Ann. Surg. 2022, 275, e286–e287. [Google Scholar] [CrossRef]
- Howard, R.; Yin, Y.S.; McCandless, L.; Wang, S.; Englesbe, M.; Machado-Aranda, D. Taking Control of Your Surgery: Impact of a Prehabilitation Program on Major Abdominal Surgery. J. Am. Coll. Surg. 2019, 228, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.E.; Liu, Y.; Hall, B.L.; Ko, C.Y. The Accuracy of the NSQIP Universal Surgical Risk Calculator Compared to Operation-Specific Calculators. Ann. Surg. Open 2023, 4, e358. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Torres, C.I.H.; Gold, R.; Kaufmann, J.; Marino, M.; Hoopes, M.J.; Totman, M.S.; Aceves, B.; Gottlieb, L.M. Social Risk Screening and Response Equity: Assessment by Race, Ethnicity, and Language in Community Health Centers. Am. J. Prev. Med. 2023, 65, 286–295. [Google Scholar] [CrossRef]
- Davis, R.E.; Couper, M.P.; Janz, N.K.; Caldwell, C.H.; Resnicow, K. Interviewer effects in public health surveys. Health Educ. Res. 2010, 25, 14–26. [Google Scholar] [CrossRef]
- Adler, R.R.; Smith, R.N.; Fowler, K.J.; Gates, J.; Jefferson, N.M.; Adler, J.T.; Patzer, R.E. Community Based Participatory Research (CBPR): An Underutilized Approach to Address Surgical Disparities. Ann. Surg. 2022, 275, 496–499. [Google Scholar] [CrossRef]
- Vest, J.R.; Mazurenko, O. Non-response Bias in Social Risk Factor Screening Among Adult Emergency Department Patients. J. Med. Syst. 2023, 47, 78. [Google Scholar] [CrossRef]
- Dupuis, M.; Strippoli, M.F.; Gholam-Rezaee, M.; Preisig, M.; Vandeleur, C.L. Mental disorders, attrition at follow-up, and questionnaire non-completion in epidemiologic research. Illustrations from the CoLaus|PsyCoLaus study. Int. J. Methods Psychiatr. Res. 2019, 28, e1805. [Google Scholar] [CrossRef]
- Bowling, A. Mode of questionnaire administration can have serious effects on data quality. J. Public Health 2005, 27, 281–291. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, E.D. Choosing the Method of Data Collection; Lawrence Erlbaum: New York, NY, USA, 2008; pp. 113–135. [Google Scholar]
- Dickson, E.; Kuhlemeier, A.; Adsul, P.; Myers, K.; Akintobi, T.; Rosas, L.; Mendoza, J.; Oetzel, J.; Castro-Reyes, P.; Alaniz, C.; et al. Developing the Engage for Equity Institutional Multi-Sector Survey: Assessing Academic Institutional Culture and Climate for Community-Based Participatory Research (CBPR). J. Clin. Transl. Sci. 2025, 9, e44. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, I.; National Institute on Aging Working, G. Multidimensional Measurement of Religiousness/Spirituality for Use in Health Research: A Report of the Fetzer Institute/National Institute on Aging Working Group, Reprinted Edition; originally published 1999, reprinted October 2003. ed.; Fetzer Institute: Kalamazoo, MI, USA, 2003. [Google Scholar]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Guide for constructing self-efficacy scales. In Self-Efficacy Beliefs of Adolescents; Pajares, F., Urdan, T., Eds.; Information Age Publishing: Greenwich, CT, USA, 2006; Volume 5, pp. 307–337. [Google Scholar]
- Siegel, J.M. The Multidimensional Anger Inventory. J. Pers. Soc. Psychol. 1986, 51, 191–200. [Google Scholar] [CrossRef]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT Alcohol Consumption Questions (AUDIT-C): An effective brief screening test for problem drinking. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services; National Institutes of Health; National Institute on Drug Abuse, and United States Department of Health and Human Services; Food and Drug Administration; Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files; Inter-University Consortium for Political and Social Research: Ann Arbor, MI, USA, 2025. [Google Scholar] [CrossRef]
- Hammond, D.; Goodman, S.; Wadsworth, E.; Rynard, V.; Boudreau, C.; Hall, W. Evaluating the impacts of cannabis legalization: The International Cannabis Policy Study. Int. J. Drug Policy 2020, 77, 102698. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.J.; Babor, T.F.; Kranzler, H.R. Validity of the Drug Abuse Screening Test (DAST-10) in inpatient substance abusers: Problems of drug dependence. In Proceedings of the 53rd Annual Scientific Meeting; Committee on Problems of Drug Dependence, Inc.: Rockville, MD, USA, 1991; p. 233. [Google Scholar]
- Hager, E.R.; Quigg, A.M.; Black, M.M.; Coleman, S.M.; Heeren, T.; Rose-Jacobs, R.; Cook, J.T.; Ettinger de Cuba, S.A.; Casey, P.H.; Chilton, M.; et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics 2010, 126, e26–e32. [Google Scholar] [CrossRef] [PubMed]
- PRAPARE® Implementation and Action Toolkit. Available online: https://prapare.org/prapare-toolkit/ (accessed on 17 August 2025).
