Impact of Structured Postoperative Surveillance on Survival in Patients with Resected Pancreatic Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Structured Surveillance
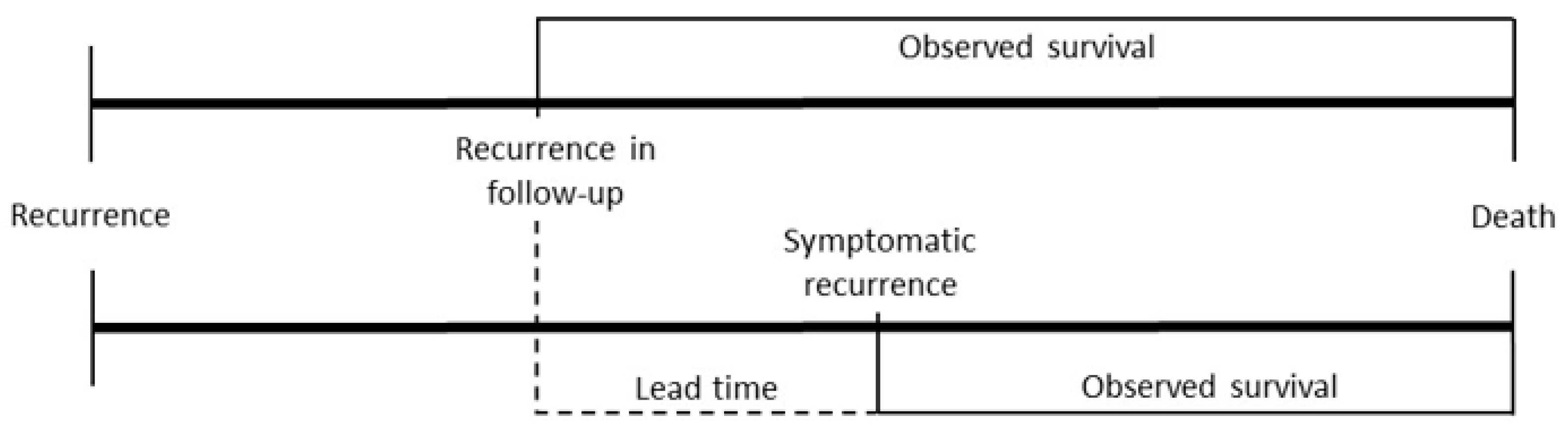
2.2. Survival Definitions
2.3. Surgical Procedures
2.4. Postoperative Course
2.5. Statistical Analysis
3. Results
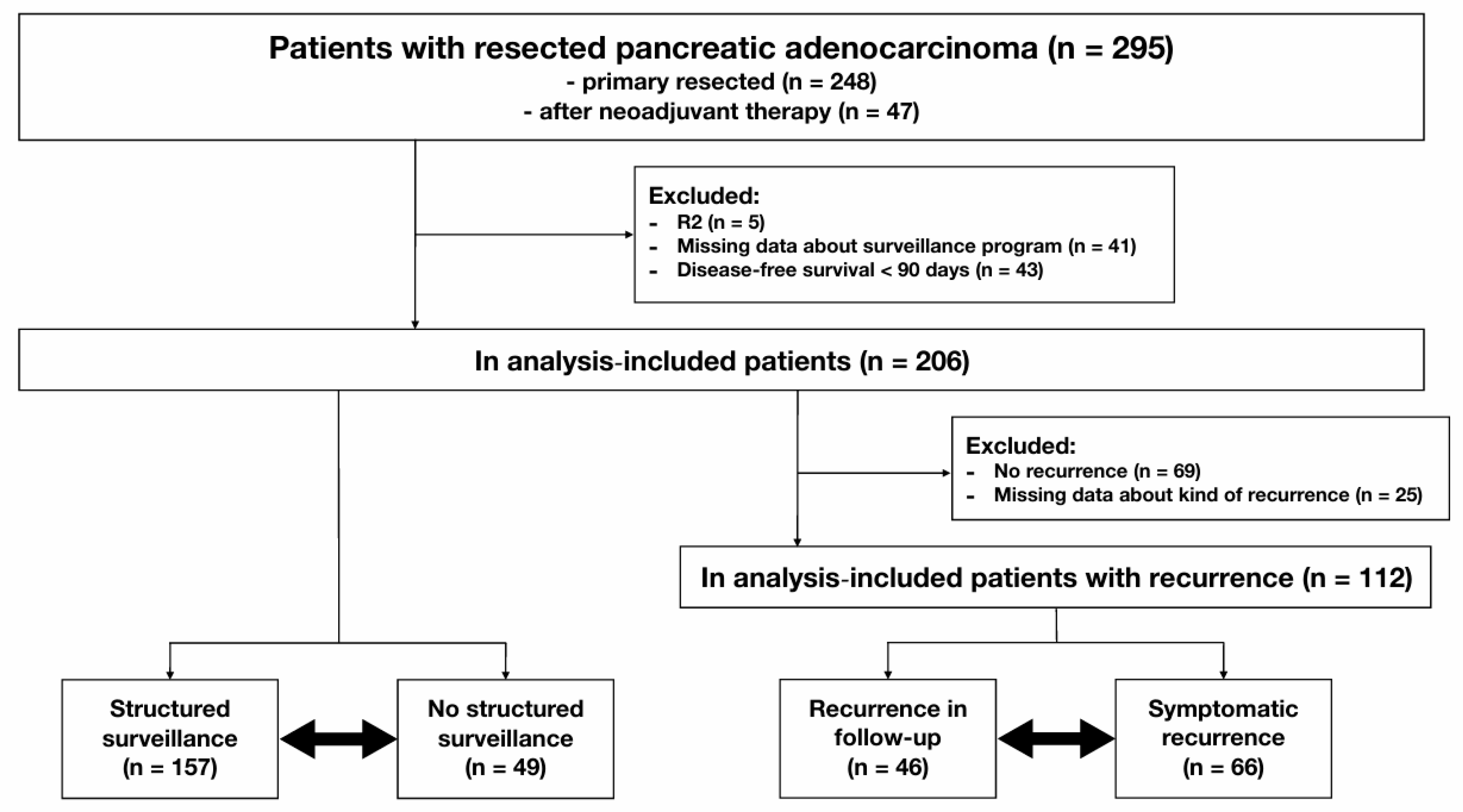
3.1. Dataset
3.2. Patient Characteristics, Surgical Details, and Histopathological Results
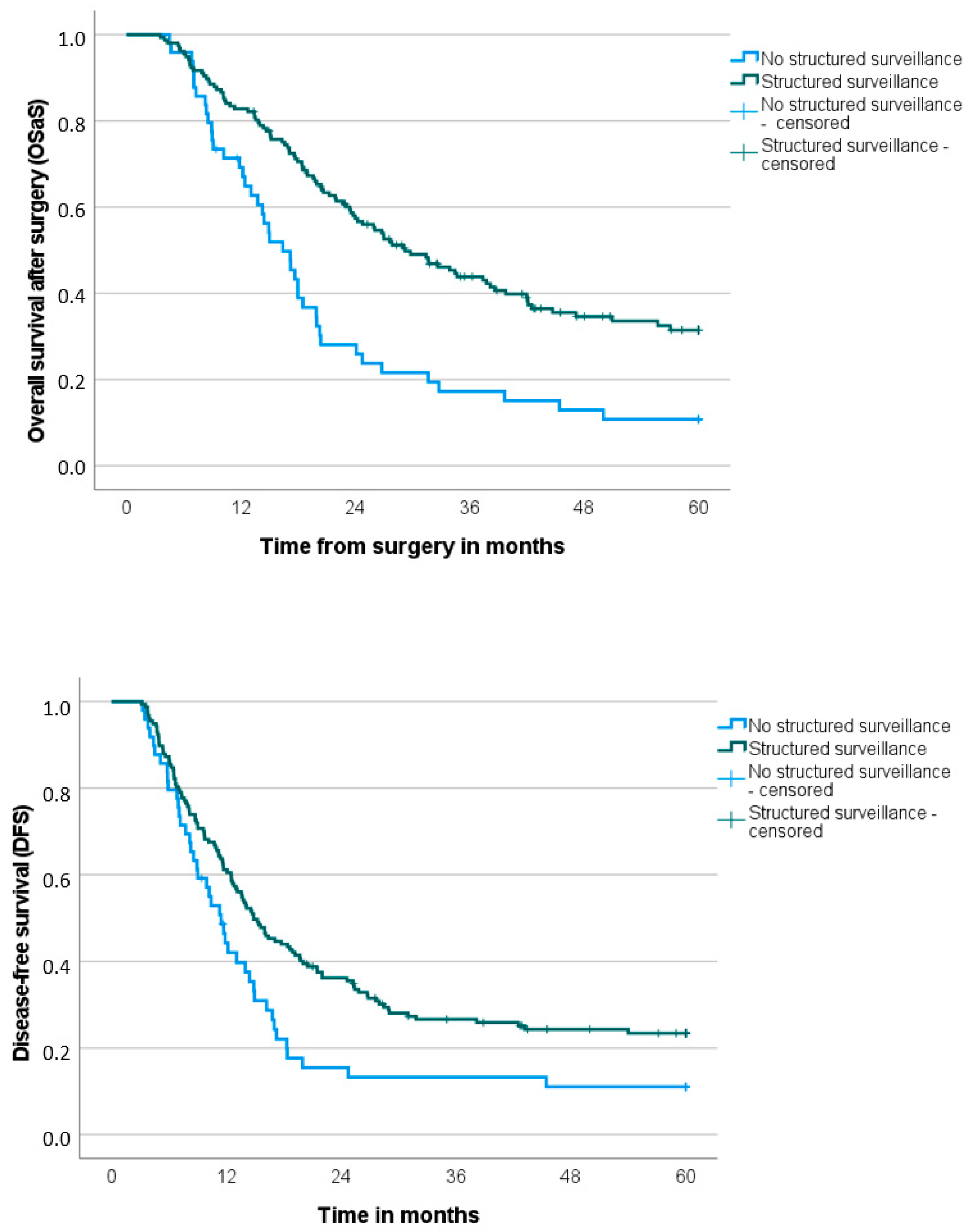
3.3. Follow-Up, Recurrence Details, and Overall and Disease-Free Survival
3.4. Prognostic Factors for Overall Survival After Surgery (OSaS) and Disease-Free Survival (DFS) in the Whole Patient Cohort (n = 206)
3.5. Prognostic Factors for Overall Survival After Surgery (OSaS) and Overall Survival After Recurrence (OSaR) in Patients with Recurrence (n = 112)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourshams, A.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Safiri, S.; Roshandel, G.; Sharif, M.; Khatibian, M.; Fitzmaurice, C.; Nixon, M.R.; et al. Global, Regional, and National Burden of Pancreatic Cancer and Its Attributable Risk Factors in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut; Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. (Eds.) Krebs in Deutschland für 2017/2018, 13th ed.; Robert Koch-Institut: Berlin, Germany, 2021. [Google Scholar]
