Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Assessment
2.4. Data Extraction and Data Synthesis
2.5. Post-Hoc Analysis
3. Results
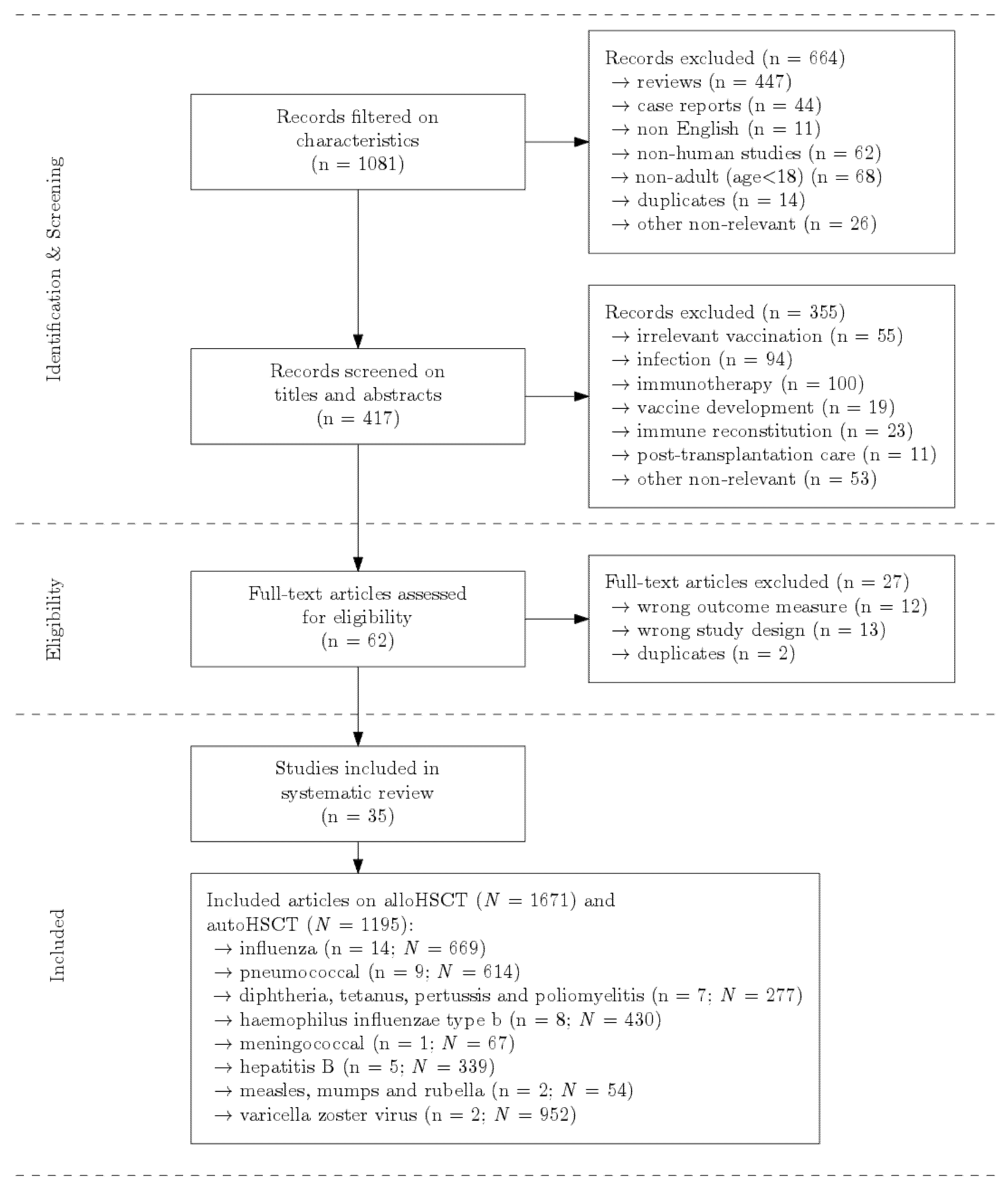
3.1. Searching, Selection and Quality Assessment
3.2. Allogeneic Hematopoietic Stem Cell Transplantation
3.2.1. Influenza
3.2.2. Pneumococcal
3.2.3. Diphtheria, Tetanus, Pertussis (DTP) and Poliomyelitis
3.2.4. Haemophilus influenzae Type b (Hib)
3.2.5. Meningococcal
3.2.6. Hepatitis B
3.2.7. Measles, Mumps and Rubella (MMR)
3.2.8. Varicella Zoster Virus (VZV)
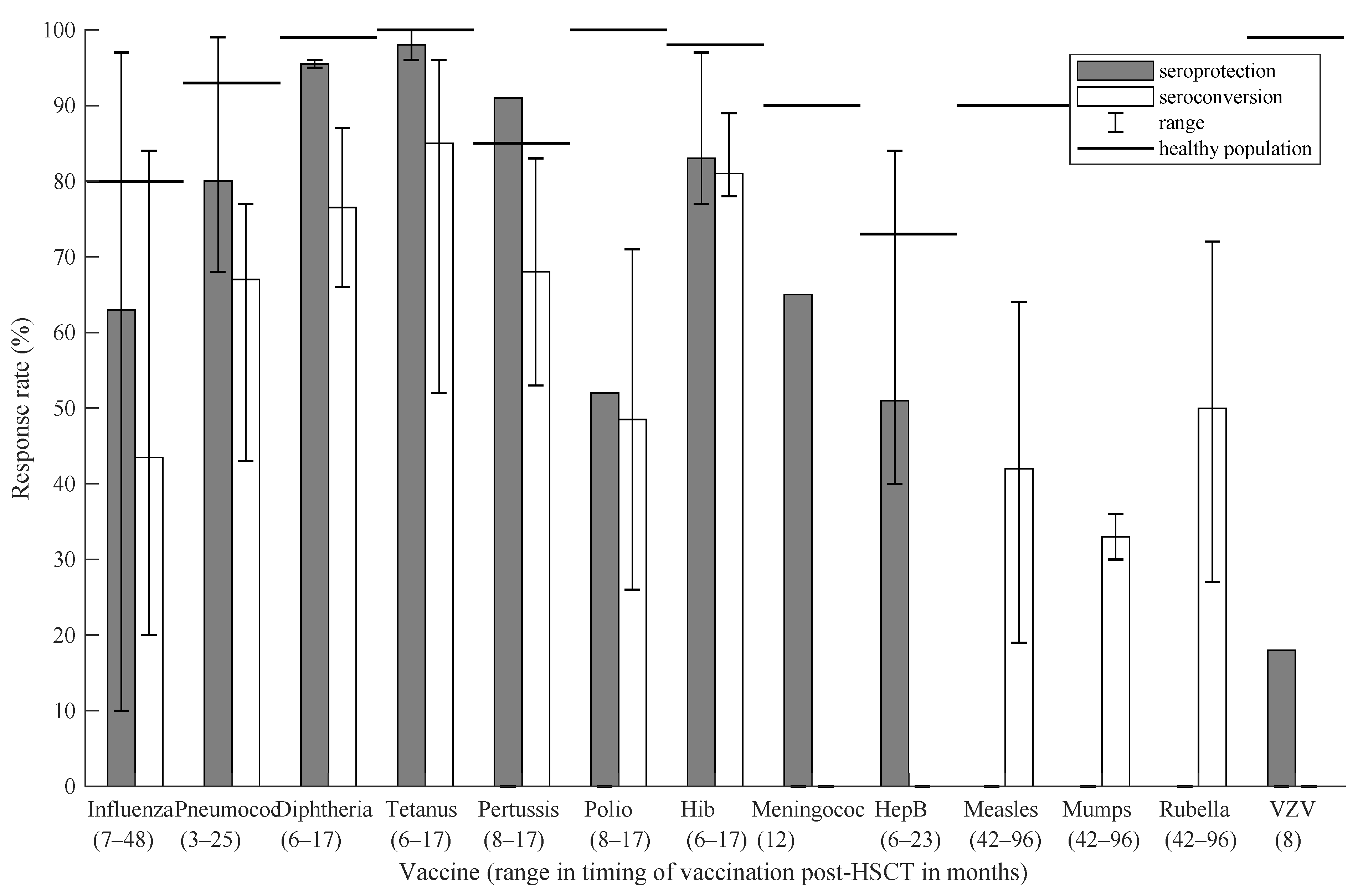
3.3. Autologous Hematopoietic Stem Cell Transplantation
3.3.1. Influenza
3.3.2. Pneumococcal
3.3.3. Diphtheria, Tetanus, Pertussis (DTP)
3.3.4. Haemophilus influenzae Type b (Hib)
3.3.5. Meningococcal
3.3.6. Hepatitis B
3.3.7. Varicella Zoster Virus (VZV)
3.4. Overview of All Results
3.5. Post-Hoc Analysis: COVID-19
4. Discussion
4.1. Allogeneic Hematopoietic Stem Cell Transplantation
- Many post-HSCT patients respond inadequately to vaccination, and responses are remarkably low compared with the healthy population. Thus, evaluating responses post-vaccination might be indicated. Suggested antibody titers for this evaluation in clinical practice are provided in Supplementary Table S5, including suggested cutoff values.
- Most studies showed improved vaccine responses when delaying revaccination for at least 6 months post-transplantation and thereby allowing better immunological recovery [8,9]. However, postponing vaccination increases risk of infection: the estimated incidence of invasive pneumococcal disease (IPD) is 347 infections per 100,000 alloHSCT recipients compared with only 7 per 100,000 persons in the general population [28]. Timing of revaccination is a balance between immune recovery versus infection risk. Insights in individual immune recovery would help to assess the risk of failure. The factors that influence the immune system and the extent of influence are unknown.