- Billioux, A.; Verlander, K.; Anthony, S.; Alley, D. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspect. 2017. [Google Scholar] [CrossRef]
- Moser, A.; Stuck, A.E.; Silliman, R.A.; Ganz, P.A.; Clough-Gorr, K.M. The eight-item modified Medical Outcomes Study Social Support Survey: Psychometric evaluation showed excellent performance. J. Clin. Epidemiol. 2012, 65, 1107–1116. [Google Scholar] [CrossRef]
- Office of Disease, P.; Health, P. National Health Interview Survey (NHIS). Available online: https://odphp.health.gov/healthypeople/objectives-and-data/data-sources-and-methods/data-sources/national-health-interview-survey-nhis (accessed on 17 August 2025).
- Hibbard, J.H.; Stockard, J.; Mahoney, E.R.; Tusler, M. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 2004, 39, 1005–1026. [Google Scholar] [CrossRef]
- Baker, D.W.; Williams, M.V.; Parker, R.M.; Gazmararian, J.A.; Nurss, J. Development of a brief test to measure functional health literacy. Patient Educ. Couns. 1999, 38, 33–42. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Haerpfer, C.; Inglehart, R.; Moreno, A.; Welzel, C.; Kizilova, K.; Diez-Medrano, J.; Lagos, M.; Norris, P.; Ponarin, E.; Puranen, B. World Values Survey: Round Seven–Country-Pooled Datafile Version 5.0. Available online: https://www.worldvaluessurvey.org/WVSDocumentationWV7.jsp (accessed on 17 August 2025).
- Hall, M.A.; Zheng, B.; Dugan, E.; Camacho, F.; Kidd, K.E.; Mishra, A.; Balkrishnan, R. Measuring patients’ trust in their primary care providers. Med. Care. Res. Rev. 2002, 59, 293–318. [Google Scholar] [CrossRef]
- Williams, D.R.; Yan, Y.; Jackson, J.S.; Anderson, N.B. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J. Health Psychol. 1997, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Bild, D.E.; Bluemke, D.A.; Burke, G.L.; Detrano, R.; Diez Roux, A.V.; Folsom, A.R.; Greenland, P.; Jacob, D.R., Jr.; Kronmal, R.; Liu, K.; et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am. J. Epidemiol. 2002, 156, 871–881. [Google Scholar] [CrossRef]
- Saelens, B.E.; Sallis, J.F.; Black, J.B.; Chen, D. Neighborhood-based differences in physical activity: An environment scale evaluation. Am. J. Public Health 2003, 93, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Sampson, R.J.; Raudenbush, S.W.; Earls, F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science 1997, 277, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Schultz, K.; Pantel, H.; Mongiu, A.; Reddy, V.; Leeds, I. Preoperative Psychosocial Risk Burden Among Patients Undergoing Major Thoracic and Abdominal Surgery. In Proceedings of the Society for Surgery of the Alimentary Tract, Washington, DC, USA, 18–21 May 2024. [Google Scholar]

| Characteristics, n (%) | Overall (n = 383) | Limited Psychosocial Vulnerability (n = 330) | Elevated Psychosocial Vulnerability a (n = 53) | p-Value |
| Age, years, (median, IQR) | 66 (57–73) | 66 (58–73) | 63 (51–70) | 0.021 |
| Sex assigned at birth | 0.568 | |||
| Male | 191 (50) | 167 (51) | 24 (45) | |
| Female | 192 (50) | 163 (49) | 29 (55) | |
| Self-identified gender | 0.596 | |||
| Man | 190 (50) | 166 (50) | 24 (45) | |
| Woman | 193 (50) | 164 (50) | 29 (55) | |
| Self-identified race | <0.001 | |||
| White | 350 (91) | 310 (94) | 40 (75) | |
| Non-white | 33 (8.6) | 20 (6.1) | 13 (25) | |
| Self-identified ethnicity | 0.500 | |||
