Factors Associated with Thyroid-Related Adverse Events in Patients Receiving PD-1 or PD-L1 Inhibitors Using Machine Learning Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Patients and Data Collection
2.2. Statistical Analysis and Machine Learning Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Afzal, M.Z.; Shirai, K. Ipilimumab and nivolumab induced immune-related adverse events in metastatic mucosal melanoma. BMJ Case Rep. 2021, 14, e243713. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Z.; Gao, P.; Song, Y.X.; Sun, J.X.; Chen, X.W.; Zhao, J.H.; Wang, Z.N. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy 2020, 12, 587–603. [Google Scholar] [CrossRef]
- Maughan, B.L.; Bailey, E.; Gill, D.M.; Agarwal, N. Incidence of Immune-Related Adverse Events with Program Death Receptor-1- and Program Death Receptor-1 Ligand-Directed Therapies in Genitourinary Cancers. Front. Oncol. 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 13 August 2021).
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef]
- Bertelsen, J.B.; Hegedüs, L. Cigarette smoking and the thyroid. Thyroid 1994, 4, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Fukata, S.; Kuma, K.; Sugawara, M. Relationship between cigarette smoking and hypothyroidism in patients with Hashimoto’s thyroiditis. J. Endocrinol. Investig. 1996, 19, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jin, B.; Liu, T.; Chen, J.; Li, G.; Dang, J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100990. [Google Scholar] [CrossRef]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Garon, E.; Gandhi, L.; Rizvi, N.; Hui, R.; Balmanoukian, A.S.; Patnaik, A.; Eder, J.P.; Blumenshein, G.R.; Aggarwal, C.; Soria, J.C.; et al. Antitumor Activity of Pembrolizumab (Pembro; Mk-3475) and Correlation with Programmed Death Ligand 1 (Pd-L1) Expression in a Pooled Analysis of Patients (Pts) with Advanced Non–Small Cell Lung Carcinoma (Nsclc). Ann. Oncol. 