- Ren, S.; Qian, L.C.; Cao, Y.Y.; Daniels, M.J.; Song, L.N.; Tian, Y.; Wang, Z.Q. Computed Tomography-Based Radiomics Diagnostic Approach for Differential Diagnosis between Early- and Late-Stage Pancreatic Ductal Adenocarcinoma. World J. Gastrointest. Oncol. 2024, 16, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Aldaoud, A.; Wolf, T.; Krammer-Steiner, B.; Linde, H.; Scheiner-Sparna, R.; Hamm, D.; Jänicke, M.; Marschner, N.; TPK-Group (Tumour Registry Pancreatic Cancer). Results from the Prospective German TPK Clinical Cohort Study: Treatment Algorithms and Survival of 1,174 Patients with Locally Advanced, Inoperable, or Metastatic Pancreatic Ductal Adenocarcinoma. Int. J. Cancer 2019, 144, 981–990. [Google Scholar] [CrossRef]
- Shibata, K.; Matsumoto, T.; Yada, K.; Sasaki, A.; Ohta, M.; Kitano, S. Factors Predicting Recurrence After Resection of Pancreatic Ductal Carcinoma. Pancreas 2005, 31, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gbolahan, O.B.; Tong, Y.; Sehdev, A.; O’Neil, B.; Shahda, S. Overall Survival of Patients with Recurrent Pancreatic Cancer Treated with Systemic Therapy: A Retrospective Study. BMC Cancer 2019, 19, 468. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Parikh, A.A.; Maiga, A.; Bentrem, D.; Squires, M.H.; Kooby, D.A.; Maithel, S.K.; Weber, S.M.; Cho, C.S.; Katz, M.; Martin, R.C.; et al. Adjuvant Therapy in Pancreas Cancer: Does It Influence Patterns of Recurrence? J. Am. Coll. Surg. 2016, 222, 448–456. [Google Scholar] [CrossRef]
- Smeenk, H.G.; Tran, T.C.K.; Erdmann, J.; Van Eijck, C.H.J.; Jeekel, J. Survival After Surgical Management of Pancreatic Adenocarcinoma: Does Curative and Radical Surgery Truly Exist? Langenbecks Arch. Surg. 2005, 390, 94–103. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Khorana, A.A.; McKernin, S.E.; Berlin, J.; Hong, T.S.; Maitra, A.; Moravek, C.; Mumber, M.; Schulick, R.; Zeh, H.J.; Katz, M.H.G. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, 37, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines®. Pancreatic Adenocarcinoma Version 1.2025—20 December 2024. © National Comprehensive Cancer Network, Inc., 2025. All Rights Reserved. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455 (accessed on 27 January 2025).
