Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Evaluation of Studies and Statistical Analysis
3. Results
3.1. Literature Review
3.2. Study and Patient Characteristics
3.3. Patient Selection for Downstaging Therapies
3.4. Should Patients with HCC Initially beyond the Listing Criteria Be Transplanted following Successful Downstaging?
3.4.1. Question 1: Comparison Based on ITT Analysis of Patients Initially beyond the Listing Criteria vs. Those within the Listing Criteria (Table 4)
- (a)
- Waiting List Drop-out and Interval on the Waiting List
- (b)
- Post-LT Survival Outcomes
- (c)
- Survival Outcomes Based on ITT Analysis
- (d)
- Sensitivity Analysis
3.4.2. Question 2: Comparison of Patients Initially beyond the Listing Criteria, Downstaged and Transplanted versus Those Not Transplanted (Table 5)
3.5. Survival Outcomes Based on ITT Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e471. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Patel, Y.A.; Aggarwal, A.; Desai, A.P.; Frenette, C.; Pillai, A.A.; Salgia, R.; Seetharam, A.; Sharma, P.; Sherman, C.; et al. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am. J. Transpl. 2020, 20, 333–347. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Toso, C.; Asthana, S.; Bigam, D.L.; Shapiro, A.M.; Kneteman, N.M. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009, 49, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e3; quiz e914–e985. [Google Scholar] [CrossRef]
- Mehta, N.; Guy, J.; Frenette, C.T.; Dodge, J.L.; Osorio, R.W.; Minteer, W.B.; Roberts, J.P.; Yao, F.Y. Excellent Outcomes of Liver Transplantation Following Down-Staging of Hepatocellular Carcinoma to Within Milan Criteria: A Multicenter Study. Clin. Gastroenterol. Hepatol. 2018, 16, 955–964. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Dodge, J.L.; Grab, J.D.; Yao, F.Y. National Experience on Down-Staging of Hepatocellular Carcinoma Before Liver Transplant: Influence of Tumor Burden, Alpha-Fetoprotein, and Wait Time. Hepatology 2020, 71, 943–954. [Google Scholar] [CrossRef]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreras, J.; Di Maira, T.; Vinaixa, C.; San Juan, F.; Rubín, Á.; Berenguer, M. Milan-out Criteria and Worse Intention-to-Treat Outcome Postliver Transplantation. Transplant. Direct. 2019, 5, e487. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Odaldi, F.; Cucchetti, A.; Trevisani, F.; Piscaglia, F.; De Pace, V.; Bertuzzo, V.R.; Neri, F.; Golfieri, R.; Cappelli, A.; et al. Long term results of down-staging and liver transplantation for patients with hepatocellular carcinoma beyond the conventional criteria. Sci. Rep. 2019, 9, 3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryce, K.; Tsochatzis, E.A. Downstaging for hepatocellular cancer: Harm or benefit? Transl. Gastroenterol. Hepatol. 2017, 2, 106. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 6 June 2022).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2021; Available online: www.training.cochrane.org/handbook (accessed on 6 June 2022).