Navigating Stomatologic Complications Secondary to Antineoplastic Agents—A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Oncology Medications with Adverse Effects on the Oral Cavity
2.1. Bone-Modifying Agents
2.1.1. Bisphosphonates (BPs)
2.1.2. Denosumab
2.2. Chemotherapy
| Oral Adverse Effect | Associated Drugs | Typical Clinical Uses |
|---|---|---|
| Oral mucositis/ulcerations | - Methotrexate - 5-Fluorouracil/Capecitabine - Doxorubicin/Epirubicin - Vinblastine - Cyclophosphamide - Docetaxel/Paclitaxel | - Methotrexate: Leukemias, lymphomas - 5-Fluorouracil/Capecitabine: breast, GI cancers - Doxorubicin/Epirubicin: Breast, ovarian, bladder, lung cancers - Vinblastine: Hodgkin’s lymphoma, testicular cancer - Cyclophosphamide: Breast cancer, lymphomas, leukemia - Docetaxel/Paclitaxel: Breast, prostate, lung, ovarian cancers |
| Xerostomia | - Methotrexate - Platinum salts - Cyclophosphamide - Docetaxel/Paclitaxel | - Platinum salts: Testicular, ovarian, bladder, lung cancers |
| Taste alterations (dysgeusia) | - Methotrexate - 5-Fluorouracil/Capecitabine - Platinum salts - Doxorubicin/Epirubicin - Docetaxel/Paclitaxel | |
| Neurotoxicity/mandibular pain | - Platinum salts - Vincristine - Vinblastine | - Vincristine: Leukemias, lymphomas, neuroblastomas |
| Pigmentations | - Doxorubicin/Epirubicin | |
| Enamel or dental defects | - Vinblastine - Cyclophosphamide |
2.3. Modern Molecules: Unraveling Their Impact on the Oral Cavity
2.3.1. Monoclonal Antibodies—Anti-VEGF/VEGFR
2.3.2. Monoclonal Antibodies—Anti-EGFR
2.3.3. Monoclonal Antibodies—Other Targets
2.3.4. Fusion Proteins/Decoy Receptors
2.3.5. Small Molecule TKIs
2.3.6. mTOR Inhibitors
3. Management of Oral Cavity Adverse Effects and Complications Secondary to Oncological Treatments
3.1. Pre-Treatment Dental Management
3.2. Diagnostic and Management
3.2.1. Xerostomia
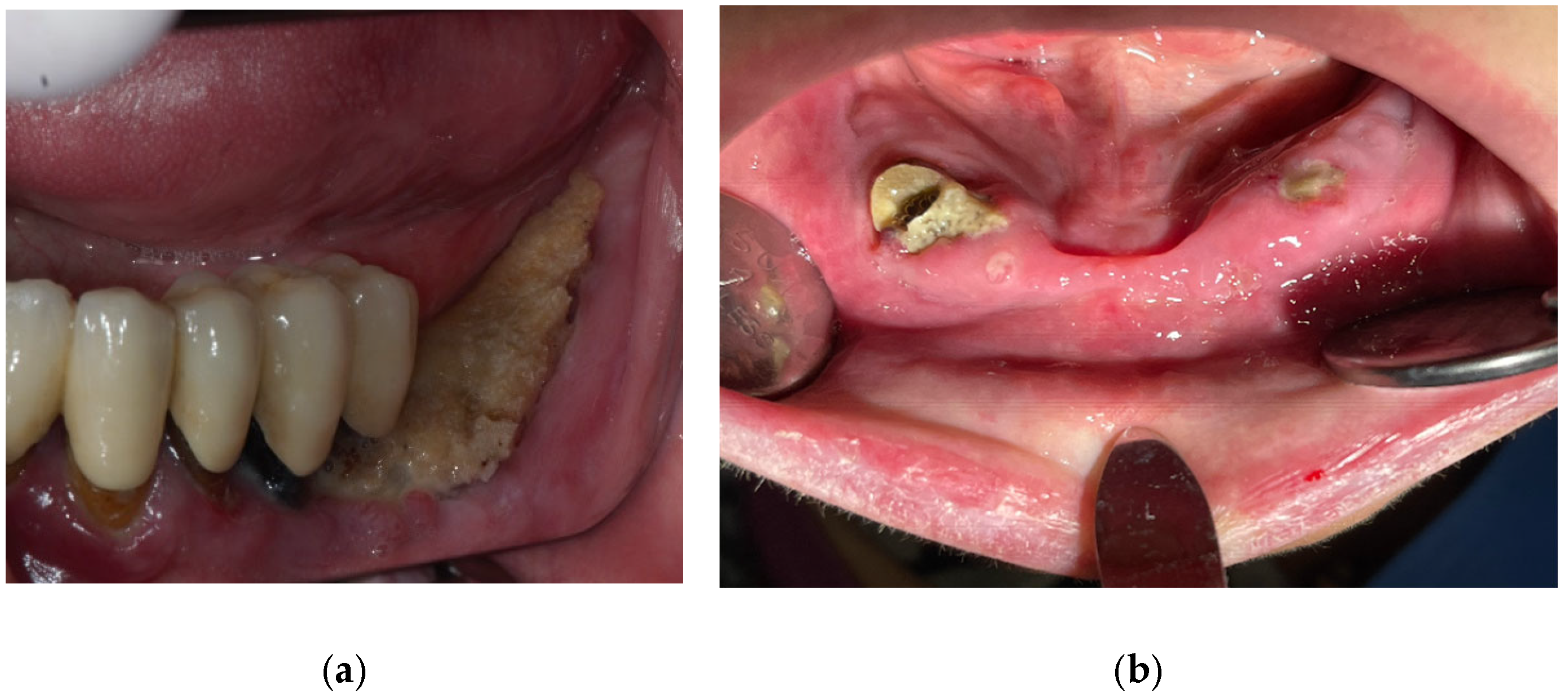
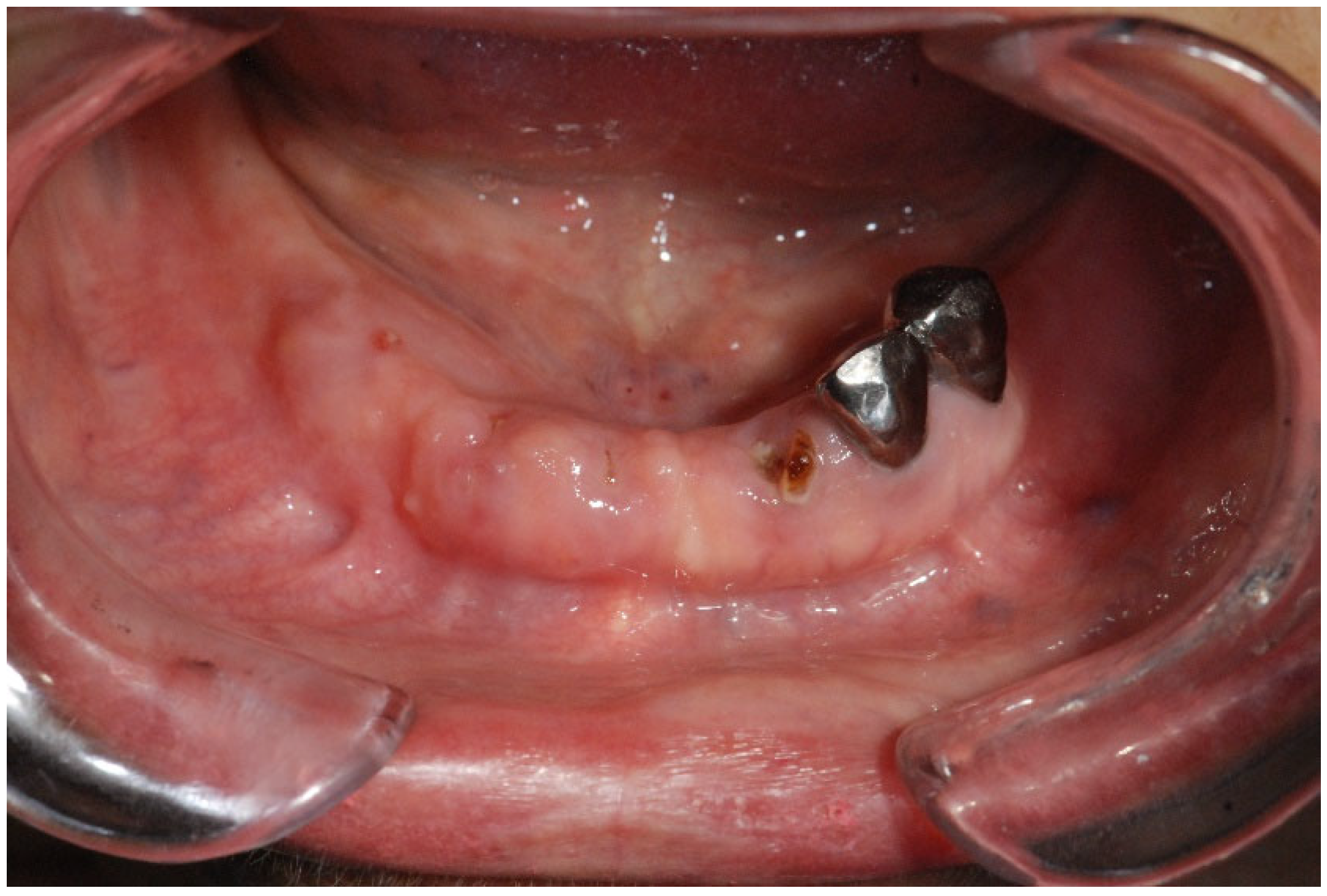
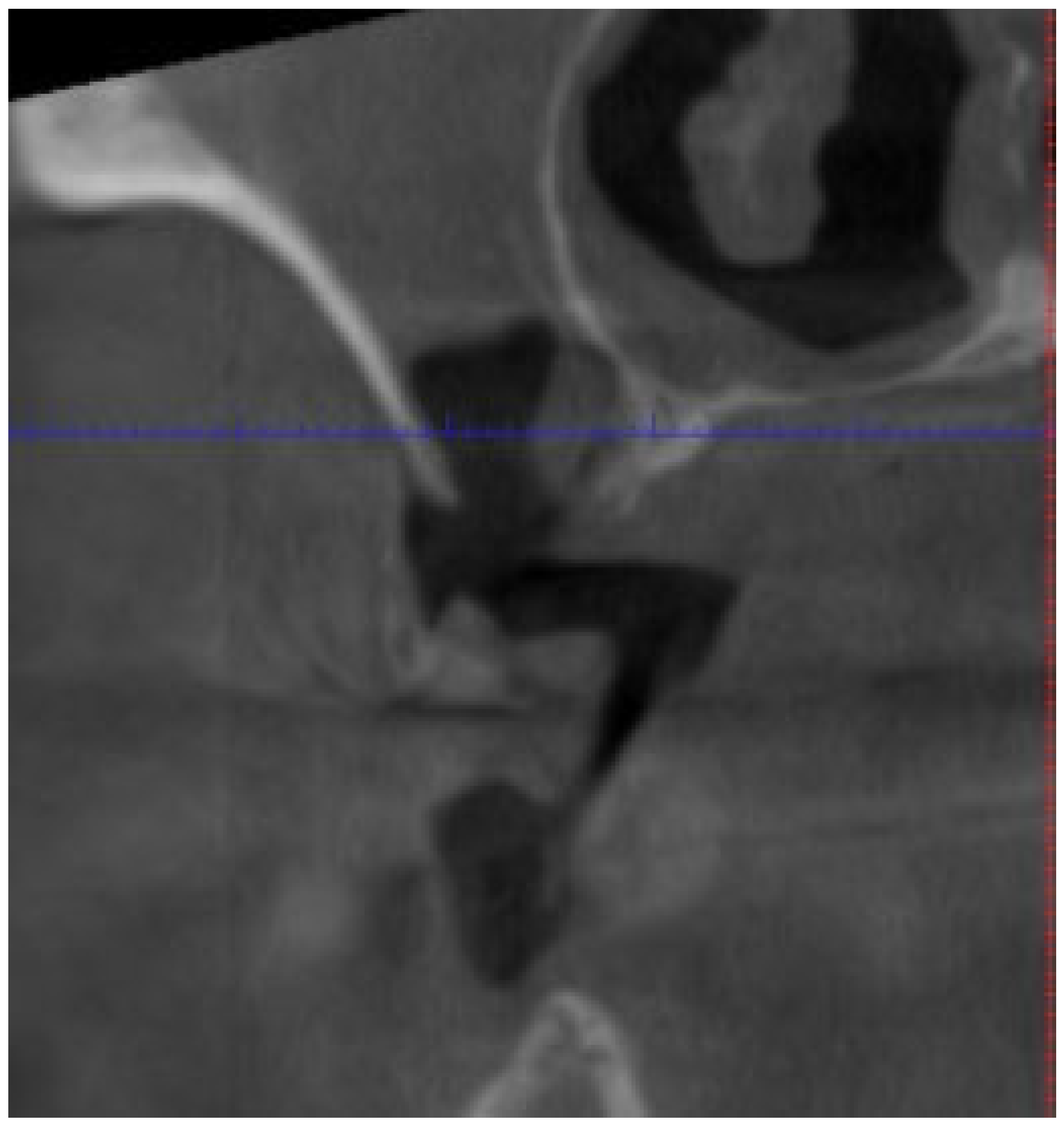
3.2.2. Osteonecrosis of the Jaw
| Sign/Symptom | Description | Stage |
|---|---|---|
| Gingival Swelling | Inflammation and swelling of the gums, which may occur in response to adjacent necrotic bone or local infection. | Early stages (Stage 0, progressing to Stage 2) |
| Dull Mandibular Pain | Persistent, non-specific pain in the mandible that may indicate early changes in bone integrity. | Stage 0 |
| Sinus Pain | Unexplained discomfort or pain in the sinus regions, suggesting possible maxillary bone involvement. | Stage 0 |
| Unexplained Tooth Mobility | Loosening of teeth without evident periodontal disease, possibly due to underlying bone necrosis. | Stage 0 |
| Odontalgia | Tooth pain occurring without an obvious dental cause, potentially reflecting underlying bone pathology. | Stage 0 |
| Exposed Bone | Visible necrotic bone in the mandible or maxilla, often detectable through an intraoral or extraoral fistula; persists for over 8 weeks. | Stage 1 and beyond |
| Fistula (Intraoral/Extraoral) | An abnormal tract that connects the oral cavity (or skin) to necrotic bone, allowing probing of the underlying bone tissue (Figure 4). | Stage 1+ |
| Signs of Infection | Presence of erythema, pus discharge, and purulent drainage around exposed bone areas, indicating an active infection. | Typically Stage 2 |
| Pathologic Fractures | Fractures of the jawbone that occur with minimal or no trauma due to significant bone compromise. | Stage 3 |
| Extraoral Fistula | Fistula formation visible on the facial skin, reflecting advanced necrotic changes in the bone. | Stage 3 |
| Osteolysis Beyond Alveolar Bone | Bone resorption that extends beyond the alveolar process (e.g., affecting the inferior border, ramus, sinus floor, or zygoma), indicating severe bone involvement. | Stage 3 |
| Oroantral/Oronasal Communication | Abnormal openings between the oral cavity and the sinus or nasal areas, which may lead to further complications. | Stage 3 |
3.2.3. Lichenoid Reactions/Lichen Planus
3.2.4. Pigmentations