- Influenza vaccine studies were extremely heterogeneic. Responses seemed to improve with postponement of vaccination. Most studies initiated their vaccination at least 12 months post-transplantation, and therefore, no proper recommendation can be made on earlier vaccination. However, in case of a pandemic such as we experienced with COVID-19, a less adequate response to vaccination, and thus poorer seroprotection, would be preferable over no seroprotection [58]. Therefore, one might then consider vaccinating all patients that are at least 3 months post-HSCT and clinically stable. Earlier post-HSCT and vaccinating family members with close contact to the patient might be considered [4].
- Pneumococcal vaccines might already be effective at 3–6 months post-transplant [27,29]. Earliest administration was three months post-transplant with response > 68%. However, responses did increase when vaccination was postponed: vaccination at 7–12 months post-transplant resulted in 69–99% responses [28,41,42,44].
- Diphtheria, tetanus, pertussis, poliomyelitis and Hib were earliest administered at 6 months post-transplant. Responses for tetanus varied widely (52–100% [41,42,43,44]); for Hib responses were slightly more comparable between studies: 77–97% [40,44]. Vaccination on DTP, poliomyelitis and Hib might be started at 6 months post-transplantation.
- Hepatitis B vaccine was administered 6–23 months post-HSCT with varying responses: 40–84% [33,34,35,43]. In the general population, responses to hepatitis B vaccine are also quite low compared with other vaccines [47,59]. Therefore, it is advised not to initiate vaccination before 12 months post-HSCT.
- Vaccine responses to MMR remain suboptimal multiple years post-HSCT, and vaccination must be considered carefully per individual. As the vaccine is live-attenuated, the clinical condition of the individual patient is very important. Vaccination must be postponed in case of active GVHD and/or usage of immunosuppressants.
- VZV vaccination has not yet been studied widely, and thus, no proper recommendation on timing can be made. However, with the recombinant subunit vaccine (Shingrix®), potential severe side effects of the live-attenuated VZV vaccine are no longer an issue, and vaccination can be considered earlier post-HSCT. Finally, COVID-19 vaccine responses are poor compared with the healthy population. However, vaccination should be considered shortly post-HSCT, as any chance for response must be taken in times of a pandemic such as COVID-19.