2014, 25, v1. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Middleton, G.; Brock, K.; Savage, J.; Mant, R.; Summers, Y.; Connibear, J.; Shah, R.; Ottensmeier, C.; Shaw, P.; Lee, S.M.; et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): A single arm, phase 2 trial. Lancet Respir. Med. 2020, 8, 895–904. [Google Scholar] [CrossRef]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Botticelli, A.; Cirillo, A.; Pomati, G.; Cerbelli, B.; Scagnoli, S.; Roberto, M.; Gelibter, A.; Mammone, G.; Calandrella, M.L.; Cerbelli, E.; et al. The role of opioids in cancer response to immunotherapy. J. Transl. Med. 2021, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Ide, S.; Sakurada, S.; Sasaki, K.; Sora, I.; Tamura, G.; Ohkawara, Y.; Takayanagi, M.; Ohno, I. μ-opioid receptor-mediated alterations of allergen-induced immune responses of bronchial lymph node cells in a murine model of stress asthma. Allergol. Int. 2012, 61, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Santamaría, A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin. Transl. Oncol. 2020, 22, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yu, H.; Ma, J.; Wang, J.; Banerjee, S.; Charboneau, R.; Barke, R.A.; Roy, S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS ONE 2013, 8, e54040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Complication (n = 23) | No Complication (n = 164) | p-Value | |
|---|---|---|---|---|
| Sex | 1.000 | |||
| Male | 18 (78.3) | 129 (78.7) | ||
| Female | 5 (21.7) | 35 (21.3) | ||
| Age | 0.420 | |||
| <65 | 11 (47.8) | 64 (39) | ||
| ≥65 | 12 (52.2) | 100 (61) | ||
| BMI | 0.921 | |||
| <23 | 13 (61.9) | 93 (60.8) | ||
| ≥23 | 8 (38.1) | 60 (39.2) | ||
| Smoking history | 0.025 | |||
| Yes | 9 (39.1) | 29 (17.7) | ||
| No | 14 (60.9) | 135 (82.3) | ||
| Alcohol history | 0.115 | |||
| Yes | 4 (17.4) | 12 (7.3) | ||