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y.; et al. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: A Synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Krebsgesellschaft; Deutsche Krebshilfe; AWMF. S3-Leitlinie Exokrines Pankreaskarzinom, Langversion 3.1—September 2024. AWMF Registernummer: 032-010OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/pankreaskarzinom/ (accessed on 27 January 2025).
- Halle-Smith, J.M.; Hall, L.; Daamen, L.A.; Hodson, J.; Pande, R.; Young, A.; Jamieson, N.B.; Lamarca, A.; van Santvoort, H.C.; Molenaar, I.Q.; et al. Clinical Benefit of Surveillance After Resection of Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kruger, S.; Schirle, K.; Heinemann, V.; Dorman, K.; Westphalen, C.B.; Weiss, L.; Gebauer, L.; Günther, M.; Ormanns, S.; et al. Clinical Impact of Structured Post-Operative Surveillance in Resected Pancreatic Adenocarcinoma: Results from a Retrospective Cohort Study. Oncol. Res. Treat. 2023, 46, 106–115. [Google Scholar] [CrossRef]
- Daamen, L.A.; Groot, V.P.; Intven, M.P.W.; Besselink, M.G.; Busch, O.R.; Koerkamp, B.G.; Mohammad, N.H.; Hermans, J.J.; van Laarhoven, H.W.M.; Nuyttens, J.J.; et al. Postoperative Surveillance of Pancreatic Cancer Patients. Eur. J. Surg. Oncol. 2019, 45, 1770–1777. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Hackner, D.; Hobbs, M.; Merkel, S.; Siepmann, T.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 3929. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Ansari, D.; Søreide, K.; Andersson, B.; Palnæs, C.; Seppänen, H.; Sparrelid, E.; Jørgen, K.; Kirkegård, J.; Kauhanen, S.; Månsson, C.; et al. Surveillance After Surgery for Pancreatic Cancer: A Global Scoping Review of Guidelines and a Nordic Survey of Contemporary Practice. Scand. J. Gastroenterol. 2024, 59, 1097–1104. [Google Scholar] [CrossRef]
- Gonzales, B.A.; Diniz, A.L.; Torres, S.M.; de Castro Ribeiro, H.S.; de Farias, I.C.; de Godoy, A.L.; Coimbra, F.J.F.; de Jesus, V.H.F. Patterns of Disease Relapse and Posttreatment Follow-Up of Patients with Resected Pancreatic Adenocarcinoma: A Single-Center Analysis. J. Surg. Oncol. 2022, 126, 708–717. [Google Scholar] [CrossRef]
- Tjaden, C.; Michalski, C.W.; Strobel, O.; Giese, N.; Hennche, A.K.; Büchler, M.W.; Hackert, T. Clinical Impact of Structured Follow-up After Pancreatic Surgery. Pancreas 2016, 45, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.M.; Belyaev, O.; Höhn, P.; Praktiknjo, M.; Janot, M.; Uhl, W.; Braumann, C. Late Recurrences of Pancreatic Cancer in Patients with Long-Term Survival After Pancreaticoduodenectomy. J. Gastrointest. Oncol. 2021, 12, 474–483. [Google Scholar] [CrossRef]
- Andel, P.C.M.; van Goor, I.W.J.M.; Augustinus, S.; Berrevoet, F.; Besselink, M.G.; Bhojwani, R.; Boggi, U.; Bouwense, S.A.W.; Cirkel, G.A.; van Dam, J.L.; et al. Routine Imaging or Symptomatic Follow-Up After Resection of Pancreatic Adenocarcinoma. JAMA Surg. 2025, 160, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.-W.D.; Abbott, D.E.; Cantor, S.B.; Fleming, J.B.; Lee, J.E.; Pisters, P.W.T.; Varadhachary, G.R.; Abbruzzese, J.L.; Wolff, R.A.; Ahmad, S.A. Frequency and Intensity of Postoperative Surveillance After Curative Treatment of Pancreatic Cancer: A Cost-Effectiveness Analysis. Ann. Surg. Oncol. 2013, 20, 2197–2203. [Google Scholar] [CrossRef]