4.2. Autologous Hematopoietic Stem Cell Transplantation
4.3. Overall Discussion
- Physicians may need to take into account the current state of immunosuppression and associated expectations for immune recovery to determine the precise timing and the estimated success of vaccination post-HSCT.
- Future studies with standardized definitions, especially on vaccine response (seroconversion/seroprotection), are necessary to increase generalizability and relevancy of results.
- More insight in predictors of vaccine response, such as immune reconstitution, GVHD-status and immunosuppressive therapy [61], might help to design an optimized and probably more individualized vaccination schedule. For example, rituximab is known to interfere with immune recovery for 6–12 months after cessation [63] and was reported to negatively influence vaccine response [13]. Therefore, postponing vaccination is necessary in these specific patients.
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gratwohl, A.; Baldomero, H.; Passweg, J.; Frassoni, F.; Niederwieser, D.; Schmitz, N.; Urbano-Ispizua, A. Hematopoietic Stem Cell Transplantation for Hematological Malignancies in Europe. Leukemia 2003, 17, 941–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.S.; Rezvani, K. Immune Reconstitution Post Allogeneic Transplant and the Impact of Immune Recovery on the Risk of Infection. Virulence 2016, 7, 901–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention Altered Immunocompetence. General Best Practice Guidelines for Immunization: Best Practice Guidance of the Advisory Committee on Immunization Practices (ACIP). Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html (accessed on 10 June 2019).
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of Haemopoietic Stem Cell Transplant Recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Ljungman, P.; Cordonnier, C.; Einsele, H.; Englund, J.; Machado, C.M.; Storek, J.; Small, T. Vaccination of Hematopoietic Cell Transplant Recipients. Bone Marrow Transplant. 2009, 44, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook, Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Li, H.W.; Sykes, M. Emerging Concepts in Haematopoietic Cell Transplantation. Nat. Rev. Immunol. 2012, 12, 403–416. [Google Scholar] [CrossRef]
- Conrad, A.; Alcazer, V.; Valour, F.; Ader, F. Vaccination Post-Allogeneic Hematopoietic Stem Cell Transplantation: What Is Feasible? Expert Rev. Vaccines 2018, 17, 299–309. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Avetisyan, G.; Aschan, J.; Hassan, M.; Ljungman, P. Evaluation of Immune Responses to Seasonal Influenza Vaccination in Healthy Volunteers and in Patients after Stem Cell Transplantation. Transplantation 2008, 86, 257–263. [Google Scholar] [CrossRef]
- Issa, N.C.; Marty, F.M.; Gagne, L.S.; Koo, S.; Verrill, K.A.; Alyea, E.P.; Cutler, C.S.; Koreth, J.; Armand, P.; Ho, V.T.; et al. Seroprotective Titers against 2009 H1N1 Influenza A Virus after Vaccination in Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Yalçin, S.S.; Kondolot, M.; Albayrak, N.; Altaş, A.B.; Karacan, Y.; Kuşkonmaz, B.; Aksu, S.; Cetin, M.; Göker, H.; Yurdakök, K.; et al. Serological Response to Influenza Vaccine after Hematopoetic Stem Cell Transplantation. Ann. Hematol. 2010, 89, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Spina, F.; Carniti, C.; Anselmi, G.; Lucini, D.; Vendramin, A.; Pregliasco, F.; Corradini, P. Long-Term Patterns of Humoral and Cellular Response after Vaccination against Influenza A (H1N1) in Patients with Hematologic Malignancies. Eur. J. Haematol. 2012, 89, 111–119. [Google Scholar] [CrossRef]
- Dhédin, N.; Krivine, A.; le Corre, N.; Mallet, A.; Lioure, B.; Bay, J.-O.; Rubio, M.-T.; Agape, P.; Thiébaut, A.; le Goff, J.; et al. Comparable Humoral Response after Two Doses of Adjuvanted Influenza A/H1N1pdm2009 Vaccine or Natural Infection in Allogeneic Stem Cell Transplant Recipients. Vaccine 2014, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Roll, D.; Ammer, J.; Holler, B.; Salzberger, B.; Schweiger, B.; Jilg, W.; Andreesen, R.; Edinger, M.; Wolff, D.; Holler, E. Vaccination against Pandemic H1N1 (2009) in Patients after Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective Analysis. Infection 2012, 40, 153–161. [Google Scholar] [CrossRef]
- Mohty, B.; Bel, M.; Vukicevic, M.; Nagy, M.; Levrat, E.; Meier, S.; Grillet, S.; Combescure, C.; Kaiser, L.; Chalandon, Y.; et al. Graft-versus-Host Disease Is the Major Determinant of Humoral Responses to the AS03-Adjuvanted Influenza A/09/H1N1 Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Haematologica 2011, 96, 896–904. [Google Scholar] [CrossRef]