| No | 19 (82.6) | 152 (92.7) | ||
| Comorbidities | ||||
| Hypertension | 0.013 | |||
| Yes | 14 (60.9) | 56 (34.1) | ||
| No | 9 (39.1) | 108 (65.9) | ||
| Hyperlipidemia | 0.052 | |||
| Yes | 5 (21.7) | 13 (7.9) | ||
| No | 18 (78.3) | 151 (92.1) | ||
| COPD | 0.477 | |||
| Yes | 1 (4.3) | 18 (11) | ||
| No | 22 (95.7) | 146 (89) | ||
| Diabetes mellitus | 0.689 | |||
| Yes | 5 (21.7) | 42 (25.6) | ||
| No | 18 (78.3) | 122 (74.4) | ||
| Gout | 1.000 | |||
| Yes | 0 (0) | 4 (2.4) | ||
| No | 23 (100) | 160 (97.6) | ||
| BPH | 0.136 | |||
| Yes | 0 (0) | 19 (11.6) | ||
| No | 23 (100) | 145 (88.4) | ||
| Parkinson’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.6) | ||
| No | 23 (100) | 163 (99.4) | ||
| Osteoporosis | 1.000 | |||
| Yes | 0 (0) | 2 (1.2) | ||
| No | 23 (100) | 162 (98.8) | ||
| MI | 0.075 | |||
| Yes | 2 (8.7) | 2 (1.2) | ||
| No | 21 (91.3) | 162 (98.8) | ||
| Heart disease | 0.044 | |||
| Yes | 4 (17.4) | 8 (4.9) | ||
| No | 19 (82.6) | 156 (95.1) | ||
| Asthma | 1.000 | |||
| Yes | 0 (0) | 3 (1.8) | ||
| No | 23 (100) | 161 (98.2) | ||
| Buger’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.6) | ||
| No | 23 (100) | 163 (99.4) | ||
| Angina | 0.123 | |||
| Yes | 1 (4.3) | 0 (0) | ||
| No | 22 (95.7) | 164 (100) | ||
| Crohn’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.6) | ||
| No | 23 (100) | 163 (99.4) | ||
| HIV | 1.000 | |||
| Yes | 0 (0) | 2 (1.2) | ||
| No | 23 (100) | 162 (98.8) | ||
| Hepatitis B | 1.000 | |||
| Yes | 0 (0) | 3 (1.8) | ||
| No | 23 (100) | 161 (98.2) | ||
| Concomitant drug | ||||
| Statins | 0.771 | |||
| Yes | 3 (13) | 29 (17.7) | ||
| No | 20 (87) | 135 (82.3) | ||
| PPIs | 0.952 | |||
| Yes | 8 (34.8) | 56 (34.1) | ||
| No | 15 (65.2) | 108 (65.9) | ||
| 5-HT₃ Antagonists | 0.625 | |||
| Yes | 3 (13) | 16 (9.8) | ||
| No | 20 (87) | 148 (90.2) | ||
| D2 antagonists | 0.231 | |||