3.2.5. Aphtous Ulcers/Stomatitis
3.2.6. Mucositis/Gingivitis
3.2.7. Dysgeusia
3.2.8. Bleeding
3.3. Post-Treatment Special Considerations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Dasooqi, N.; Gibson, R.J.; Bowen, J.M.; Keefe, D.M. Matrix metalloproteinases: Key regulators in the pathogenesis of chemotherapy-induced mucositis? Cancer Chemother. Pharmacol. 2009, 64, 1–9. [Google Scholar] [PubMed]
- Taylor, K.H.; Middlefell, L.S.; Mizen, K.D. Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br. J. Oral Maxillofac. Surg. 2010, 48, 221–223. [Google Scholar]
- Harris, M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004, 5, 292–302. [Google Scholar] [PubMed]
- Basu, B.; Eisen, T. Perspectives in drug development for metastatic renal cell cancer. Target. Oncol. 2010, 5, 139–156. [Google Scholar]
- Mukai, H. Targeted therapy in breast cancer: Current status and future directions. Jpn. J. Clin. Oncol. 2010, 40, 711–716. [Google Scholar]
- Herbst, R.S.; Shin, D.M. Monoclonal antibodies to target epidermal growth factor receptor–positive tumors. Cancer 2002, 94, 1593–1611. [Google Scholar]
- John, A.M.; Thomas, N.S.B.; Mufti, G.J.; Padua, R.A. Targeted therapies in myeloid leukemia. Semin. Cancer Biol. 2004, 14, 41–62. [Google Scholar]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Watters, A.L.; Epstein, J.B.; Agulnik, M. Oral complications of targeted cancer therapies: A narrative literature review. Oral. Oncol. 2011, 47, 441–448. [Google Scholar]
- Holen, I.; Coleman, R.E. Bisphosphonates as treatment of bone metastases. Curr. Pharm. Des. 2010, 16, 1262–1271. [Google Scholar]
- Im, G.I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.C.; Pillai, G.; Papapoulos, S.E. Pharmacokinetics/pharmacodynamics of bisphosphonates: Use for optimisation of intermittent therapy for osteoporosis. Clin. Pharmacokinet. 2005, 44, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Santini, D.; Vincenzi, B.; Avvisati, G.; Dicuonzo, G.; Battistoni, F.; Gavasci, M.; Salerno, A.; Denaro, V.; Tonini, G. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin. Cancer Res. 2002, 8, 1080–1084. [Google Scholar] [CrossRef]
- Dodson, T.B.; Raje, N.S.; Caruso, P.A.; Rosenberg, A.E. Case 9–2008—A 65-year-old woman with a nonhealing ulcer of the jaw. N. Engl. J. Med. 2008, 358, 1214–1291. [Google Scholar] [CrossRef]
- Woo, S.B.; Hellstein, J.W.; Kalmar, J.R. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann. Intern. Med. 2006, 144, 753–756. [Google Scholar] [CrossRef]
- Palaska, P.K.; Cartsos, V.; Zavras, A.I. Bisphosphonates and time to osteonecrosis development. Oncologist 2009, 14, 1154–1166. [Google Scholar] [CrossRef]
- Epstein, M.S.; Ephros, H.D.; Epstein, J.B. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e437–e442. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Toulis, K.A. Denosumab-related osteonecrosis of the jaws. Osteoporos. Int. 2011, 22, 369–370. [Google Scholar] [CrossRef]
- Tassone, P.; Tagliaferri, P.; Rossi, M.; Calimeri, T.; Bulotta, A.; Abbruzzese, A.; Caraglia, M.; Neri, P. Challenging the current approaches to multiple myeloma-related bone disease: From BPs to target therapy. Curr. Cancer Drug Targets 2009, 9, 854–870. [Google Scholar] [PubMed]
- Amgen. Highlights of Prescribing Information. Xgeva (Deno-Sumab) Injection, for Subcutaneous Use; Amgen: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Santini, D.; Fratto, M.E.; Vincenzi, B.; Napoli, N.; Galluzzo, S.; Tantardini, M.; Abbruzzese, A.; Caraglia, M.; Tonini, G. Denosumab: The era of targeted therapies in bone metastatic diseases. Curr. Cancer Drug Targets 2009, 9, 834–842. [Google Scholar] [PubMed]
- Van Poznak, C.H.; Temin, S.; Yee, G.C.; Janjan, N.A.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Geoghegan, C.; Hillner, B.E.; Theriault, R.L.; et al. American Society of Clinical Oncology executive summary of the clinical practice Guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011, 29, 1221–1227. [Google Scholar]