- Witkowski, E.R.; Smith, J.K.; Ragulin-Coyne, E.; Ng, S.C.; Shah, S.A.; Tseng, J.F. Is It Worth Looking? Abdominal Imaging After Pancreatic Cancer Resection: A National Study. J. Gastrointest. Surg. 2012, 16, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nong, M.Z.; Dove, D.; Fischer, D.A.; Hourdequin, K.C.; Ripple, G.H.; Amin, M.A.; McGrath, E.B.; Zaki, B.I.; Smith, K.D.; Brooks, G.A. Surveillance with Serial Imaging and CA 19-9 Tumor Marker Testing After Resection of Pancreatic Cancer: A Single-Center Retrospective Study. Am. J. Clin. Oncol. 2024, 47, 25–29. [Google Scholar] [CrossRef]
- Duffy, S.W.; Nagtegaal, I.D.; Wallis, M.; Cafferty, F.H.; Houssami, N.; Warwick, J.; Allgood, P.C.; Kearins, O.; Tappenden, N.; O’Sullivan, E.; et al. Correcting for Lead Time and Length Bias in Estimating the Effect of Screen Detection on Cancer Survival. Am. J. Epidemiol. 2008, 168, 98–104. [Google Scholar] [CrossRef]
- Hutchison, G.B.; Shapiro, S. Lead Time Gained by Diagnostic Screening for Breast Cancer. J. Natl. Cancer Inst. 1968, 41, 665–681. [Google Scholar]
- Thompson, C.A.; Charlson, M.E.; Schenkein, E.; Wells, M.T.; Furman, R.R.; Elstrom, R.; Ruan, J.; Martin, P.; Leonard, J.P. Surveillance CT Scans Are a Source of Anxiety and Fear of Recurrence in Long-Term Lymphoma Survivors. Ann. Oncol. 2010, 21, 2262–2266. [Google Scholar] [CrossRef]
- Deobald, R.G.; Cheng, E.S.; Ko, Y.; Wright, F.C.; Karanicolas, P.J. A Qualitative Study of Patient and Clinician Attitudes Regarding Surveillance After Resection of Pancreatic and Peri-Ampullary Cancer. HPB 2015, 17, 409–415. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 206) | |||
|---|---|---|---|
| Structured Surveillance | No Structured Surveillance | p-Value | |
| Number | 157 | 49 | |
| Age (years), median (IQR) | 67 (14) | 70 (11) | 0.051 |
| Sex, n (%) | 0.513 | ||
| Female | 77 (49) | 21 (43) | |
| Male | 80 (51) | 28 (57) | |
| ASA (n = 202/110) *, n (%) | 0.198 | ||
| I | 4 (3) | 0 (0) | |
| II | 97 (64) | 29 (59) | |
| III | 52 (34) | 20 (41) | |
| BMI (kg/m2), median (IQR) | 25.4 (5.0) | 25.4 (5.4) | 0.387 |
| Comorbidity, n (%) | |||
| Hypertension | 83 (53) | 22 (45) | 0.413 |
| Diabetes | 39 (25) | 15 (31) | 0.459 |
| Cardiovascular | 22 (14) | 13 (27) | 0.051 |
| Pulmonary | 10 (6) | 3 (6) | 1.000 |
| Cerebrovascular | 6 (4) | 2 (4) | 1.000 |
| Liver disease | 16 (10) | 5 (10) | 0.281 |
| Neoadjuvant therapy, n (%) | 42 (27) | 5 (10) | 0.018 |
| Preoperative albumin (g/L), median (IQR) | 40.2 (7.5) | 38.8 (6.4) | 0.623 |
| Preoperative CA19-9 (U/mL) (n = 135) *, median (IQR) | 90 (326) | 64 (227) | 0.484 |
| Preoperative CEA (ng/mL), median (IQR) | 2.3 (3.0) | 2.2 (2.7) | 0.986 |
| Only Patients with Recurrence (n = 112) * | |||
|---|---|---|---|
| Recurrence in Follow-Up | Symptomatic Recurrence | p-Value | |
| Number | 46 | 66 | |