- De Lavallade, H.; Garland, P.; Sekine, T.; Hoschler, K.; Marin, D.; Stringaris, K.; Loucaides, E.; Howe, K.; Szydlo, R.; Kanfer, E.; et al. Repeated Vaccination Is Required to Optimize Seroprotection against H1N1 in the Immunocompromised Host. Haematologica 2011, 96, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Karras, N.A.; Weeres, M.; Sessions, W.; Xu, X.; Defor, T.; Young, J.-A.H.; Stefanski, H.; Brunstein, C.; Cooley, S.; Miller, J.S.; et al. A Randomized Trial of One versus Two Doses of Influenza Vaccine after Allogeneic Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2013, 19, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Natori, Y.; Humar, A.; Lipton, J.; Kim, D.D.; Ashton, P.; Hoschler, K.; Kumar, D. A Pilot Randomized Trial of Adjuvanted Influenza Vaccine in Adult Allogeneic Hematopoietic Stem Cell Transplant Recipients. Bone Marrow Transplant. 2017, 52, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Halasa, N.B.; Savani, B.N.; Asokan, I.; Kassim, A.; Simons, R.; Summers, C.; Bourgeois, J.; Clifton, C.; Vaughan, L.A.; Lucid, C.; et al. Randomized Double-Blind Study of the Safety and Immunogenicity of Standard-Dose Trivalent Inactivated Influenza Vaccine versus High-Dose Trivalent Inactivated Influenza Vaccine in Adult Hematopoietic Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Gueller, S.; Allwinn, R.; Mousset, S.; Martin, H.; Wieters, I.; Herrmann, E.; Serve, H.; Bickel, M.; Bug, G. Enhanced Immune Response after a Second Dose of an AS03-Adjuvanted H1N1 Influenza A Vaccine in Patients after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1546–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhard, D.; Zakay-Rones, Z.; Shapira, M.Y.; Resnick, I.; Averbuch, D.; Grisariu, S.; Dray, L.; Djian, E.; Strauss-Liviatan, N.; Grotto, I.; et al. The Humoral Immune Response of Hematopoietic Stem Cell Transplantation Recipients to AS03-Adjuvanted A/California/7/2009 (H1N1)v-like Virus Vaccine during the 2009 Pandemic. Vaccine 2011, 29, 1777–1782. [Google Scholar] [CrossRef]
- Villa, D.; Gubbay, J.; Sutherland, D.R.; Laister, R.; McGeer, A.; Cooper, C.; Fortuno, E.S., 3rd; Xu, W.; Shi, L.; Kukreti, V.; et al. Evaluation of 2009 Pandemic H1N1 Influenza Vaccination in Adults with Lymphoid Malignancies Receiving Chemotherapy or Following Autologous Stem Cell Transplant. Leuk. Lymphoma 2013, 54, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Okinaka, K.; Akeda, Y.; Kurosawa, S.; Fuji, S.; Tajima, K.; Oishi, K.; Fukuda, T. Pneumococcal Polysaccharide Vaccination in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Prospective Single-Center Study. Microbes Infect. 2017, 19, 553–559. [Google Scholar] [CrossRef]
- Cordonnier, C.; Ljungman, P.; Juergens, C.; Maertens, J.; Selleslag, D.; Sundaraiyer, V.; Giardina, P.C.; Clarke, K.; Gruber, W.C.; Scott, D.A.; et al. Immunogenicity, Safety, and Tolerability of 13-Valent Pneumococcal Conjugate Vaccine Followed by 23-Valent Pneumococcal Polysaccharide Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant Aged ≥2 Years: An Open-Label Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Langedijk, A.C.; van Aalst, M.; Meek, B.; van Leeuwen, E.M.M.; Zeerleder, S.; Meijer, E.; Hazenberg, M.D.; Grobusch, M.P.; Goorhuis, A. Long-Term Pneumococcal Vaccine Immunogenicity Following Allogeneic Hematopoietic Stem Cell Transplantation. Vaccine 2019, 37, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Labopin, M.; Chesnel, V.; Ribaud, P.; de La Camara, R.; Martino, R.; Ullmann, A.J.; Parkkali, T.; Locasciulli, A.; Yakouben, K.; et al. Randomized Study of Early versus Late Immunization with Pneumococcal Conjugate Vaccine after Allogeneic Stem Cell Transplantation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 1392–1401. [Google Scholar] [CrossRef]
- Small, T.N.; Zelenetz, A.D.; Noy, A.; Rice, R.D.; Trippett, T.M.; Abrey, L.; Portlock, C.S.; McCullagh, E.J.; Vanak, J.M.; Mulligan, A.M.; et al. Pertussis Immunity and Response to Tetanus-Reduced Diphtheria-Reduced Pertussis Vaccine (Tdap) after Autologous Peripheral Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2009, 15, 1538–1542. [Google Scholar] [CrossRef] [Green Version]
- Van der Velden, A.M.T.; Claessen, A.M.E.; van Velzen-Blad, H.; Biesma, D.H.; Rijker, G.T. Development of Functional Haemophilus influenzae Type b Antibodies after Vaccination. Haematologica 2005, 90, 1582–1584. [Google Scholar]
- Cheng, M.P.; Pandit, A.; Antin, J.H.; Walsh, S.R.; Huynh, D.; Ghobrial, I.M.; Baden, L.R.; Marty, F.M.; Issa, N.C. Safety and Immunogenicity of Conjugate Quadrivalent Meningococcal Vaccination after Hematopoietic Cell Transplantation. Blood Adv. 2018, 2, 1272–1276. [Google Scholar] [CrossRef] [Green Version]
- Onozawa, M.; Hashino, S.; Darmanin, S.; Okada, K.; Morita, R.; Takahata, M.; Shigematsu, A.; Kahata, K.; Kondo, T.; Tanaka, J.; et al. HB Vaccination in the Prevention of Viral Reactivation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients with Previous HBV Infection. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2008, 14, 1226–1230. [Google Scholar] [CrossRef]