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral mucositis induced by anticancer therapies. Curr. Oral. Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef]
- Hong, C.H.; Nape-as, J.J.; Hodgson, B.D.; Stokman, M.A.; Mathers-Stauffer, V.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T.; Dental Disease Section, Oral Care Study Group, MASCC/ISOO. A systematic review on patients undergoing cancer therapy. Support. Care Cancer 2010, 18, 1007–1021. [Google Scholar] [CrossRef]
- National Cancer Institute. Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ®) Health Professional Version. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65881.5/?report=printable (accessed on 5 February 2025).
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar]
- Van Poznak, C. Osteonecrosis of the jaw and bevacizumab therapy. Breast Cancer Res. Treat. 2010, 122, 189–191. [Google Scholar]
- Kudva, A.; Koshy, J.; Jacob, J.G. Oral mucosal pseudotumor—Novelty complication in a patient undergoing bevacizumab therapy. Oral Oncol. 2021, 122, 105543. [Google Scholar] [CrossRef]
- Maluf, G.; Caldas, R.J.; Fregnani, E.R.; da Silva Santos, P.S. A rare case of bevacizumab-related osteonecrosis of the jaw associated with dental implants. Int. J. Implant. Dent. 2019, 5, 34. [Google Scholar]
- Santos-Silva, A.R.; Rosa, G.A.B.; de Castro Júnior, G.; Dias, R.B.; Ribeiro, A.C.P.; Brandão, T.B. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e32–e36. [Google Scholar]
- Katsenos, S.; Christophylakis, C.; Psathakis, K. Osteonecrosis of the jaw in a patient with advanced non-small-cell lung cancer receiving bevacizumab. Arch. Bronconeumol. 2012, 48, 218–219. [Google Scholar] [PubMed]
- Singh, A.; Pischek, A.; Randazzo, J.R.; Huryn, J.M.; Estilo, C.L.; Preeshagul, I.; Yom, S.K. Ramucirumab-related osteonecrosis of the jaw. Oral Oncol. 2021, 125, 105660. [Google Scholar] [CrossRef] [PubMed]
- Yorulmaz, A.; Yalcin, B. Panitumumab-induced paronychia: A case report and a brief review of the literature. Skin. Appendage Disord. 2021, 7, 123–126. [Google Scholar] [PubMed]
- Bartak, H.; Fareh, T.; Ben Othman, N.; Viard, D.; Cohen, M.; Rocher, F.; Ewig, E.; Drici, M.-D.; Lebrun-Frenay, C. Dental Adverse Effects of Anti-CD20 Therapies. Neurol. Ther. 2024, 13, 917–930. [Google Scholar]
- Jackson, L.K.; Johnson, D.B.; Sosman, J.A.; Murphy, B.A.; Epstein, J.B. Oral health in oncology: Impact of immunotherapy. Support. Care Cancer 2015, 23, 1–3. [Google Scholar] [CrossRef]
- Davila, A.; Magee, R.; France, K. Complications After Dental Extractions In Patients Taking Biologic Agents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, e39–e40. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Migkou, M.; Psyrri, A.; Bamias, A.; Pectasides, D.; Economopoulos, T.; Raber-Durlacher, J.E.; Dimitriadis, G.; Dimopoulos, M.A. Gingival bleeding and jaw bone necrosis in patients with metastatic renal cell carcinoma receiving sunitinib: Report of 2 cases with clinical implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 234–238. [Google Scholar]
- Monteiro, L.; Vasconcelos, C.; Pacheco, J.J.; Salazar, F. Photobiomodulation laser therapy in a Lenvatinib-related osteonecrosis of the jaw: A case report. J. Clin. Exp. Dent. 2021, 13, e626. [Google Scholar] [CrossRef]
- Marino, R.; Orlandi, F.; Arecco, F.; Gandolfo, S.; Pentenero, M. Osteonecrosis of the jaw in a patient receiving cabozantinib. Aust. Dent. J. 2015, 60, 528–531. [Google Scholar] [CrossRef]
- Benhima, N.; El Fadli, M.; Essâdi, I.; Belbaraka, R. Pazopanib-induced trismus in a young male with metastatic renal cell carcinoma: A case report and literature review. Oxford Med. Case Rep. 2024, 2024, omad146. [Google Scholar] [CrossRef]
- van Cann, T.; Loyson, T.; Verbiest, A.; Clement, P.M.; Bechter, O.; Willems, L.; Spriet, I.; Coropciuc, R.; Politis, C.; Vandeweyer, R.O.; et al. Incidence of medication-related osteonecrosis of the jaw in patients treated with both bone resorption inhibitors and VEGFR tyrosine kinase inhibitors. Support. Care Cancer 2018, 26, 869–878. [Google Scholar] [PubMed]