| Non-Hispanic | 363 (95) | 314 (95) | 49 (92) | |
| Hispanic | 20 (5.2) | 16 (4.8) | 4 (7.5) | |
| Sexual identity | 0.014 | |||
| Heterosexual | 367 (96) | 320 (97) | 47 (89) | |
| Non-heterosexual | 16 (4.2) | 10 (3.0) | 6 (11) | |
| Marital status | <0.001 | |||
| Partnered | 252 (66) | 230 (70) | 22 (42) | |
| Non-partnered | 131 (34) | 100 (30) | 31 (58) | |
| Education | 0.057 | |||
| <Four-year college degree | 167 (44) | 137 (42) | 30 (57) | |
| ≥Four-year college degree | 216 (56) | 193 (58) | 23 (43) | |
| Employment status | 0.152 | |||
| Employed | 161 (42) | 144 (44) | 17 (32) | |
| Not employed | 222 (58) | 186 (56) | 36 (68) | |
| Primary insurance coverage | 0.040 | |||
| Government | 224 (58) | 189 (57) | 35 (66) | |
| Private insurance | 155 (40) | 139 (42) | 16 (30) | |
| Uninsured | 4 (1.0) | 2 (0.6) | 2 (3.8) | |
| Socioeconomic status b | <0.001 | |||
| <U.S. FPL | 13 (3.4) | 7 (2.1) | 6 (11) | |
| 100–200% U.S. FPL | 36 (9.4) | 30 (9.1) | 6 (11) | |
| ≥200% U.S. FPL | 308 (80) | 276 (84) | 32 (60) | |
| Unknown | 26 (6.8) | 17 (5.2) | 9 (17) | |
| High-risk medical comorbidity c | ||||
| ≥1 comorbidity | 190 (50) | 158 (48) | 32 (60) | 0.123 |
| Number of high-risk medical comorbidities | 0.323 | |||
| 0 | 193 (50) | 172 (52) | 21 (40) | |
| 1 | 110 (29) | 90 (27) | 20 (38) | |
| 2 | 45 (12) | 39 (12) | 6 (11) | |
| 3+ | 35 (9.1) | 29 (8.8) | 6 (11) | |
| Primary surgical service | 0.449 | |||
| Thoracic | 137 (36) | 119 (36) | 18 (34) | |
| Surgical oncology | 93 (24) | 83 (25) | 10 (19) | |
| Colorectal | 153 (40) | 128 (39) | 25 (47) |
| Neighborhood-Level Indices, Median (IQR) | Overall (n = 383) | Limited Psychosocial Vulnerability (n = 330) | Elevated Psychosocial Vulnerability (n = 53) | p-Value | q-Value * |
| ADI percentile | 30.0 (20.0–43.0) | 29.00 (20.0–43.0) | 34.0 (23.0–50.0) | 0.035 | --- |
| SVI percentile (overall) | 0.28 (0.13–0.52) | 0.27 (0.11–0.51) | 0.35 (0.23–0.82) | 0.005 | --- |
| Theme 1—Socioeconomic Status | 0.26 (0.13–0.50) | 0.24 (0.13–0.46) | 0.38 (0.17–0.75) | 0.005 | 0.008 |
| Theme 2—Household Composition and Disability | 0.39 (0.22–0.63) | 0.38 (0.20–0.60) | 0.49 (0.35–0.77) | 0.006 | 0.008 |
| Theme 3—Minority Status and Language | 0.33 (0.19–0.52) | 0.32 (0.19–0.49) | 0.41 (0.27–0.71) | 0.001 | 0.005 |
| Theme 4—Housing Type and Transportation | 0.34 (0.15–0.57) | 0.34 (0.15–0.56) | 0.38 (0.20–0.73) | 0.211 | 0.211 |
| Psychosocial Domains, n (%) | Overall (n = 383) | Low Income (n = 13) | Middle Income (n = 36) | High Income (n = 308) | Unknown * (n = 26) | p-Value | q-Value † |
| Psychological domains | |||||||
| ≥Moderate anxiety | 60 (16) | 4 (31) | 3 (8.3) | 48 (16) | 5 (19) | 0.239 | 0.293 |
| ≥Moderate depression | 67 (17) | 5 (38) | 6 (17) | 49 (16) | 7 (27) | 0.097 | 0.164 |
| Lack of spirituality/religion | 206 (54) | 3 (23) | 17 (47) | 173 (56) | 13 (50) | 0.093 | 0.164 |
| Low resilience | 31 (8.1) | 2 (15) | 3 (8.3) | 22 (7.1) | 4 (15) | 0.250 | 0.293 |
| Limited resourcefulness | 9 (2.3) | 3 (23) | 0 (0) | 4 (1.3) | 2 (7.7) | <0.001 | 0.004 |
| Anger | 18 (4.7) | 2 (15) | 0 (0) | 10 (3.2) | 6 (23) | <0.001 | 0.003 |
| High-risk alcohol use | 104 (27) | 0 (0) | 8 (22) | 89 (29) | 7 (27) | 0.124 | 0.186 |
| History of tobacco use | 198 (52) | 6 (46) | 22 (61) | 154 (50) | 16 (62) | 0.423 | 0.431 |