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Affonso, B.B.; Galastri, F.L.; da Motta Leal Filho, J.M.; Nasser, F.; Falsarella, P.M.; Cavalcante, R.N.; de Almeida, M.D.; Felga, G.E.G.; Valle, L.G.M.; Wolosker, N. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J. Gastroenterol. 2019, 25, 5687–5701. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Grigoletto, F.; Gringeri, E.; D’Amico, F.; Valmasoni, M.; Brolese, A.; Zanus, G.; Srsen, N.; Carraro, A.; et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am. J. Transplant. 2007, 7, 972–981. [Google Scholar] [CrossRef]
- Graziadei, I.W.; Sandmueller, H.; Waldenberger, P.; Koenigsrainer, A.; Nachbaur, K.; Jaschke, W.; Margreiter, R.; Vogel, W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transplant. 2003, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.; Herber, S.; Heise, M.; Lohse, A.W.; Monch, C.; Bittinger, F.; Hoppe-Lotichius, M.; Schuchmann, M.; Victor, A.; Pitton, M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transplant. 2006, 12, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Grazi, G.L.; Piscaglia, F.; Trevisani, F.; Cescon, M.; Ercolani, G.; Vivarelli, M.; Golfieri, R.; D’Errico Grigioni, A.; Panzini, I.; et al. Liver transplantation for hepatocellular carcinoma: Results of down-staging in patients initially outside the Milan selection criteria. Am. J. Transplant. 2008, 8, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef] [Green Version]
- Heinzow, H.S.; Brockmann, J.G.; Köhler, M.; Wolters, H.H.; Senninger, N.; Schmidt, H.; Meister, T. Liver transplantation versus supraselective transarterial chemoembolization in palliative patients with hepatocellular carcinoma exceeding the Milan Criteria—Is it time for a more individual approach? Ann. Transplant. 2013, 18, 515–524. [Google Scholar] [CrossRef]
- Lei, J.Y.; Yan, L.N.; Wang, W.T. Transplantation vs. resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J. Gastroenterol. 2013, 19, 4400–4408. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Schaubel, D.E.; Guidinger, M.K.; Biggins, S.W.; Kalbfleisch, J.D.; Pomfret, E.A.; Sharma, P.; Merion, R.M. Survival benefit-based deceased-donor liver allocation. Am. J. Transplant. 2009, 9, 970–981. [Google Scholar] [CrossRef] [Green Version]
- Merion, R.M.; Schaubel, D.E.; Dykstra, D.M.; Freeman, R.B.; Port, F.K.; Wolfe, R.A. The survival benefit of liver transplantation. Am. J. Transplant. 2005, 5, 307–313. [Google Scholar] [CrossRef]