- Myers, A.L.; Kiat-Amnuay, S.; Wang, B.Y. Effect of imatinib on oral wound healing after extraction: A rare case report. J. Am. Dent. Assoc. 2022, 153, 805–811. [Google Scholar] [PubMed]
- Singh, A.; Na, S.; Huryn, J.M.; Estilo, C.L.; Horwitz, S.; Yom, S.K. Ibrutinib-associated osteonecrosis of the jaw. Oral Oncol. Rep. 2024, 9, 100228. [Google Scholar]
- Akkach, S.; Shukla, L.; Morgan, D. Everolimus-induced osteonecrosis of the jaw in the absence of bisphosphonates: A case report. Br. J. Oral Maxillofac. Surg. 2019, 57, 688–690. [Google Scholar] [PubMed]
- Lo Muzio, L.; Arena, C.; Troiano, G.; Villa, A. Oral stomatitis and mTOR inhibitors: A review of current evidence in 20,915 patients. Oral Dis. 2018, 24, 144–171. [Google Scholar]
- Geiger-Gritsch, S.; Stollenwerk, B.; Miksad, R.; Guba, B.; Wild, C.; Siebert, U. Safety of bevacizumab in patients with advanced cancer: A meta-analysis of randomized controlled trials. Oncologist 2010, 15, 1179–1191. [Google Scholar]
- Estilo, C.L.; Fornier, M.; Farooki, A.; Carlson, D.; Bohle, G., III; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar]
- Guarneri, V.; Miles, D.; Robert, N.; Diéras, V.; Glaspy, J.; Smith, I.; Thomssen, C.; Biganzoli, L.; Taran, T.; Conte, P. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010, 122, 181–188. [Google Scholar]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar]
- Abdel-Rahman, O.; ElHalawani, H. Risk of oral and gastrointestinal mucosal injury in patients with solid tumors treated with ramucirumab: A systematic review and meta-analysis. Expert. Opin. Drug Saf. 2015, 14, 1495–1506. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Siena, S.; Humblet, Y.; Canon, J.-L.; Maurel, J.; Bajetta, E.; Neyns, B.; Kotasek, D.; Santoro, A.; Scheithauer, W.; et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann. Oncol. 2008, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Tejwani, A.; Wu, S.; Jia, Y.; Agulnik, M.; Millender, L.; Lacouture, M.E. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor inhibitor therapy. Cancer 2009, 115, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Pryor, D.I.; Porceddu, S.V.; Burmeister, B.H.; Guminski, A.; Thomson, D.B.; Shepherdson, K.; Poulsen, M. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother. Oncol. 2009, 90, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Giusti, R.M.; Shastri, K.A.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA drug approval summary: Panitumumab (Vectibix™). Oncologist 2007, 12, 577–583. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y. Immunotherapy-related oral adverse effects: Immediate sequelae, chronicity and secondary cancer. Cancers 2023, 15, 4781. [Google Scholar] [CrossRef]
- Ponzetti, A.; Pinta, F.; Spadi, R.; Mecca, C.; Fanchini, L.; Zanini, M.; Ciuffreda, L.; Racca, P. Jaw osteonecrosis associated with aflibercept, irinotecan and fluorouracil: Attention to Oral District. Tumori 2016, 102, S74–S77. [Google Scholar] [CrossRef]
- Zarringhalam, P.; Brizman, E.; Shakib, K. Medication-related osteonecrosis of the jaw associated with aflibercept. Br. J. Oral Maxillofac. Surg. 2017, 55, 314–315. [Google Scholar] [CrossRef]
- Vallina, C.; Ramirez, L.; Torres, J.; Casanas, E.; Hernandez, G.; Lopez-Pintor, R.M. Osteonecrosis of the jaws produced by sunitinib: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e326. [Google Scholar] [CrossRef]
- Villa, A.; Kuten-Shorrer, M. Pathogenesis of oral toxicities associated with targeted therapy and immunotherapy. Int. J. Mol. Sci. 2023, 24, 8188. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Vardas, E.; Tziveleka, S.; Georgaki, M.; Kouri, M.; Katoumas, K.; Piperi, E.; Nikitakis, N.G. Oral side effects in patients with metastatic renal cell carcinoma receiving the antiangiogenic agent pazopanib—Report of three cases. Dent. J. 2022, 10, 232. [Google Scholar] [CrossRef]