| Yes | 1 (4.3) | 1 (0.6) | ||
| No | 22 (95.7) | 163 (99.4) | ||
| Corticosteroids | 1.000 | |||
| Yes | 1 (4.3) | 7 (4.3) | ||
| No | 22 (95.7) | 157 (95.7) | ||
| Antihistamines | 0.204 | |||
| Yes | 3 (13) | 10 (6.1) | ||
| No | 20 (87) | 154 (93.9) | ||
| Diuretics | 1.000 | |||
| Yes | 1 (4.3) | 12 (7.3) | ||
| No | 22 (95.7) | 152 (92.7) | ||
| β-blockers | 0.061 | |||
| Yes | 3 (13) | 5 (3) | ||
| No | 20 (87) | 159 (97) | ||
| P2Y12 inhibitors | 0.032 | |||
| Yes | 4 (17.4) | 7 (4.3) | ||
| No | 19 (82.6) | 157 (95.7) | ||
| 5HT₄ agonists | 0.327 | |||
| No | 22 (95.7) | 162 (98.8) | ||
| Yes | 1 (4.3) | 2 (1.2) | ||
| Antiepileptics | 1.000 | |||
| Yes | 0 (0) | 1 (0.6) | ||
| No | 23 (100) | 163 (99.4) | ||
| Antibiotics | 0.744 | |||
| Yes | 2 (8.7) | 21 (12.8) | ||
| No | 21 (91.3) | 143 (87.2) | ||
| Alpha-blockers | 0.476 | |||
| Yes | 1 (4.3) | 19 (11.6) | ||
| No | 22 (95.7) | 145 (88.4) | ||
| 5α-Reductase inhibitors | 1.000 | |||
| Yes | 1 (4.3) | 12 (7.3) | ||
| No | 22 (95.7) | 152 (92.7) | ||
| NSAIDs | 0.261 | |||
| Yes | 2 (8.7) | 32 (19.5) | ||
| No | 21 (91.3) | 132 (80.5) | ||
| Metformin | 0.185 | |||
| Yes | 5 (21.7) | 19 (11.6) | ||
| No | 18 (78.3) | 145 (88.4) | ||
| Antipsychotics | 0.327 | |||
| Yes | 1 (4.3) | 2 (1.2) | ||
| No | 22 (95.7) | 162 (98.8) | ||
| Anticoagulants | 0.350 | |||
| Yes | 5 (21.7) | 23 (14) | ||
| No | 18 (78.3) | 141 (86) | ||
| ACE inhibitors/ARBs | 0.684 | |||
| Yes | 2 (8.7) | 12 (7.3) | ||
| No | 21 (91.3) | 152 (92.7) | ||
| Zolpidem | 0.327 | |||
| Yes | 1 (4.3) | 2 (1.2) | ||
| No | 22 (95.7) | 162 (98.8) | ||
| TCAs | 1.000 | |||
| Yes | 0 (0) | 1 (0.6) | ||
| No | 23 (100) | 163 (99.4) | ||
| Opioids | 0.038 | |||
| Yes | 12 (52.2) | 120 (73.2) | ||
| No | 11 (47.8) | 44 (26.8) | ||
| Aspirin | 1.000 | |||
| Yes | 0 (0) | 5 (3) | ||
| No | 23 (100) | 159 (97) | ||
| Dopamine | 0.327 | |||