- Vitale, A.; Morales, R.R.; Zanus, G.; Farinati, F.; Burra, P.; Angeli, P.; Frigo, A.C.; Del Poggio, P.; Rapaccini, G.; Di Nolfo, M.A.; et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: A multicentre, cohort study. Lancet Oncol. 2011, 12, 654–662. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Volk, M.L.; Frigo, A.C.; Grigoletto, F.; Brolese, A.; Zanus, G.; D’Amico, F.; Farinati, F.; Burra, P.; et al. The survival benefit of liver transplantation in hepatocellular carcinoma patients. Dig. Liver Dis. 2010, 42, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Volk, M.; Cillo, U. Transplant benefit for patients with hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 9183–9188. [Google Scholar] [CrossRef]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Kudo, M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int. J. Clin. Oncol. 2022, 27, 1110–1119. [Google Scholar] [CrossRef]
- Bteich, F.; Di Bisceglie, A.M. Current and Future Systemic Therapies for Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2019, 15, 266–272. [Google Scholar]
- Lo, C. Downstaging of hepatocellular carcinoma before transplantation: An advance in therapy or just another selection criterion. Am. J. Transplant. 2008, 8, 2485–2486. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, S.R.; Russo, M.W. Liver transplantation beyond or downstaging within the Milan criteria for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 265–275. [Google Scholar] [CrossRef]
- Wallace, D.; Cowling, T.E.; Walker, K.; Suddle, A.; Gimson, A.; Rowe, I.; Callaghan, C.; Sapisochin, G.; Mehta, N.; Heaton, N.; et al. Liver transplantation outcomes after transarterial chemotherapy for hepatocellular carcinoma. Br. J. Surg. 2020, 107, 1183–1191. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]





| Question | Study Group | Intervention | Control | Outcomes |
|---|---|---|---|---|
| 1 | Patients with liver cirrhosis and HCC within and beyond listing criteria awaiting LT | Any downstaging therapy before LT (patients beyond listing criteria) | Any downstaging therapy before LT (patients within listing criteria) | Waitlist dropout, time on the waiting list, post-LT and ITT survival (1, 3 and 5 year overall survival) |
| 2 | Patients with liver cirrhosis and HCC beyond listing criteria | Any downstaging therapy before LT | Any downstaging therapy not followed by LT | ITT survival (1, 3 and 5 year overall survival) |
| Study ID | Country | Type of Study | Total N | Inclusion Criteria Used | Criteria for Successful Downstaging | Study Group | Details | N | Control Group | Details | N | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affonso 2019 | Brazil | Obs. Prosp. | 200 | No upper limit: HCC above MC | Patients with HCC within MC | Downstaging | TACE | 64 | Bridging | TACE | 136 | |
| Cillo 2007 | Italy | Obs. Prosp. | 100 | No upper limit: HCC above MC, with no extrahepatic spread, macrovascular invasion or poor differentiation. | Downstaging | Various | 40 | Bridging | Various | 60 | 21 | |
| Graziadei 2003 | Austria | RCT | 63 | No upper limit: HCC above MC, with no extrahepatic disease or vascular invasion | Patients with HCC within MC | Downstaging | TACE | 15 | Bridging | TACE | 48 | |