- Wang, F.; Wei, S.; Zhang, Z.; Zhang, Y.; He, J.; Sun, B. Osimertinib: Another medication related to osteonecrosis of the jaws? A case report and literature review. Front. Pharmacol. 2022, 13, 947947. [Google Scholar]
- Subramanian, G.; Kashikar, S.; Fitzhugh, V.; Quek, S.Y.P. Osimertinib and jaw osteonecrosis? A case report. Int. J. Cancer 2019, 145, 2003–2005. [Google Scholar] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008, 372, 449–456. [Google Scholar]
- Epstein, J.B.; Güneri, P.; Barasch, A. Appropriate and necessary oral care for people with cancer: Guidance to obtain the right oral and dental care at the right time. Support. Care Cancer 2014, 22, 1981–1988. [Google Scholar]
- Caribé-Gomes, F.; Chimenos-Küstner, E.; López-López, J.; Finestres-Zubeldia, F.; Guix-Melcior, B. Dental management of the complications of radio and chemotherapy in oral cancer. Med. Oral 2003, 8, 178–187. [Google Scholar]
- McCaul, L.K. Oral and dental management for head and neck cancer patients treated by chemotherapy and radiotherapy. Dent. Update 2012, 39, 135–138, 140. [Google Scholar]
- Cardona, A.; Balouch, A.; Abdul, M.M.; Sedghizadeh, P.P.; Enciso, R. Efficacy of chlorhexidine for the prevention and treatment of oral mucositis in cancer patients: A systematic review with meta-analyses. J. Oral Pathol. Med. 2017, 46, 680–688. [Google Scholar] [CrossRef]
- Barclay, S.C.; Turani, D. Current practice in dental oncology in the UK. Dent. Update 2010, 37, 560–561. [Google Scholar]
- Parra-Rojas, S.; Velázquez-Cayón, R.T.; Borges-Gil, A.; Mejías-Torrus, J.L.; Cassol-Spanemberg, J. Oral Complications and Management Strategies for Cancer Patients: Principles of Supportive Oncology in Dentistry. Curr. Oncol. Rep. 2024, 26, 391–399. [Google Scholar]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef]
- McCaul, L.K.; Barclay, S.; Nixon, P.; Yule, P.L.; Trainor, J.; Stevenson, B.; Paterson, A.; Nicol, A.; Keys, W.; Donachie, M.; et al. Oral prehabilitation for patients with head and neck cancer: Getting it right—The Restorative Dentistry-UK consensus on a multidisciplinary approach. Br. Dent. J. 2022, 233, 794–800. [Google Scholar] [PubMed]
- Sari, J.; Nasiloski, K.; Gomes, A. Oral complications in patients receiving head and neck radiation therapy: A literature review. RGO—Rev. Gaúcha Odontol. 2014, 62, 395–400. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [PubMed]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw—Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar]
- Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Vidović Juras, D.; Andabak Rogulj, A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. [Google Scholar]
- Kün-Darbois, J.D.; Fauvel, F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current management. Morphologie 2021, 105, 170–187. [Google Scholar]
- Yong, C.W.; Robinson, A.; Hong, C. Dental Evaluation Prior to Cancer Therapy. Front. Oral Health 2022, 3, 876941. [Google Scholar]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Patano, A.; Viapiano, F.; Netti, A.; Azzollini, D.; Ciocia, A.M.; de Ruvo, E.; Campanelli, M.; et al. MRONJ Treatment Strategies: A Systematic Review and Two Case Reports. Appl. Sci. 2023, 13, 4370. [Google Scholar] [CrossRef]
- Scribante, A.; Ghizzoni, M.; Pellegrini, M.; Pulicari, F.; Spadari, F. Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of MRONJ: A Systematic Review. Medicina 2023, 59, 972. [Google Scholar] [CrossRef]
- Schlosser, B.J. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol. Ther. 2010, 23, 251–267. [Google Scholar]
- Kuten-Shorrer, M.; Hochberg, E.P.; Woo, S.B. Lichenoid mucosal reaction to rituximab. Oncologist 2014, 19, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, V.; Muzio, F.; Manna, G.; Moggio, E.; Vassallo, C.; Orlandi, E.; Fiandrino, G.; Lucioni, M.; Borroni, G. Photoinduced dermatitis and oral lichenoid reaction in a CML patient treated with imatinib mesylate. Photodermatol. Photoimmunol. Photomed. 2012, 28, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Sankar, V.; Xu, X.; Barrett, A.W.; High, A.S.; Odell, E.W.; Speight, P.M.; Farthing, P.M. The role of histopathological characteristics in distinguishing amalgam-associated oral lichenoid reactions and oral lichen planus. J. Oral Pathol. Med. 2006, 35, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Woo, S.B. Adverse drug events in the oral cavity. Dermatol. Clin. 2020, 38, 523–533. [Google Scholar] [CrossRef]