- Takahata, M.; Hashino, S.; Onozawa, M.; Shigematsu, A.; Sugita, J.; Fujimoto, K.; Endo, T.; Kondo, T.; Tanaka, J.; Imamura, M.; et al. Hepatitis B Virus (HBV) Reverse Seroconversion (RS) Can Be Prevented Even in Non-Responders to Hepatitis B Vaccine after Allogeneic Stem Cell Transplantation: Long-Term Analysis of Intervention in RS with Vaccine for Patients with Previous HBV Infection. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2014, 16, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.; Papadopoulos, E.B.; Young, J.W.; O’Reilly, R.J.; Prockop, S.; Kernan, N.A.; Jakubowski, A.; Boulad, F.; Perales, M.-A.; Castro-Malaspina, H.; et al. Immunogenicity of Recombinant Hepatitis B Vaccine (RHBV) in Recipients of Unrelated or Related Allogeneic Hematopoietic Cell (HC) Transplants. Blood 2006, 108, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kamimura, T.; Yoshida, S.; Mori, Y.; Kadowaki, M.; Kohno, K.; Ishihara, D.; Urata, S.; Sugio, T.; Kamezaki, K.; et al. Safety and Seropositivity after Live Attenuated Vaccine in Adult Patients Receiving Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, 1576–1585. [Google Scholar] [CrossRef]
- Kawamura, K.; Wada, H.; Nakasone, H.; Akahoshi, Y.; Kawamura, S.; Takeshita, J.; Yoshino, N.; Misaki, Y.; Yoshimura, K.; Gomyo, A.; et al. Immunity and Vaccination Against Measles, Mumps, and Rubella in Adult Allogeneic Hematopoietic Stem Cell Transplant Recipients. Transplant. Cell. Ther. 2021, 27, 436.e1–436.e8. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.F.; Lin, R.Y.; Natori, Y.; Anderson, A.D.; Alencar, M.C.; Wang, T.P.; Morris, M.I.; Komanduri, K.V. Reduced Immunogenicity of the Adjuvanted Recombinant Zoster Vaccine after Hematopoietic Cell Transplant: A Pilot Study. Blood Adv. 2020, 4, 4618–4622. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Sullivan, K.M.; el Idrissi, M.; Salaun, B.; Alonso Alonso, A.; Andreadis, C.; Anttila, V.-J.; Bloor, A.J.; Broady, R.; Cellini, C.; et al. Adjuvanted Recombinant Zoster Vaccine in Adult Autologous Stem Cell Transplant Recipients: Polyfunctional Immune Responses and Lessons for Clinical Practice. Hum. Vaccines Immunother. 2021, 1–11. [Google Scholar] [CrossRef]
- Pao, M.; Papadopoulos, E.B.; Chou, J.; Glenn, H.; Castro-Malaspina, H.; Jakubowski, A.A.; Kernan, N.A.; Perales, M.A.; Prokop, S.; Scaradavou, A.; et al. Response to Pneumococcal (PNCRM7) and Haemophilus influenzae Conjugate Vaccines (HIB) in Pediatric and Adult Recipients of an Allogeneic Hematopoietic Cell Transplantation (AlloHCT). Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2008, 14, 1022–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerveld-Eggink, A.; van der Velden, A.M.T.; Ossenkoppele, G.J.; van de Loosdrecht, A.A.; Biesma, D.H.; Rijkers, G.T. Antibody Response to Polysaccharide Conjugate Vaccines after Nonmyeloablative Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. Am. Soc. Blood Marrow Transplant. 2009, 15, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, G.L.; Shune, L.; Purtill, D.; Devlin, S.; Lauer, E.; Lubin, M.; Bhatt, V.; McElrath, C.; Kernan, N.A.; Scaradavou, A.; et al. Robust Vaccine Responses in Adult and Pediatric Cord Blood Transplantation Recipients Treated for Hematologic Malignancies. Biol. Blood Marrow Transplant. Am. Soc. Blood Marrow Transplant. 2015, 21, 2160–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, A.; Perry, M.; Langlois, M.-E.; Labussière-Wallet, H.; Barraco, F.; Ducastelle-Leprêtre, S.; Larcher, M.-V.; Balsat, M.; Boccard, M.; Chidiac, C.; et al. Efficacy and Safety of Revaccination against Tetanus, Diphtheria, Haemophilus influenzae Type b and Hepatitis B Virus in a Prospective Cohort of Adult Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2020, 26, 1729–1737. [Google Scholar] [CrossRef]
- Winkler, J.; Tittlbach, H.; Schneider, A.; Buchstaller, C.; Mayr, A.; Vasova, I.; Roesler, W.; Mach, M.; Mackensen, A.; Winkler, T.H. Measuring the Cellular Memory B Cell Response after Vaccination in Patients after Allogeneic Stem Cell Transplantation. Ann. Hematol. 2020, 99, 1895–1906. [Google Scholar] [CrossRef]
- Van der Velden, A.M.T.; Claessen, A.M.E.; van Velzen-Blad, H.; de Groot, M.R.; Kramer, M.H.H.; Biesma, D.H.; Rijkers, G.T. Vaccination Responses and Lymphocyte Subsets after Autologous Stem Cell Transplantation. Vaccine 2007, 25, 8512–8517. [Google Scholar] [CrossRef]
- Palazzo, M.; Shah, G.L.; Copelan, O.; Seier, K.; Devlin, S.M.; Maloy, M.; Kenny, S.; Hassoun, H.; Korde, N.S.; Lendvai, N.; et al. Revaccination after Autologous Hematopoietic Stem Cell Transplantation Is Safe and Effective in Patients with Multiple Myeloma Receiving Lenalidomide Maintenance. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The British Columbia Centre for Disease Control (BCCDC) Vaccine Immunogenicity, Efficacy and Effectiveness. Available online: http://www.bccdc.ca/resource-gallery/Documents/GuidelinesandForms/GuidelinesandManuals/Epid/CDManual/Chapter2-Imms/VaccineImmunogenicity.pdf (accessed on 27 November 2021).