| Yes | 1 (4.3) | 2 (1.2) | ||
| No | 22 (95.7) | 162 (98.8) | ||
| Benzodiazepines | 0.738 | |||
| Yes | 3 (13) | 19 (11.6) | ||
| No | 20 (87) | 145 (88.4) | ||
| Antivirals | 1.000 | |||
| Yes | 0 (0) | 3 (1.8) | ||
| No | 23 (100) | 161 (98.2) | ||
| SSRIs, SNRIs | 0.600 | |||
| Yes | 0 (0) | 7 (4.3) | ||
| No | 23 (100) | 157 (95.7) | ||
| Cancer stage | 0.428 | |||
| 1 | 0 (0.0) | 1 (0.6) | ||
| 2 | 0 (0.0) | 3 (1.8) | ||
| 3 | 3 (13.0) | 9 (5.5) | ||
| 4 | 20 (87.0) | 150 (92.0) | ||
| Diagnosis | 0.223 | |||
| Bladder cancer | 0 (0) | 15 (9.1) | ||
| Colon cancer | 0 (0) | 3 (1.8) | ||
| Gastric cancer | 0 (0) | 8 (4.9) | ||
| Hepatocellular cancer | 1 (4.3) | 12 (7.3) | ||
| Lung cancer | 19 (82.6) | 75 (45.7) | ||
| Pancreatic cancer | 0 (0) | 2 (1.2) | ||
| Rectal cancer | 0 (0) | 3 (1.8) | ||
| Renal cancer | 0 (0) | 3 (1.8) | ||
| Stomach cancer | 0 (0) | 3 (1.8) | ||
| Other | 2 (8.7) | 38 (23.2) | ||
| ECOGPS | 0.464 | |||
| 0 | 0 (0) | 1 (0.6) | ||
| 1 | 21 (91.3) | 129 (79.6) | ||
| 2 | 2 (8.7) | 18 (11.1) | ||
| 3 | 0 (0) | 14 (8.6) | ||
| Characteristics | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Sex | 1.024 (0.355–2.952) | 0.965 | ||
| Age < 65 | 0.698 (0.291–1.677) | 0.422 | ||
| BMI | 0.954 (0.373–2.438) | 0.921 | ||
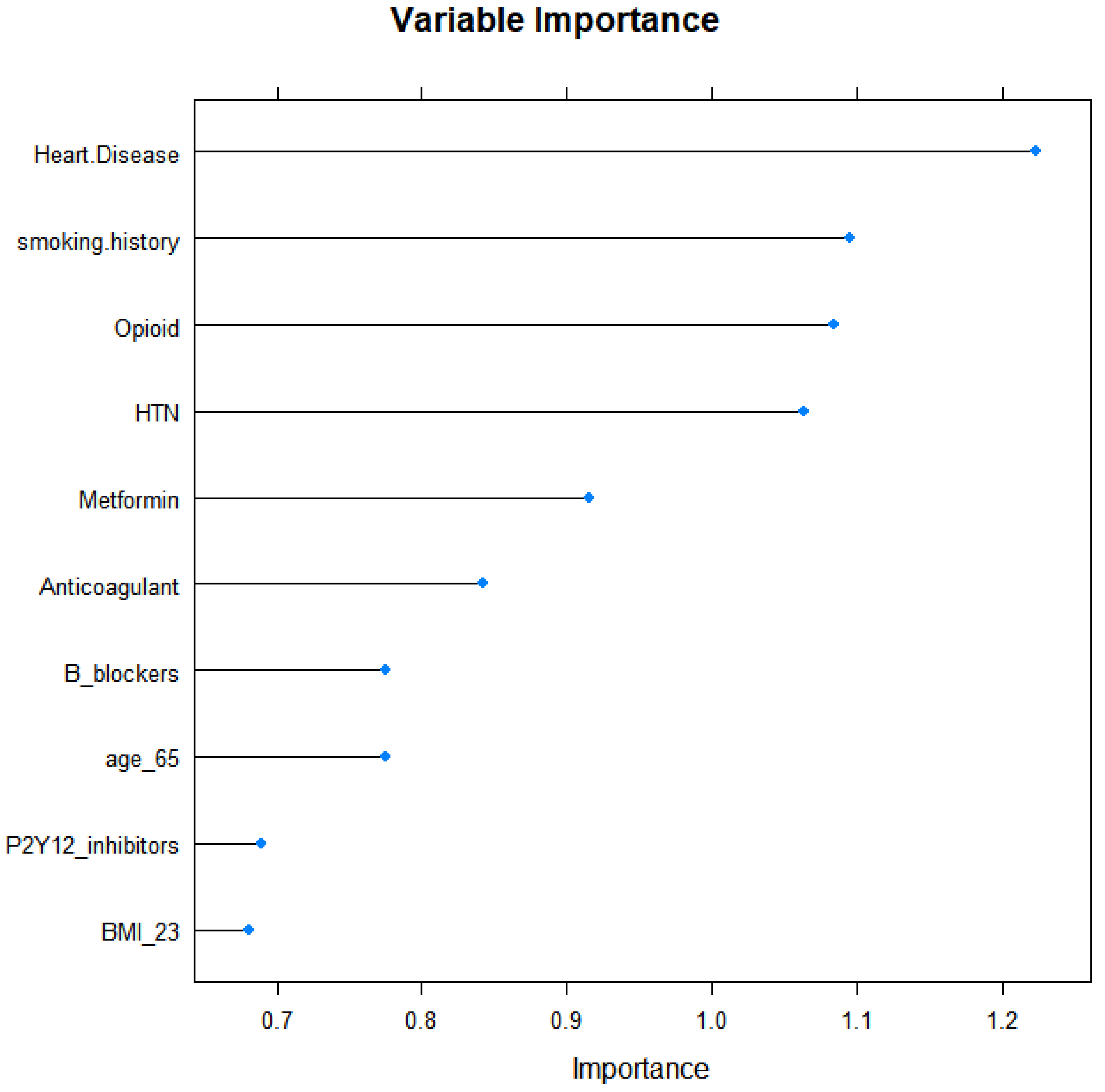
| Heart disease | 4.105 (1.129–14.932) | 0.032 | ||
| P2Y12 inhibitors | 4.722 (1.265–17.631) | 0.021 | ||
| Smoking history | 2.993 (1.183–7.574) | 0.021 | 3.748 (1.338–10.496) | 0.012 |
| Hypertension | 3.000 (1.223–7.360) | 0.016 | 4.093 (1.478–11.332) | 0.007 |
| Opioids | 0.400 (0.165–0.972) | 0.043 | 0.248 (0.090–0.683) | 0.007 |
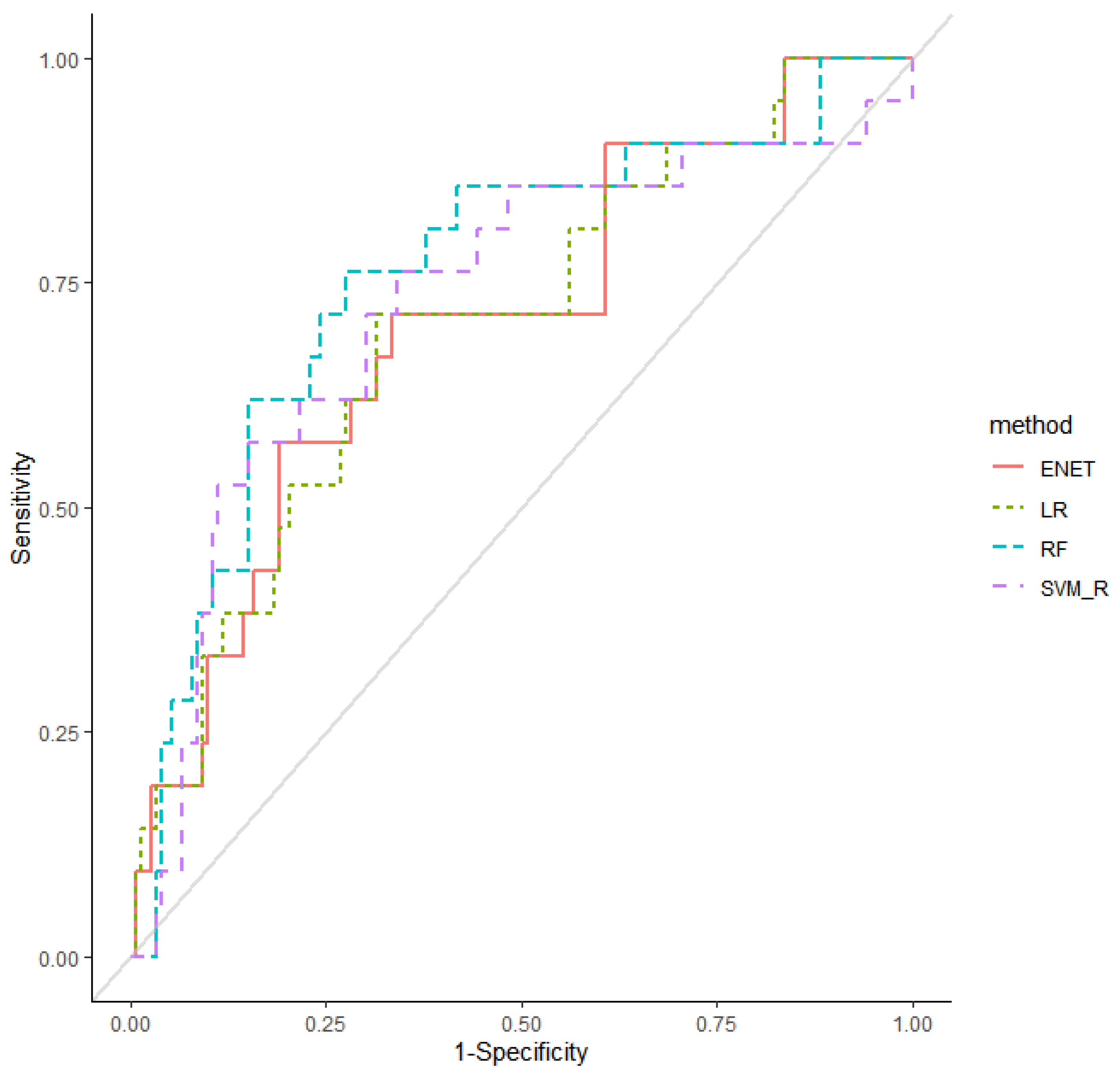
| Machine Learning Model | AUROC (95% CI) | AUPRC (95% CI) |
|---|---|---|
| Logistic regression | 0.71 (0.587–0.827) | 0.47 (0.312–0.622) |
| Elastic net | 0.71 (0.588–0.829) | 0.47 (0.314–0.625) |