| Age (years), median (IQR) | 64 (19) | 69 (13) | 0.069 |
| Sex, n (%) | 0.081 | ||
| Female | 24 (52) | 23 (35) | |
| Male | 22 (48) | 43 (65) | |
| ASA (n = 202/110) **, n (%) | 0.904 | ||
| I | 0 (0) | 1 (1) | |
| II | 30 (67) | 44 (68) | |
| III | 15 (33) | 20 (31) | |
| BMI (kg/m2), median (IQR) | 26.3 (6.9) | 25.6 (5.0) | 0.280 |
| Comorbidity, n (%) | |||
| Hypertension | 21 (46) | 32 (49) | 0.848 |
| Diabetes | 7 (15) | 20 (30) | 0.076 |
| Cardiovascular | 2 (4) | 13 (20) | 0.023 |
| Pulmonary | 3 (7) | 4 (6) | 1.000 |
| Cerebrovascular | 1 (2) | 2 (3) | 1.000 |
| Liver disease | 5 (11) | 5 (8) | 0.846 |
| Neoadjuvant therapy, n (%) | 14 (30) | 14 (21) | 0.375 |
| Preoperative albumin (g/L), median (IQR) | 41.1 (6.2) | 39.1 (6.9) | 0.188 |
| Preoperative CA19-9 (U/mL) (n = 135) **, median (IQR) | 72 (443) | 133 (489) | 0.775 |
| Preoperative CEA (ng/mL), median (IQR) | 2.9 (3.5) | 2.3 (2.7) | 0.485 |
| All Patients (n = 206) | |||
|---|---|---|---|
| Structured Surveillance (n = 157) | No Structured Surveillance (n = 49) | p-Value | |
| Kind of surgery, n (%) | 0.874 | ||
| Pancreatic head resection | 119 (76) | 39 (80) | |
| Distal pancreatectomy | 34 (22) | 9 (18) | |
| Total pancreatectomy | 4 (3) | 1 (2) | |
| Vascular resection, n (%) | 56 (36) | 15 (31) | 0.607 |
| Multivisceral resection, n (%) | 40 (26) | 13 (27) | 1.000 |
| Postoperative In-hospital morbidity, n (%) | 75 (48) | 28 (57) | 0.326 |
| Postoperative in-hospital major morbidity, n (%) | 43 (27) | 10 (20) | 0.357 |
| T category (n = 205/112) *, n (%) | 0.193 | ||
| (y)pT0 | 3 (1) | 1 (2) | |
| (y)pT1 | 14 (9) | 9 (18) | |
| (y)pT2 | 48 (31) | 10 (20) | |
| (y)pT3 | 89 (57) | 29 (59) | |
| (y)pT4 | 3 (2) | 0 (0) | |
| N category, n (%) | 0.518 | ||
| (y)pN0 | 79 (50) | 22 (45) | |
| (y)pN+ | 78 (50) | 27 (55) | |
| M category, n (%) | 0.485 | ||
| M0 | 149 (95) | 45 (92) | |
| (y)pM1 | 8 (5) | 4 (8) | |
| R status, n (%) | 0.301 | ||
| R0 | 146 (93) | 48 (98) | |
| R1 | 11 (7) | 1 (2) | |
| Differentiation (n = 182/96) *, n (%) | 0.859 | ||
| G1/2 | 54 (39) | 16 (36) | |
| G3 | 84 (61) | 28 (64) | |
| Adjuvant chemotherapy, n (%) | 127 (83) | 23 (55) | <0.001 |
| Recurrence, n (%) | 105 (67) | 32 (65) | 0.863 |
| Location of recurrence (n = 130/106) *, n (%) | 0.140 | ||
| Locoregional only | 19 (18) | 1 (4) | |
| Metastatic disease | 56 (54) | 19 (70) | |
| Both | 28 (27) | 7 (26) | |
| Kind of recurrence (n = 112), n (%) | <0.001 | ||
| In follow-up | 46 (57) | 0 (0) | |
| Symptomatic | 35 (43) | 31 (100) | |
| Time to recurrence (months), median (SD) | 12.1 (11.4) | 9.9 (8.3) | 0.029 |
| Overall survival from surgery (months), median (SD) | 29.2 (3.2) | 16.4 (1.8) | <0.001 |
| Overall survival from recurrence (months) (n = 137/112), median (SD) | 10.8 (1.0) | 3.6 (2.1) | <0.001 |
| Disease-free survival (months), median (SD) | 14.8 (1.3) | 11.4 (1.1) | 0.010 |
| Only Patients with Recurrence (n = 112) * | |||
|---|---|---|---|
| Recurrence in Follow-Up (n = 46) | Symptomatic Recurrence (n = 66) | p-Value | |
| Kind of surgery, n (%) | 1.000 | ||