- Chiarucci, M.; Paolasini, S.; Isidori, A.; Guiducci, B.; Loscocco, F.; Capalbo, M.; Visani, G. Immunological Response Against SARS-COV-2 After BNT162b2 Vaccine Administration Is Impaired in Allogeneic but Not in Autologous Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 737300. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Canti, L.; Humblet-Baron, S.; Desombere, I.; Neumann, J.; Pannus, P.; Heyndrickx, L.; Henry, A.; Servais, S.; Willems, E.; Ehx, G.; et al. Predictors of Neutralizing Antibody Response to BNT162b2 Vaccination in Allogeneic Hematopoietic Stem Cell Transplant Recipients. J. Hematol. Oncol. 2021, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-Based CART Therapy-A Single-Center Prospective Cohort Study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Tamari, R.; Politikos, I.; Knorr, D.A.; Vardhana, S.A.; Young, J.C.; Marcello, L.T.; Doddi, S.; Devlin, S.M.; Ramanathan, L.V.; Pessin, M.S.; et al. Predictors of Humoral Response to SARS-CoV-2 Vaccination after Hematopoietic Cell Transplantation and CAR T-Cell Therapy. Blood Cancer Discov. 2021, 2, 577–585. [Google Scholar] [CrossRef]
- Shem-Tov, N.; Yerushalmi, R.; Danylesko, I.; Litachevsky, V.; Levy, I.; Olmer, L.; Lusitg, Y.; Avigdor, A.; Nagler, A.; Shimoni, A.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Haematopoietic Stem Cell Transplantation Recipients. Br. J. Haematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Piñana, J.L.; López-Corral, L.; Martino, R.; Montoro, J.; Vazquez, L.; Pérez, A.; Martin-Martin, G.; Facal-Malvar, A.; Ferrer, E.; Pascual, M.-J.; et al. SARS-CoV-2-Reactive Antibody Detection after SARS-CoV-2 Vaccination in Hematopoietic Stem Cell Transplant Recipients: Prospective Survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am. J. Hematol. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matkowska-Kocjan, A.; Owoc-Lempach, J.; Chruszcz, J.; Kuźnik, E.; Szenborn, F.; Jurczenko, L.; Wójcik, M.; Banyś, D.; Szenborn, L.; Ussowicz, M. The COVID-19 MRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation. Vaccines 2021, 9, 1209. [Google Scholar] [CrossRef] [PubMed]
- Attolico, I.; Tarantini, F.; Carluccio, P.; Schifone, C.P.; Delia, M.; Gagliardi, V.P.; Perrone, T.; Gaudio, F.; Longo, C.; Giordano, A.; et al. Serological Response Following BNT162b2 Anti-SARS-CoV-2 MRNA Vaccination in Haematopoietic Stem Cell Transplantation Patients. Br. J. Haematol. 2021. ahead of print. [Google Scholar] [CrossRef]
- Easdale, S.; Shea, R.; Ellis, L.; Bazin, J.; Davis, K.; Dallas, F.; Thistlethwayte, E.; Ethell, M.; Potter, M.; Arias, C.; et al. Serologic Responses Following a Single Dose of SARS-Cov-2 Vaccination in Allogeneic Stem Cell Transplantation Recipients. Transplant. Cell. Ther. 2021, 27, 880.e1–880.e4. [Google Scholar] [CrossRef] [PubMed]
- Rijksinstituut voor Volkgezondheid en Milieu: Landelijke Coördinatie Infectieziektebestrijding. COVID-19-Vaccinatie van Immuungecompromitteerde Patiënten. Available online: https://lci.rivm.nl/handleiding-covid-19-vaccinatie-van-immuungecompromitteerde-patienten#:~:text=Voor%20de%20prioritering%20van%20COVID,met%20toename%20van%20de%20leeftijd (accessed on 1 October 2021).
- Mahanty, S.; Prigent, A.; Garraud, O. Immunogenicity of Infectious Pathogens and Vaccine Antigens. BMC Immunol. 2015, 16, 31. [Google Scholar] [CrossRef] [Green Version]
- Wiegering, V.; Eyrich, M.; Winkler, B.; Schlegel, P.G. Comparison of Immune Reconstitution After Allogeneic Versus Autologous Stem Cell Transplantation in 182 Pediatric Recipients. J. Pediatr. Hematol. Oncol. 2019, 41, e302–e307. [Google Scholar] [CrossRef]
- Janssen, M.J.M.; Bruns, A.H.W.; Verduyn Lunel, F.M.; Raijmakers, R.A.P.; de Weijer, R.J.; Nanlohy, N.M.; Smits, G.P.; van Baarle, D.; Kuball, J. Predictive Factors for Vaccine Failure to Guide Vaccination in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Bone Marrow Transplant. 2021, 56, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Kneppers, E.; van der Holt, B.; Kersten, M.-J.; Zweegman, S.; Meijer, E.; Huls, G.; Cornelissen, J.J.; Janssen, J.J.; Huisman, C.; Cornelisse, P.B.; et al. Lenalidomide Maintenance after Nonmyeloablative Allogeneic Stem Cell Transplantation in Multiple Myeloma Is Not Feasible: Results of the HOVON 76 Trial. Blood 2011, 118, 2413–2419. [Google Scholar] [CrossRef]