- Arora, B.; Kumar, L.; Sharma, A.; Wadhwa, J.; Kochupillai, V. Pigmentary changes in CML patients treated with imatinib mesylate. Ann. Oncol. 2004, 15, 358–359. [Google Scholar] [CrossRef]
- Donnell, C.C.; Walton, R.L.; Carrozzo, M. The blue palate—A case series of imatinib-related oral pigmentation and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 49–61. [Google Scholar] [CrossRef]
- McPherson, T.; Sherman, V.; Turner, R. Imatinib-associated hyperpigmentation, a side effect that should be recognized. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 82–83. [Google Scholar] [CrossRef]
- Oliveira, S.R.; de Azevedo Branco, L.G.; Rocha, A.L.; Travassos, D.V.; Magalhães, G.H.R.; Fonseca, F.P.; Mesquita, R.A.; Abreu, L.G.; da Silva, T.A. Association of oral mucosa hyperpigmentation with imatinib mesylate use: A cross-sectional study and systematic literature review. Clin. Oral. Investig. 2019, 23, 4371–4382. [Google Scholar] [CrossRef]
- Pancholi, N.; Taneja, P. Intraoral hyperpigmentation due to imatinib mesylate. Oral Surg. 2016, 9, 206–214. [Google Scholar] [CrossRef]
- Alexandrescu, D.T.; Dasanu, C.A.; Farzanmehr, H.; Kauffman, L. Persistent cutaneous hyperpigmentation after tyrosine kinase inhibition with imatinib for GIST. Dermatol. Online J. 2008, 14, 7. [Google Scholar] [CrossRef]
- Casamiquela, K.M.; Cohen, P.R. Chemotherapy-associated tongue hyperpigmentation and blue lunula. J. Drugs Dermatol. 2013, 12, 223–226. [Google Scholar] [PubMed]
- Alfreijat, M. Tongue hyperpigmentation associated with chemotherapy. J. Community Hosp. Intern. Med. Perspect. 2013, 3, 21047. [Google Scholar]
- Sonis, S.; Treister, N.; Chawla, S.; Demetri, G.; Haluska, F. Preliminary characterization of oral lesions associated with mTOR inhibitors in cancer patients. Cancer 2010, 116, 210–215. [Google Scholar] [CrossRef]
- Martins, F.; de Oliveira, M.A.; Wang, Q.; Sonis, S.; Gallottini, M.; George, S.; Treister, N. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013, 49, 293–298. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2022, 21, 495–508. [Google Scholar]
- Xu, Y.; Wen, N.; Sonis, S.T.; Villa, A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients. Cancer 2021, 127, 1796–1804. [Google Scholar]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Thompson, J.A. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline summary. J. Oncol. Pract. 2018, 14, 247–249. [Google Scholar]
- Dimitriou, F.; Hogan, S.; Menzies, A.M.; Dummer, R.; Long, G.V. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur. J. Cancer 2021, 157, 214–224. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Nape-as, J.J.; Brennan, M.T.; Bahrani-Mougeot, F.K.; Fox, P.C.; Lockhart, P.B. Relationship between mucositis and changes in oral microflora during cancer chemotherapy. Oral Surg. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 48–59. [Google Scholar]
- Chavelli-Lopez, B.; Bagan-Sebastian, J.V. Treatment of oral mucositis due to chemotherapy. J. Clin. Exp. Dent. 2016, 8, e201–e209. [Google Scholar] [CrossRef] [PubMed]
- Potting, C.M.; Uitterhoeve, R.; Op Reimer, W.S.; Van Achterberg, T. The effectiveness of commonly used mouthwashes for the prevention of chemotherapy-induced oral mucositis: A systematic review. Eur. J. Cancer Care 2006, 15, 431–439. [Google Scholar]
- Chaveli López, B.; Gavaldá Esteve, C.; Sarrión Pérez, M.G. Dental treatment considerations in the chemotherapy patient. J. Clin. Exp. Dent. 2011, 3, e31–e42. [Google Scholar]
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T. Interventions for preventing oral mucositis in cancer patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2011, 13, CD000978. [Google Scholar]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar]