| Herreras 2019 | Spain | Obs. Retrosp. | 177 | No upper limit: HCC above MC, with no vascular invasion, extrahepatic disease, or alpha-fetoprotein (AFP) higher than 1000 g/dL | Patients with tumour response; patients with AFP values > 400 mg/dL after downstaging procedure were excluded. | Downstaging | Various | 29 | Bridging | Various | 148 | |
| Otto 2006 | Germany | Obs.Retrosp. | 96 | No upper limit: HCC above MC | Patients with tumour response. | Downstaging | TACE | 62 | Bridging | TACE | 34 | 29 |
| Ravaioli 2008 | Italy | Obs. Retrosp. | 177 | Patient with HCC within Bologna Downstaging Protocol | Patients with HCC within MC | Downstaging | Various | 48 | Bridging + Obs | Various | 129 | |
| Ravaioli 2019 | Italy | Obs. Retrosp. | 281 | Patient with HCC within Bologna Downstaging Criteria | Patients with HCC within MC | Downstaging | Various | 95 | Bridging + Obs | Various | 186 | 60 |
| Yao 2015 | USA | Obs. Retrosp. | 606 | Patients with HCC within UCSF Downstaging Protocol | Patients with HCC within MC/UNOS T2 Criteria | Downstaging | Various | 118 | Bridging | Various | 488 |
| Study ID | Country | Type of Study | Total N | Inclusion Criteria Used | Criteria for Successful Downstaging | Study Group | Details | N | Control Group | Details | N | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heinzow 2013 | Germany | Obs. Retrosp. | 63 | No upper limit: HCC above MC | Patients with HCC within MC | Downstaging and LT | TACE | 23 | Loregional therapy | TACE | 40 | |
| Lei 2013 | China | Obs. Retrosp. | 66 | Patients with HCC within UCSF Downstaging Protocol | Patients with HCC within MC/UNOS T2 Criteria | Downstaging and LT | Various | 31 | Lororegional therapy and liver resection | Various | 35 | 43 |
| Mazzaferro 2020 | Italy | RCT | 45 | No upper limit: HCC above MC | Patients with HCC within MC | Downstaging and LT | Various | 23 | Loregional therapy | Various | 22 | 71 |
| Outcomes | Studies | Patients | OR (95% CI) | I2 | GRADE | |
|---|---|---|---|---|---|---|
| Entire cohort | Drop-out due to all causes | 7 | 1537 | 2.05 (1.45–2.88) | 42% | ⨁⨁◯◯ Low |
| Drop-out due to tumour progression | 4 | 550 | 3.07 (1.83–5.14) | 0% | ⨁⨁◯◯ Low | |
| Drop-out due to liver deterioration | 2 | 273 | 3.93 (1.11–13.88) | 0% | ⨁⨁◯◯ Low | |
| Time from initial assessment to LT | 4 | 1002 | 1.93 * (0.91–2.94) | 0% | ⨁⨁◯◯ Low | |
| Post-LT 1y-suvival | 6 | 1048 | 1.03 (0.55–1.96) | 37% | ⨁◯◯◯ Very low | |
| Post-LT 3y-suvival | 6 | 1048 | 1.03 (0.67–1.57) | 19% | ⨁◯◯◯ Very low | |
| Post-LT 5y-suvival | 4 | 860 | 0.76 (0.50–1.14) | 11% | ⨁◯◯◯ Very low | |
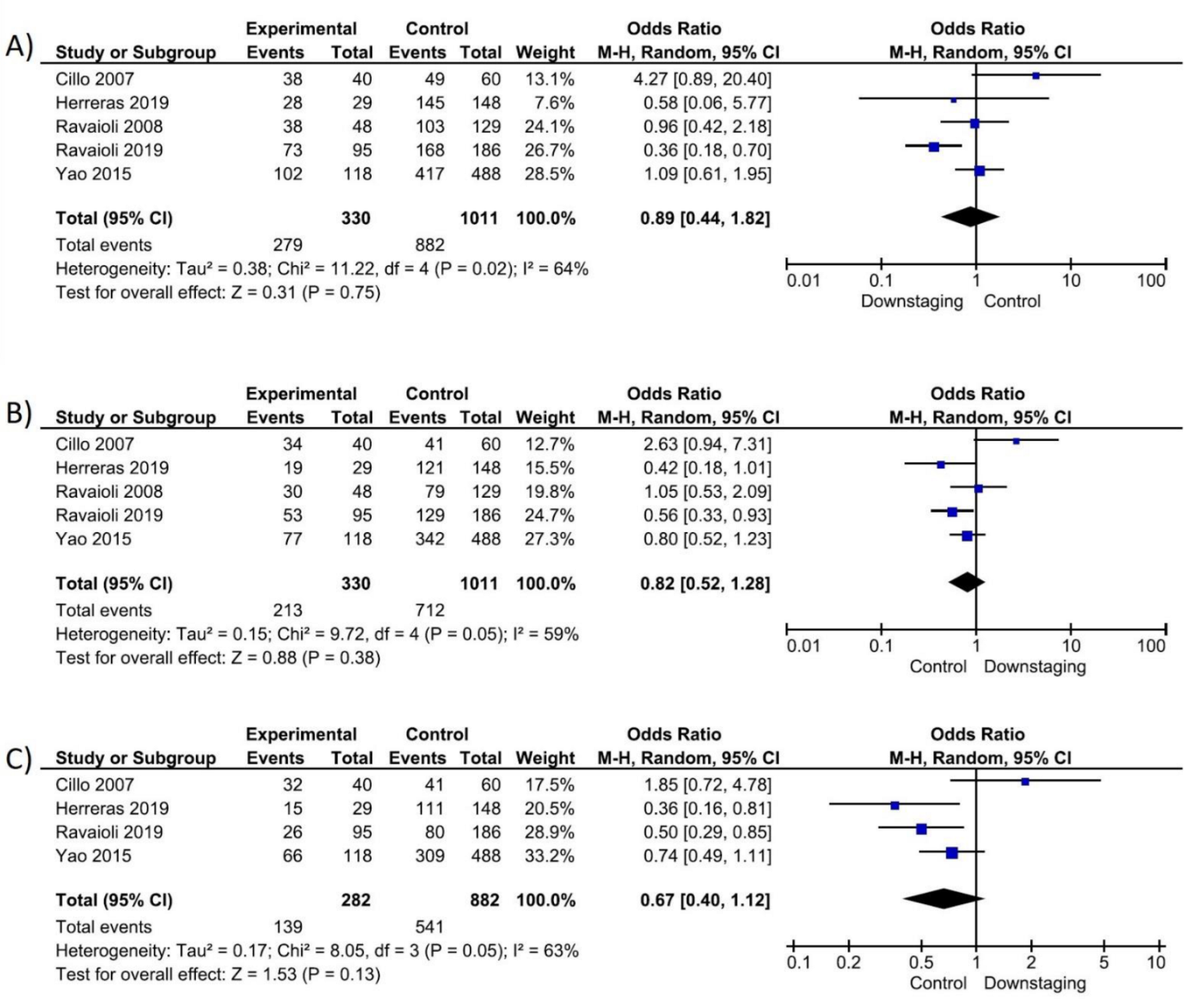
| ITT 1y-suvival | 5 | 1341 | 0.89 (0.44–1.82) | 64% | ⨁◯◯◯ Very low | |
| ITT 3y-suvival | 5 | 1341 | 0.82 (0.52–1.28) | 11% | ⨁◯◯◯ Very low | |
| ITT 5y-suvival | 4 | 1164 | 0.67 (0.40–1.12) | 11% | ⨁◯◯◯ Very low | |
| Manuscript based on a downstaging protocol | Drop-out due to all causes | 3 | 1064 | 1.64 (1.22–2.21) | 42% | ⨁⨁◯◯ Low |
| Drop-out due to tumour progression | 1 | 177 | 2.82 (1.23–6.50) | NA | ⨁⨁◯◯ Low | |