| Random Forest | 0.77 (0.648–0.883) | 0.51 (0.357–0.666) |
| SVM (Linear) | 0.57 (0.394–0.752) | 0.36 (0.216–0.497) |
| SVM (Radial) | 0.69 (0.539–0.838) | 0.45 (0.310–0.600) |
| Method | Hyperparameter | |
|---|---|---|
| Model Specification and Search Grids | Selected Values | |
| Elastic net | λ: 100 equally spaced values in logarithmic scale between 10−4 and 0 | λ: 0.01261857 |
| α: 0, 0.2, 0.4, 0.6, 0.8, 1 | α: 0.6 | |
| Random forests | mtry: 1, 2, 3, 4, 5, 6, 7 | mtry: 1 |
| SVM with linear kernel | C: 0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 5 | C: 1 |
| SVM with radial kernel | Sigma: 2−15, 2−13, 2−11, 2−9, 2−7, 2−5, 2−3, 2−1, 2, 23 | Sigma: 0.125 |
| C: 2−5, 2−3, 2−1, 2, 23, 25, 27, 29, 211, 213, 215 | C: 128 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Cho, Y.-A.; Kim, D.-C.; Jo, A.-R.; Min, K.-H.; Lee, K.-E. Factors Associated with Thyroid-Related Adverse Events in Patients Receiving PD-1 or PD-L1 Inhibitors Using Machine Learning Models. Cancers 2021, 13, 5465. https://doi.org/10.3390/cancers13215465
Kim W, Cho Y-A, Kim D-C, Jo A-R, Min K-H, Lee K-E. Factors Associated with Thyroid-Related Adverse Events in Patients Receiving PD-1 or PD-L1 Inhibitors Using Machine Learning Models. Cancers. 2021; 13(21):5465. https://doi.org/10.3390/cancers13215465
Chicago/Turabian StyleKim, Woorim, Young-Ah Cho, Dong-Chul Kim, A-Ra Jo, Kyung-Hyun Min, and Kyung-Eun Lee. 2021. "Factors Associated with Thyroid-Related Adverse Events in Patients Receiving PD-1 or PD-L1 Inhibitors Using Machine Learning Models" Cancers 13, no. 21: 5465. https://doi.org/10.3390/cancers13215465
APA StyleKim, W., Cho, Y.-A., Kim, D.-C., Jo, A.-R., Min, K.-H., & Lee, K.-E. (2021). Factors Associated with Thyroid-Related Adverse Events in Patients Receiving PD-1 or PD-L1 Inhibitors Using Machine Learning Models. Cancers, 13(21), 5465. https://doi.org/10.3390/cancers13215465