- Arbabi-kalati, F.; Arbabi-kalati, F.; Deghatipour, M.; Ansari Moghadam, A. Evaluation of zinc sulfate in prevention of chemotherapy-induced mucositis: A double-blind RCT. Arch. Iran. Med. 2012, 15, 413–417. [Google Scholar]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines. Ann. Oncol. 2015, 26 (Suppl. S5), v139–v151. [Google Scholar]
- Grushka, M.; Epstein, J.B.; Gorsky, M. Burning mouth syndrome. Am. Fam. Physician 2002, 65, 615–620. [Google Scholar]
- Mistretta, C.M.; Kumari, A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. Int. J. Mol. Sci. 2019, 20, 1341. [Google Scholar] [CrossRef]
- Kumari, A.; Ermilov, A.N.; Allen, B.L.; Bradley, R.M.; Dlugosz, A.A.; Mistretta, C.M. Hedgehog pathway blockade with LDE225 disrupts taste organs and sensation. J. Neurophysiol. 2015, 113, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.A.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in advanced basal cell carcinoma: Primary analysis of STEVIE trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Soeda, H.; Komine, K.; Otsuka, K.; Shibata, H. Preliminary estimation of chemotherapy-induced dysgeusia in Japanese cancer patients. BMC Palliat. Care 2013, 12, 38. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and vismodegib in locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Koizumi, T.; Fukushima, T.; Tatai, T.; Kobayashi, T.; Sekiguchi, N.; Sakamoto, A.; Sasaki, S. Successful treatment of crizotinib-induced dysgeusia by switching to alectinib in ALK-positive NSCLC. Lung Cancer 2015, 88, 112–113. [Google Scholar] [CrossRef]
- Farooq, M.Z.; Aqeel, S.B.; Lingamaneni, P.; Pichardo, R.C.; Jawed, A.; Khalid, S.; Banskota, S.U.; Fu, P.; Mangla, A. Association of immune checkpoint inhibitors with neurologic adverse events: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e227722. [Google Scholar] [CrossRef]
- Albarran, V.; Chamorro, J.; Rosero, D.I.; Saavedra, C.; Soria, A.; Carrato, A.; Gajate, P. Neurologic toxicity of immune checkpoint inhibitors: A review. Front. Pharmacol. 2022, 13, 774170. [Google Scholar] [CrossRef]
- Rahnama, M.; Madej-Czerwonka, B.; Jastrzębska-Jamrogiewicz, I.; Jamrogiewicz, R. Influence of parenteral chemotherapy on oral mucosa health. Contemp. Oncol. 2015, 19, 77–82. [Google Scholar]

| Therapeutic Class | Drug | Mechanism | Common Clinical Uses | Common Oral Side Effects | Source |
|---|---|---|---|---|---|
| Monoclonal Antibodies—Anti-VEGF/VEGFR | Bevacizumab | Binds VEGF ligand to inhibit angiogenesis | Metastatic colorectal cancer, NSCLC, glioblastoma, renal cell carcinoma, cervical cancer | Stomatitis, mucositis, gingival bleeding; medication-related osteonecrosis of the jaw (MRONJ) | [29,30,31,32,33] |
| Ramucirumab | Binds VEGFR2 to block VEGF signaling | Advanced gastric cancer, NSCLC, colorectal cancer | Stomatitis, mucosal irritation, bleeding, ONJ | [34] | |
| Monoclonal Antibodies—Anti-EGFR | Cetuximab | Binds EGFR, preventing receptor activation | Metastatic colorectal cancer (KRAS wild-type), head and neck cancers | Stomatitis, mucositis, taste alterations, oral mucosal irritation | [9] |
| Panitumumab | Binds EGFR, inhibiting receptor signaling | Metastatic colorectal cancer (KRAS wild-type) | Stomatitis, mucosal irritation, dryness, taste changes | [9,35] | |
| Monoclonal Antibodies—Other Targets | Rituximab | Targets CD20 on B-cells | Non-Hodgkin’s lymphoma, CLL | Oral mucosal dryness or mild irritation; secondary infections may occur | [36] |
| Ipilimumab | Inhibits CTLA-4, enhancing immune response | Metastatic melanoma (with other checkpoint inhibitors) | Stomatitis, mucosal inflammation, lichenoid reactions in the oral mucosa, MRONJ, xerostomia | [37] | |
| Fusion Proteins/Decoy Receptors | Aflibercept | “VEGF trap” binding VEGF and placental growth factor | Metastatic colorectal cancer | Stomatitis, mucositis, MRONJ | [38] |