| Time from initial assessment to LT | 1 | 606 | 1.80 * (0.73–2.87) | NA | ⨁⨁◯◯ Low | |
| Post-LT 1y-suvival | 3 | 724 | 0.93 (0.53–1.64) | 0% | ⨁◯◯◯ Very low | |
| Post-LT 3y-suvival | 3 | 724 | 0.93 (0.62–1.41) | 0% | ⨁◯◯◯ Very low | |
| Post-LT 5y-suvival | 2 | 418 | 0.83 (0.54–1.30) | 0% | ⨁◯◯◯ Very low | |
| ITT 1y-suvival | 3 | 1064 | 0.72 (0.35–1.47) | 69% | ⨁◯◯◯ Very low | |
| ITT 3y-suvival | 3 | 1064 | 0.75 (0.54–1.04) | 15% | ⨁◯◯◯ Very low | |
| ITT 5y-suvival | 2 | 887 | 0.63 (0.44–0.91) | 21% | ⨁◯◯◯ Very low | |
| Manuscript not based on a downstaging protocol (no strict inclusion criteria for downstaging selection) | Drop-out due to all causes | 4 | 573 | 2.93 (1.84–4.67) | 14% | ⨁⨁◯◯ Low |
| Drop-out due to tumour progression | 3 | 373 | 3.13 (1.46–6.71) | 24% | ⨁⨁◯◯ Low | |
| Time from initial assessment to LT | 3 | 396 | 3.00 * (−0.84–6.84) | 3% | ⨁⨁◯◯ Low | |
| Post-LT 1y-suvival | 3 | 324 | 1.56 (0.23–10.65) | 75% | ⨁◯◯◯ Very low | |
| Post-LT 3y-suvival | 3 | 324 | 1.51 (0.40–5.72) | 64% | ⨁◯◯◯ Very low | |
| Post-LT 5y-suvival | 2 | 196 | 0.61 (0.18–2.12) | 64% | ⨁◯◯◯ Very low | |
| ITT 1y-suvival | 2 | 277 | 1.88 (0.27–13.16) | 50% | ⨁◯◯◯ Very low | |
| ITT 3y-suvival | 2 | 277 | 1.03 (0.17–6.24) | 86% | ⨁◯◯◯ Very low | |
| ITT 5y-suvival | 2 | 199 | 0.80 (0.16–4.03) | 85% | ⨁◯◯◯ Very low |
| Outcomes | Studies | Patients | OR (95% CI) | I2 | GRADE |
|---|---|---|---|---|---|
| ITT 1y-suvival | 3 | 154 | 1.05 (0.31, 3.62) | 44% | ⨁◯◯◯ Very low |
| ITT 3y-suvival | 3 | 154 | 3.77 (1.26, 11.32) | 54% | ⨁◯◯◯ Very low |
| ITT 5y-suvival | 2 | 129 | 3.08 (1.15, 8.23) | 0% | ⨁◯◯◯ Very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martino, M.; Vitale, A.; Ferraro, D.; Maniscalco, M.; Pisaniello, D.; Arenga, G.; Falaschi, F.; Terrone, A.; Iacomino, A.; Galeota Lanza, A.; et al. Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers 2022, 14, 5102. https://doi.org/10.3390/cancers14205102
Di Martino M, Vitale A, Ferraro D, Maniscalco M, Pisaniello D, Arenga G, Falaschi F, Terrone A, Iacomino A, Galeota Lanza A, et al. Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers. 2022; 14(20):5102. https://doi.org/10.3390/cancers14205102
Chicago/Turabian StyleDi Martino, Marcello, Alessandro Vitale, Daniele Ferraro, Marilisa Maniscalco, Donatella Pisaniello, Giuseppe Arenga, Federica Falaschi, Alfonso Terrone, Alessandro Iacomino, Alfonso Galeota Lanza, and et al. 2022. "Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes" Cancers 14, no. 20: 5102. https://doi.org/10.3390/cancers14205102
APA StyleDi Martino, M., Vitale, A., Ferraro, D., Maniscalco, M., Pisaniello, D., Arenga, G., Falaschi, F., Terrone, A., Iacomino, A., Galeota Lanza, A., Esposito, C., Cillo, U., & Vennarecci, G. (2022). Downstaging Therapies for Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation: A Systematic Review and Meta-Analysis on Intention-to-Treat Outcomes. Cancers, 14(20), 5102. https://doi.org/10.3390/cancers14205102