| Current marijuana use | 67 (17) | 3 (23) | 3 (8.3) | 56 (18) | 5 (19) | 0.431 | 0.431 |
| History of SUD | 37 (9.7) | 4 (31) | 7 (19) | 24 (7.8) | 2 (7.7) | 0.010 | 0.023 |
| Social domains | |||||||
| Food insecurity | 22 (5.7) | 3 (23) | 5 (14) | 9 (2.9) | 5 (19) | <0.001 | 0.001 |
| Transportation needs | 17 (4.4) | 2 (15) | 2 (5.6) | 9 (2.9) | 4 (15) | 0.006 | 0.017 |
| Housing insecurity | 85 (22) | 5 (38) | 11 (31) | 61 (20) | 8 (31) | 0.134 | 0.191 |
| Utility difficulties | 49 (13) | 5 (38) | 9 (25) | 29 (9.4) | 6 (23) | <0.001 | 0.003 |
| Intimate partner violence | 6 (1.6) | 1 (7.7) | 0 (0) | 1 (0.3) | 4 (15) | <0.001 | 0.001 |
| Limited social support | 65 (17) | 5 (38) | 9 (25) | 43 (14) | 8 (31) | 0.009 | 0.021 |
| Limited access to care | 87 (23) | 2 (15) | 9 (25) | 63 (20) | 13 (50) | 0.006 | 0.017 |
| Low patient activation | 10 (2.6) | 3 (23) | 0 (0) | 5 (1.6) | 2 (7.7) | 0.002 | 0.006 |
| Limited health literacy | 28 (7.3) | 3 (23) | 7 (19) | 14 (4.5) | 4 (15) | <0.001 | 0.003 |
| High perceived stress | 23 (6.0) | 3 (23) | 2 (5.6) | 15 (4.9) | 3 (12) | 0.031 | 0.064 |
| Limited community involvement | 64 (17) | 5 (38) | 7 (19) | 47 (15) | 5 (19) | 0.148 | 0.200 |
| Limited surgeon trust | 127 (33) | 7 (54) | 10 (28) | 100 (32) | 10 (38) | 0.337 | 0.379 |
| Everyday discrimination | 17 (4.4) | 2 (15) | 3 (8.3) | 12 (3.9) | 0 (0) | 0.076 | 0.146 |
| Lack of access to healthy foods | 101 (26) | 2 (15) | 10 (28) | 87 (28) | 2 (7.7) | 0.108 | 0.172 |
| Limited neighborhood recreation infrastructure | 233 (61) | 6 (46) | 17 (47) | 195 (63) | 15 (58) | 0.181 | 0.233 |
| Lack of community cohesion and informal social control | 282 (74) | 12 (92) | 28 (78) | 222 (72) | 20 (77) | 0.364 | 0.394 |
| Other HRSNs ‡ | 9 (2.3) | 2 (15) | 2 (5.6) | 3 (1.0) | 2 (7.7) | 0.002 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, K.S.; Linhares, S.M.; Park, E.Y.; Godfrey, E.L.; Dhanda, U.; Epstein, E.J.; Blake, K.B.T.; Huang, Y.; Zaheer, H.; Leeds, I.L. Insult to Injury: Cross-Sectional Analysis of Preoperative Psychosocial Vulnerabilities in Adult Patients Undergoing Major Elective Cancer Surgery. Cancers 2025, 17, 2859. https://doi.org/10.3390/cancers17172859
Schultz KS, Linhares SM, Park EY, Godfrey EL, Dhanda U, Epstein EJ, Blake KBT, Huang Y, Zaheer H, Leeds IL. Insult to Injury: Cross-Sectional Analysis of Preoperative Psychosocial Vulnerabilities in Adult Patients Undergoing Major Elective Cancer Surgery. Cancers. 2025; 17(17):2859. https://doi.org/10.3390/cancers17172859
Chicago/Turabian StyleSchultz, Kurt S., Samantha M. Linhares, Emily Y. Park, Elizabeth L. Godfrey, Uday Dhanda, Eliza J. Epstein, Kathryn Bailey Thomson Blake, Yuqing Huang, Haadia Zaheer, and Ira L. Leeds. 2025. "Insult to Injury: Cross-Sectional Analysis of Preoperative Psychosocial Vulnerabilities in Adult Patients Undergoing Major Elective Cancer Surgery" Cancers 17, no. 17: 2859. https://doi.org/10.3390/cancers17172859
APA StyleSchultz, K. S., Linhares, S. M., Park, E. Y., Godfrey, E. L., Dhanda, U., Epstein, E. J., Blake, K. B. T., Huang, Y., Zaheer, H., & Leeds, I. L. (2025). Insult to Injury: Cross-Sectional Analysis of Preoperative Psychosocial Vulnerabilities in Adult Patients Undergoing Major Elective Cancer Surgery. Cancers, 17(17), 2859. https://doi.org/10.3390/cancers17172859