| Pancreatic head resection | 35 (76) | 51 (77) | |
| Distal pancreatectomy | 11 (24) | 15 (23) | |
| Total pancreatectomy | 0 (0) | 0 (0) | |
| Vascular resection, n (%) | 14 (30) | 25 (38) | 0.430 |
| Multivisceral resection, n (%) | 14 (30) | 15 (23) | 0.387 |
| Postoperative In-hospital morbidity, n (%) | 21 (46) | 31 (47) | 1.000 |
| Postoperative in-hospital major morbidity, n (%) | 15 (33) | 12 (18) | 0.063 |
| T category (n = 205/112) **, n (%) | 0.045 | ||
| (y)pT0 | 0 (0) | 2 (3) | |
| (y)pT1 | 8 (17) | 7 (11) | |
| (y)pT2 | 12 (26) | 12 (18) | |
| (y)pT3 | 23 (50) | 45 (68) | |
| (y)pT4 | 3 (7) | 0 (0) | |
| N category, n (%) | 0.699 | ||
| (y)pN0 | 22 (48) | 28 (42) | |
| (y)pN+ | 24 (52) | 38 (58) | |
| M category, n (%) | 0.020 | ||
| M0 | 46 (100) | 58 (88) | |
| (y)pM1 | 0 (0) | 8 (12) | |
| R status, n (%) | 1.000 | ||
| R0 | 43 (94) | 63 (94) | |
| R1 | 3 (6) | 4 (6) | |
| Differentiation (n = 182/96) **, n (%) | 0.058 | ||
| G1/2 | 20 (49) | 16 (29) | |
| G3 | 21 (51) | 39 (71) | |
| Adjuvant chemotherapy, n (%) | 40 (87) | 47 (72) | 0.100 |
| Recurrence, n (%) | 46 (100) | 66 (100) | - |
| Location of recurrence (n = 130/106) **, n (%) | 0.020 | ||
| Locoregional only | 13 (28) | 5 (8) | |
| Metastatic disease | 23 (50) | 35 (58) | |
| Both | 10 (22) | 20 (33) | |
| Kind of recurrence (n = 112), n (%) | - | ||
| In follow-up | 46 (100) | 0 (0) | |
| Symptomatic | 0 (0) | 66 (100) | |
| Time to recurrence (months), median (SD) | 12.6 (12.3) | 10.1 (8.6) | 0.054 |
| Overall survival from surgery (months), median (SD) | 24.8 (2.2) | 17.2 (1.1) | <0.001 |
| Overall survival from recurrence (months) (n = 137/112), median (SD) | 12.6 (1.6) | 6.5 (0.7) | <0.001 |
| Disease-free survival (months), median (SD) | - | - | - |
| Overall Survival After Surgery (OSaS) | Disease-Free Survival (DFS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| n | Median OS | p | OR | 95% CI | p | Median DFS | p | OR | 95% CI | p | |
| Age | 0.007 | 1.6 | 1.1–2.3 | 0.022 | 0.140 | 1.3 | 0.9–1.9 | 0.138 | |||
| ≤70 years | 127 | 29.0 | 13.8 | ||||||||
| >70 years | 79 | 20.3 | 14.6 | ||||||||
| Neoadjuvant therapy | 0.623 | 0.4 | 0.2–0.7 | 0.001 | 0.500 | 0.6 | 0.3–1.1 | 0.083 | |||
| Yes | 47 | 26.0 | 11.0 | ||||||||
| No | 159 | 24.1 | 14.0 | ||||||||
| T category (n = 205) * | 0.144 | 1.0 | 0.7–1.6 | 0.889 | 0.313 | 1.0 | 0.7–1.4 | 0.853 | |||
| (y)pT0/(y)pT1/(y)pT2 | 84 | 31.7 | 16.0 | ||||||||
| (y)pT3/(y)pT4 | 121 | 22.0 | 13.1 | ||||||||
| N category | <0.001 | 2.2 | 1.4–3.4 | <0.001 | 0.001 | 1.9 | 1.3–2.9 | 0.001 | |||
| (y)pN0 | 101 | 33.9 | 17.1 | ||||||||
| (y)pN+ | 105 | 19.9 | 11.9 | ||||||||
| M category | <0.001 | 2.7 | 1.3–5.7 | 0.009 | 0.042 | 1.5 | 0.7–3.0 | 0.307 | |||
| M0 | 194 | 26.0 | 14.4 | ||||||||
| (y)pM1 | 12 | 10.2 | 9.0 | ||||||||
| Differentiation (n = 182) * | 0.039 | 1.7 | 1.1–2.5 | 0.012 | 0.087 | 1.4 | 1.0–2.0 | 0.087 | |||
| G1/G2 | 70 | 31.7 | 18.4 | ||||||||
| G3 | 112 | 20.3 | 13.6 | ||||||||
| Adjuvant chemotherapy | 0.195 | 1.8 | 1.1–2.9 | 0.017 | 0.602 | 1.3 | 0.9–2.1 | 0.184 | |||