- Visser, L.G. The Immunosuppressed Traveler. Infect. Dis. Clin. N. Am. 2012, 26, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ferreira, V.H.; Blumberg, E.; Silveira, F.; Cordero, E.; Perez-Romero, P.; Aydillo, T.; Danziger-Isakov, L.; Limaye, A.P.; Carratala, J.; et al. A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 1322–1329. [Google Scholar] [CrossRef]
| Study | N | Vaccine Schedule | Response Rate (%) * | |||
|---|---|---|---|---|---|---|
| Start of Vaccination, in Months (Range) | Schedule in Days | Seroprotection | Seroconversion | |||
| Influenza | Avetisyan et al. [12] | 14 | +9.2 (3–24) | 0 | 29%, 0%, 0% ** | |
| Issa et al. [13] | 82 | +19 (2.5–94) | 0 | 51% | ||
| Yalçin et al. [14] | 58 | +24.2 (6–48) | 0 | 91% ***, 100%, 100% ** | 59.1%, 54.5%, 47.7% ** | |
| Mariotti et al. [15] | 15 | +48 (7–116) | 0 | 71.4% | 57.1% | |
| Dhédin et al. [16] | 59 | +11 (5.1–24.8) | 0, 21 | After 1st 53% After 2nd 66% | After 1st 51% After 2nd 66% | |
| Roll et al. [17] | 38 | +15 (5.1–96.4) | 0, 35 (range 14–70) | After 1st 42% After 2nd 47% | After 1st 42% | |
| Mohty et al. [18] | 57 | +30 (2–192) | 0, 21 | After 1st 64.3% After 2nd 84.2% | After 1st 53.8% After 2nd 84.2% | |
| De Lavallade et al. [19] | 26 | +36 (6–127) | 0, 21 | After 1st 46% After 2nd 73% | After 1st 46% After 2nd 73% | |
| Karras et al. [20] **** | 65 | Arm I: +8.4 (2.4–236.4) Arm II: +12 (2.4–84) | Arm I: 0 Arm II: 0, 30 | Arm I: 32%, 19%, 32% ** Arm II: 32%, 19%, 23% ** | Arm I: 31% 13% 16% ** Arm II: 31% 22% 25% ** | |
| Natori et al. [21] | 73 | +12.6 (2.8–270) | Arm I: 0 (adjuvant vaccine) Arm II: 0 (non-adjuvant vaccine) | Arm I: 57.1%, 71.4%, 57.1% ** Arm II: 59.4%, 71.9%, 68.8% ** | Arm I: 31.4%, 57.1%, 37.1% ** Arm II: 21.9%, 40.6%, 25.0% ** | |
| Halasa et al. [22] | 44 | Arm I: +8.5 Arm II: +7.1 | Arm I: 0 (60 µg) Arm II: 0 (15 µg) | Arm I: 69%, 81%, 42% ** Arm II: 57%, 36%, 43% ** | Arm I: 50%, 42%, 42% ** Arm II: 36%, 36%, 43% ** | |
| Pneumococcal | Pao et al. [40] | 76 | +15.6 | 0 (PCV7) | 44.7% | |
| Okinaka et al. [26] | 30 | +25.2 (13.0–63.4) | 0 (PPV23) | 43% | ||
| Cordonnier et al. [27] | 162 | +4.9 (3.2–6.8) | 0, 1, 2, 8 (PCV13), 9 (PPV23) | After 3× PCV13 89.7–98%, after 4× PCV13 82.6–98.8% ***** | ||
| Winkler et al. [44] | 27 | +7.5 (6.0–14.3) | 0, 1, 2 (PCV13) | Serotype 1 96%, 14 100%, 23 100% | Serotype 1 74%, 14 65%, 23 91% | |
| Meerveld-Eggink et al. [41] | 26 | +15 (12–36) | 0, 0.5, 2 (PCV7) | Serotype 4 73%, 6B 28% 9V 78%, 14 86%, 18C 82%, 19F 73%, 23F 82% | Serotype 4 85%, 6B 46%, 9V 73%, 14 69%, 18C 69%, 19F 69%, 23F 62% | |
| Shah et al. [42] | 63 | +17 (7–45) | 0, 1, 2 (PCV7/PCV13) | Serotype 14 65%, 19F 77%, 23F 58% | ||
| Langedijk et al. [28] | 103 | +20.3 (2.2–164.4) | 0, 1, 2 (PCV13), 6 (PPV23) | PCV13 serotypes 85%, PPV23 serotypes 23–92% | ||
| Cordonnier et al. [29] ****** | 158 | Arm I: +3 Arm II: +9 | Arm I: 0, 1, 2, PCV13, 9 (PPV23) Arm II: 0, 1, 2 (PCV13), 9 (PPV23) | Arm I before PPV23 59%, after PPV23 68% Arm II before PPV23 69%, after PPV23 88% | ||
| DTP and poliomyelitis | Conrad et al. [43] | 91 | +6 | 0, 1, 2 | Diphtheria 95%, tetanus 98% | |
| Winkler et al. [44] | 27 | +7.5 (6.0–14.3) | 0, 1, 2 | Diphtheria 96%, tetanus 100%, pertussis 91%, poliomyelitis 52% | Diphtheria 87%, tetanus 96%, pertussis 83%, poliomyelitis 26% | |
| Meerveld-Eggink et al. [41] | 26 | +15 (12–36) DTP | 0, 2 | Tetanus 96% | Tetanus 85% | |
| Shah et al. [42] | 63 | +17 (7–45) DTP +17 (7–45) poliomyelitis | 0, 1, 2 DTP; 0, 1 poliomyelitis | Diphtheria 66%, tetanus 52%, pertussis 53%, poliomyelitis 71% | ||
| Hib | Conrad et al. [43] | 91 | +6 | 0, 1, 2 | 97% | |
| Winkler et al. [44] | 27 | +7.5 (6.0–14.3) | 0, 1, 2 | 83% | 83% | |
| Meerveld-Eggink et al. [41] | 26 | +15 (12–36) | 0 | 77% | 78% | |
| Pao et al. [40] | 65 | +15.6 | 0 | 79% | ||
| Shah et al. [42] | 63 | +17 (7–45) | 0, 1, 2 | 89% | ||
| Men ACYW | Cheng et al. [32] | 54 | +12.3 (8.4–13.5) | 0 | Serotype A 83.3%, C 61.1%, Y 63.0%, W-135 53.7% | |
| Hepatitis B | Conrad et al. [43] | 15 | +6 | 0, 1, 2 | 84% | |
| Onozawa et al. [33] | 13 | +12 | 0, 1, 6 | 40% | ||
| Takahata et al. [34] | 21 | +15 (6–79) | 0, 1, 6 | 42.9% | ||
| Jaffe et al. [35] | 168 | +23 (5–102) | 0, 1, 6 | 59% | ||
| MMR | Kawamura et al. [37] | 25 | +41.7 (24.4–99.1) | 0 | Measles 64%, mumps 36%, rubella 72% | |
| Aoki et al. [36] | 29 | +69.3 (25.8–212.6) | 0, 1 | Measles 19%, mumps 30%, rubella 27% ******* | ||