| Small Molecule TKIs | Sunitinib | Inhibits multiple kinases (VEGFR, PDGFR, c-kit, etc.) | Renal cell carcinoma, GIST, pancreatic neuroendocrine tumors | Stomatitis, mucositis, dysgeusia; MRONJ | [39] |
| Lenvatinib | Multi-targeted TKI (VEGFR, FGFR, etc.) | Radioiodine-refractory thyroid cancer, renal cell carcinoma, hepatocellular carcinoma | Stomatitis, mucositis, taste alterations | [40] | |
| Cabozantinib | Inhibits multiple kinases (VEGFR, MET, RET, etc.) | Renal cell carcinoma, medullary thyroid cancer, hepatocellular carcinoma | Stomatitis, mucositis, dysgeusia | [41] | |
| Pazopanib | Inhibits VEGFR, PDGFR, c-kit | Renal cell carcinoma, soft tissue sarcomas | Stomatitis, mucositis, oral discomfort | [42] | |
| Axitinib | Selectively inhibits VEGFRs | Renal cell carcinoma | Stomatitis, mucosal irritation, altered taste, MRONJ | [43] | |
| Sorafenib | Inhibits VEGFR, PDGFR, and Raf kinases | Hepatocellular carcinoma, renal cell carcinoma, thyroid carcinoma | Stomatitis, mucositis, dry mouth and taste alterations | [9] | |
| Small Molecule TKIs—EGFR-targeted | Erlotinib | Inhibits EGFR tyrosine kinase | NSCLC, pancreatic cancer | Stomatitis, mucositis, dry mouth, altered taste | [9] |
| Small Molecule TKIs—BCR-ABL | Imatinib | Inhibits BCR-ABL, c-kit, and PDGFR | Chronic myelogenous leukemia (CML), GIST, dermatofibrosarcoma protuberans | Gingival hyperplasia, mucosal pigmentation, stomatitis | [44] |
| Small Molecule TKIs—BTK Inhibitor | Ibrutinib | Inhibits Bruton’s tyrosine kinase (BTK) | Chronic lymphocytic leukemia (CLL), mantle cell lymphoma, Waldenström’s macroglobulinemia | Mucositis, oral bleeding, and dry mouth | [45] |
| mTOR Inhibitors | Everolimus | Inhibits mTOR signaling pathway | Renal cell carcinoma, HR-positive breast cancer, neuroendocrine tumors | High incidence of stomatitis and mucositis, oral ulcers, and discomfort | [46] |
| Temsirolimus | Inhibits mTOR signaling pathway | Advanced renal cell carcinoma | Stomatitis, mucositis, and oral ulcerations | [47] |
| TKI Name | Oral Adverse Effects | Frequency |
|---|---|---|
| Imatinib | Oral mucositis, gingival bleeding, taste changes | Rare |
| Dasatinib | Oral ulcers, stomatitis | Common |
| Nilotinib | Dry mouth, dysgeusia, oral pain | Uncommon |
| Bosutinib | Stomatitis, oral ulcers | Rare |
| Ponatinib | Mucositis, xerostomia, tongue pain | Rare |
| Erlotinib | Stomatitis, oral ulcers | Common |
| Gefitinib | Dry mouth, mucositis | Common |
| Afatinib | Stomatitis, oral ulcers | Common |
| Osimertinib | Oral ulcers, taste disturbances | Uncommon |
| Lapatinib | Stomatitis, taste changes | Uncommon |
| Sorafenib | Mucositis, mouth ulcers | Common |
| Sunitinib | Stomatitis, dysgeusia, mucositis | Common |
| Pazopanib | Oral ulcers, dysgeusia | Uncommon |
| Cabozantinib | Stomatitis, gingival bleeding | Common |
| Axitinib | Stomatitis, oral pain | Common |
| Regorafenib | Mucositis, oral pain | Common |
| Vandetanib | Stomatitis, dysgeusia | Uncommon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, I.A.; Kajanto, L.A.; Popovici, L.R.; Augustin, I.G.; Gales, L.N. Navigating Stomatologic Complications Secondary to Antineoplastic Agents—A Comprehensive Review. Cancers 2025, 17, 1061. https://doi.org/10.3390/cancers17071061
Popovici IA, Kajanto LA, Popovici LR, Augustin IG, Gales LN. Navigating Stomatologic Complications Secondary to Antineoplastic Agents—A Comprehensive Review. Cancers. 2025; 17(7):1061. https://doi.org/10.3390/cancers17071061
Chicago/Turabian StylePopovici, Ion Alexandru, Lidia Anca Kajanto, Laura Roxana Popovici, Iolanda Georgiana Augustin, and Laurentia Nicoleta Gales. 2025. "Navigating Stomatologic Complications Secondary to Antineoplastic Agents—A Comprehensive Review" Cancers 17, no. 7: 1061. https://doi.org/10.3390/cancers17071061
APA StylePopovici, I. A., Kajanto, L. A., Popovici, L. R., Augustin, I. G., & Gales, L. N. (2025). Navigating Stomatologic Complications Secondary to Antineoplastic Agents—A Comprehensive Review. Cancers, 17(7), 1061. https://doi.org/10.3390/cancers17071061