| Yes | 150 | 25.9 | 14.0 | ||||||||
| No | 45 | 18.5 | 13.1 | ||||||||
| Structured surveillance | <0.001 | 1.8 | 1.2–2.9 | 0.006 | 0.010 | 1.4 | 1.0–2.2 | 0.048 | |||
| Yes | 157 | 29.2 | 14.8 | ||||||||
| No | 49 | 16.4 | 11.4 | ||||||||
| Overall Survival After Surgery (OSaS) | Overall Survival After Recurrence (OSaR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| n | Median OS | p | OR | 95% CI | p | Median OS | p | OR | 95% CI | p | |
| Age | 0.160 | 1.4 | 0.9–2.3 | 0.139 | 0.024 | 1.6 | 1.0–2.6 | 0.038 | |||
| ≤70 years | 69 | 23.4 | 10.7 | ||||||||
| >70 years | 43 | 18.5 | 7.9 | ||||||||
| Neoadjuvant therapy | 0.330 | 0.3 | 0.1–0.5 | <0.001 | 0.844 | 0.3 | 0.1–0.6 | <0.001 | |||
| Yes | 28 | 18.9 | 10.0 | ||||||||
| No | 84 | 20.3 | 9.2 | ||||||||
| T category | 0.133 | 0.9 | 0.5–1.7 | 0.932 | 0.014 | 1.3 | 0.8–2.3 | 0.296 | |||
| (y)pT0/(y)pT1/(y)pT2 | 41 | 23.4 | 12.7 | ||||||||
| (y)pT3/(y)pT4 | 71 | 19.9 | 8.4 | ||||||||
| N category | 0.013 | 2.2 | 1.3–3.7 | 0.002 | 0.022 | 1.8 | 1.1–3.0 | 0.030 | |||
| (y)pN0 | 50 | 27.0 | 12.2 | ||||||||
| (y)pN+ | 62 | 18.0 | 7.1 | ||||||||
| M category | <0.001 | 2.5 | 1.0–6.4 | 0.052 | <0.001 | 5.9 | 2.2–15.6 | <0.001 | |||
| M0 | 104 | 20.4 | 12.2 | ||||||||
| (y)pM1 | 8 | 9.9 | 0.9 | ||||||||
| Differentiation (n = 96) * | 0.088 | 1.5 | 0.9–2.6 | 0.105 | 0.034 | 2.0 | 1.2–3.3 | 0.008 | |||
| G1/G2 | 36 | 25.9 | 12.1 | ||||||||
| G3 | 60 | 18.5 | 7.9 | ||||||||
| Adjuv. chemoth. | 0.280 | 2.2 | 1.2–4.1 | 0.013 | 0.186 | 2.0 | 1.1–3.6 | 0.024 | |||
| Yes | 87 | 20.7 | 10.2 | ||||||||
| No | 24 | 15.0 | 6.3 | ||||||||
| Kind of recurrence | <0.001 | 2.2 | 1.3–3.7 | 0.003 | <0.001 | 1.9 | 1.2–3.2 | 0.007 | |||
| In follow-up | 46 | 24.8 | 12.6 | ||||||||
| Symptomatic | 66 | 17.2 | 6.5 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobsen, A.; Flessa, M.; Abels, A.-L.; Czubayko, F.; Mittelstädt, A.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. Impact of Structured Postoperative Surveillance on Survival in Patients with Resected Pancreatic Adenocarcinoma. Cancers 2025, 17, 1424. https://doi.org/10.3390/cancers17091424
Jacobsen A, Flessa M, Abels A-L, Czubayko F, Mittelstädt A, Krautz C, Weber GF, Grützmann R, Brunner M. Impact of Structured Postoperative Surveillance on Survival in Patients with Resected Pancreatic Adenocarcinoma. Cancers. 2025; 17(9):1424. https://doi.org/10.3390/cancers17091424
Chicago/Turabian StyleJacobsen, Anne, Maarten Flessa, Anna-Lena Abels, Franziska Czubayko, Anke Mittelstädt, Christian Krautz, Georg F. Weber, Robert Grützmann, and Maximilian Brunner. 2025. "Impact of Structured Postoperative Surveillance on Survival in Patients with Resected Pancreatic Adenocarcinoma" Cancers 17, no. 9: 1424. https://doi.org/10.3390/cancers17091424
APA StyleJacobsen, A., Flessa, M., Abels, A.-L., Czubayko, F., Mittelstädt, A., Krautz, C., Weber, G. F., Grützmann, R., & Brunner, M. (2025). Impact of Structured Postoperative Surveillance on Survival in Patients with Resected Pancreatic Adenocarcinoma. Cancers, 17(9), 1424. https://doi.org/10.3390/cancers17091424