| VZV | Camargo et al. [38] | 17 | +8 (7–12) | 0, 2 | 18% | |
| Study | N | Vaccination Schedule and Timing | Response Rate (%) * | |||
|---|---|---|---|---|---|---|
| Start of Vaccination, in Months (Range) | Schedule, in Weeks | Seroprotection | Seroconversion | |||
| Influenza | Gueller et al. [23] | 17 ** | +19.7 (4.7–49.3) | 0, 3 | After 1st 52.9% After 2nd 90.9% | After 1st 41.2% After 2nd 81.8% |
| Yalçin et al. [14] | 3 | +24.2 (6–48) | 91% ***, 100%, 100% **** | 100%, 100%, 50% **** | ||
| Engelhard et al. [24] | 78 * | +27 (1–290) ***** | 0, 3–4 | After 1st 44.2% After 2nd 48.8% | After 1st 32.5% After 2nd 41.9% | |
| Villa et al. [25] | 40 | Arm I: +12 months Arm II: +12 months | Arm I: 0 Arm II: 0, 3 | Arm I: 40% Arm II: after 1st 15%, after 2nd 40% | Arm I: 30% Arm II: after 1st 5%, after 2nd 30% | |
| Pneumococcal | Van der Velden et al. [45] | 20 | +6 | 0, 2 (PCV7), 8 (PPV23) | PCV7 serotypes 78%, PPV23 serotypes 61% | |
| Palazzo et al. [46] | 122 | +12.6 (8.1–26.4) | 0, 1, 2 (PCV13) | 58% | ||
| DTP | Van der Velden et al. [45] | 20 | +6 | 0, 2, 8 | Tetanus 100% | |
| Palazzo et al. [44] | 122 | +12.6 (8.1–26.4) | 0, 1, 2 | Tetanus 92%, diphtheria 100%, pertussis 89% | Tetanus 60%, diphtheria 70%, pertussis 76% | |
| Small et al. [30] | 28 | +36 (15.6–99.9) | 0 | Tetanus: 85% * | Tetanus 32.1%, diphtheria 32.1, pertussis 7.1% | |
| Hib | Van der Velden et al. [31] | 16 | +6 | 0, 2, 8 | 100% | |
| Van der Velden et al. [45] | 20 | +6 | 0, 2, 8 | After 1st 33% After 2nd 72% After 3rd 94% | ||
| Palazzo et al. [46] | 122 | +12.6 (8.1–26.4) | 0, 1, 2 | 95% | 71% | |
| Men ACYW | Cheng et al. [32] | 13 * | +12.3 (8.4–13.5) | Serotype A 69.2%, C 84.6%, Y 76.9%, W-135 69.2% | ||
| HepB | Palazzo et al. [46] | 122 | +12.6 (8.1–26.4) | 0, 1, 6 | 43% | 40% |
| VZV | Stadtmauer et al. [39] | 922 | +2 | 0, 1–2 | 57.7–71.4% | |
| Camargo et al. [38] | 13 | +8 (7–12) | 0, 2 | 62% | ||
| Study | N | Allo/Auto | Start of Vaccination, in Months (Range) | Vaccine | Schedule, in Weeks | Seroconversion |
|---|---|---|---|---|---|---|
| Chiarucci et al. [48] | 50 | 12 allo + 38 auto | 12.3 (0.2–24.5) | Pfizer-BioNTech | 0, 3 | Allo: 50% Auto: 84% |
| Lindemann et al. [49] | 117 | Allo | 30 (5–391) | Pfizer-BioNTech | 0, 3 | 68% |
| Canti et al. [50] | 37 | Allo | 31 (5–51) | Pfizer-BioNTech | 0, 3 | 86% |
| Ram et al. [51] | 66 | Allo | 32 (3–263) | Pfizer-BioNTech | 0, 3 | 75% |
| Tamari et al. [52] | 217 | 149 allo + 61 auto | 34 (16–59) | Pfizer-BioNTech/Moderna | 0, 3 | 87% |
| Shem-Tov et al. [53] | 152 | Allo | +41 (24–76) | Pfizer-BioNTech | 0, 3 | 77.6% |
| Luis Pinana et al. [54] | 397 | 311 allo + 86 auto | Allo: 98 (4–646) Auto: 88 (3–763) | Pfizer-BioNTech/ AstraZeneca/Moderna | 0, 3 | Allo: 78% Auto: 85% |
| Matkowska-Kocjan et al. [55] | 65 | Allo | 126 (36–324) | Pfizer-BioNTech | 0, 5 | 96.5% |
| Attolico et al. [56] | 114 | 52 allo + 52 auto | Non available * | Pfizer-BioNTech | 0,3 | Allo: 75.8% Auto: 94.2% |
| Easdale et al. [57] | 55 | Allo | Non available ** | Pfizer-BioNTech/AstraZeneca | 0 | 38.2% |
| Vaccine | Number of Vaccines | Schedule | |
|---|---|---|---|
| First Vaccination, in Months Post-HSCT | Schedule, in Months | ||
| Influenza | 1 | 12 months ** | Yearly in influenza season |
| Pneumococcal (PCV13) | 3× PCV13, 1× PPV23 | PCV13: 3–6 months *** | PCV13: 0, 1, 2 months PPV23: 9 months |
| Diphtheria, tetanus, pertussis and poliomyelitis | 3 | 6 months | 0, 1, 2 months |
| Haemophilus influenzae type b | 3 | 6 months | 0, 1, 2 months |
| Hepatitis B | 3 | 12 months | 0, 1, 6 months |
| Meningococcal | 2 | 12 months | 0, 2–8 months |
| Measles, mumps and rubella | 1 | 24 months **** | 0 |
| Varicella zoster virus (Shingrix®) | 2 | 12 months | 0, 1 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, M.; Bruns, A.; Kuball, J.; Raijmakers, R.; van Baarle, D. Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review. Cancers 2021, 13, 6140. https://doi.org/10.3390/cancers13236140
Janssen M, Bruns A, Kuball J, Raijmakers R, van Baarle D. Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review. Cancers. 2021; 13(23):6140. https://doi.org/10.3390/cancers13236140
Chicago/Turabian StyleJanssen, Michelle, Anke Bruns, Jürgen Kuball, Reinier Raijmakers, and Debbie van Baarle. 2021. "Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review" Cancers 13, no. 23: 6140. https://doi.org/10.3390/cancers13236140






