Neuropeptides, Altruism, and Adverse Childhood Experiences: Investigating Biological and Behavioral Correlations in Medical Students
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Neuropeptide Saliva Level
2.4. Questionnaires
2.5. Data Analysis
3. Results
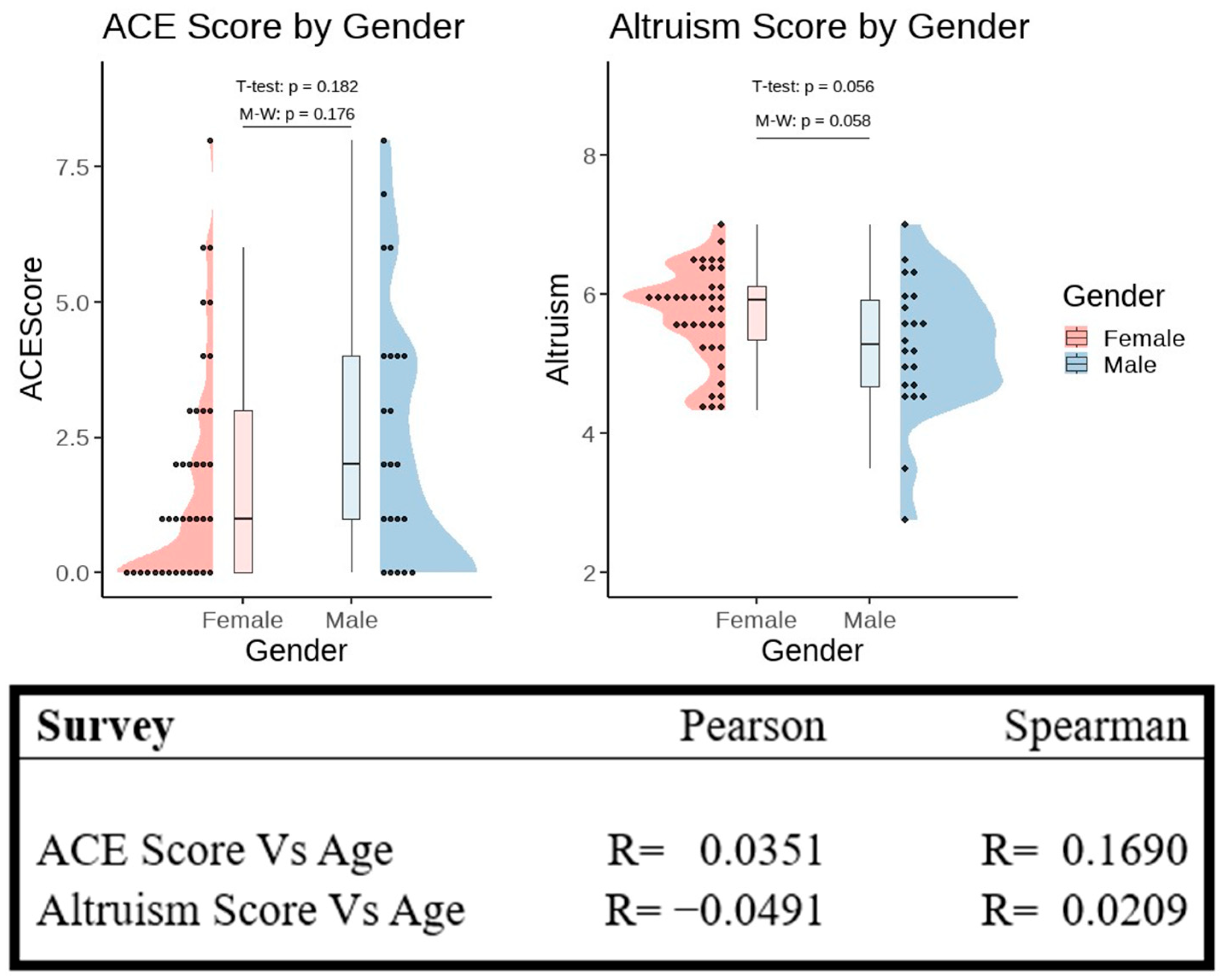
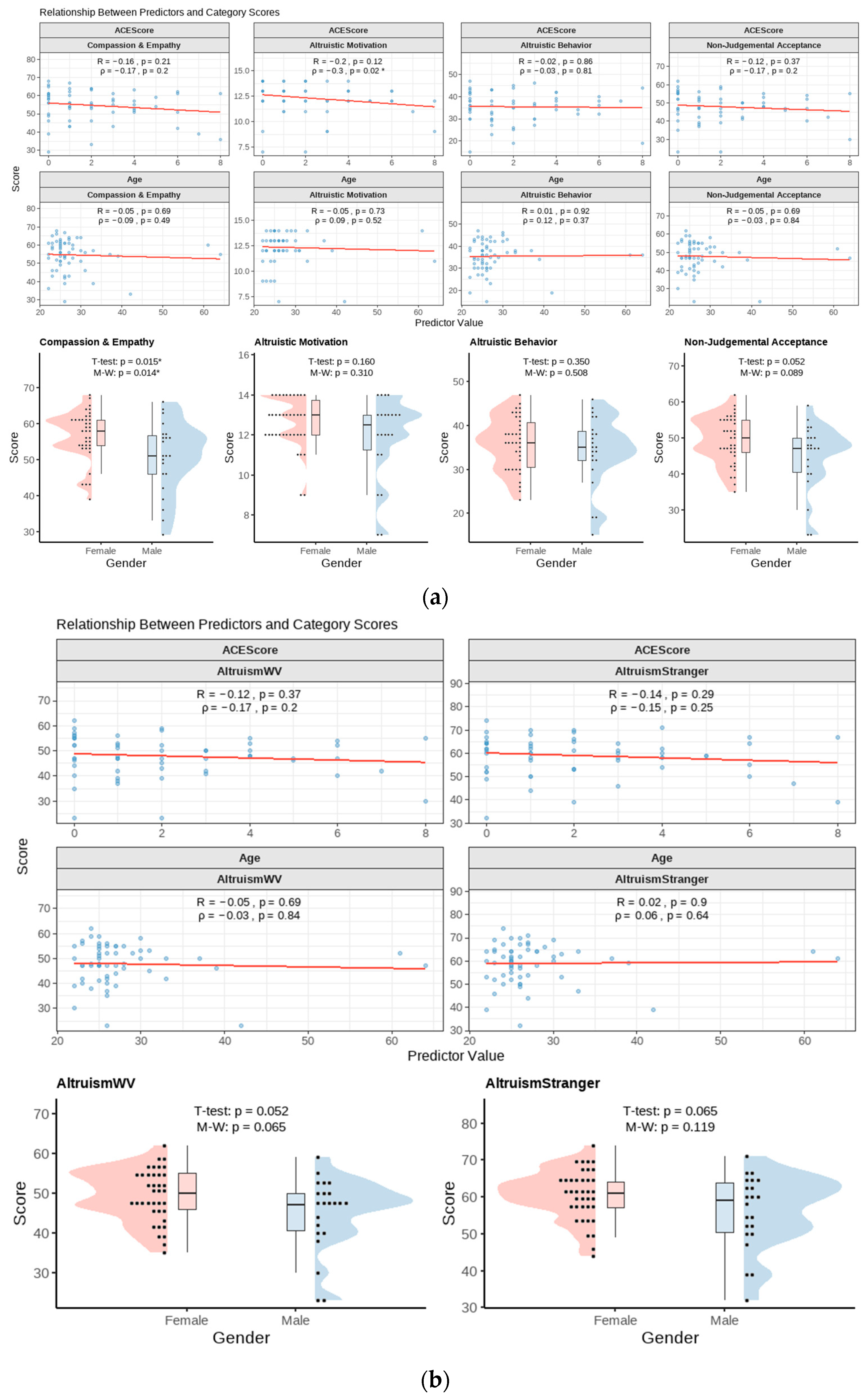
3.1. Survey Data Analysis
3.2. Neuropeptide Evaluation and Interrelationships
3.3. Identifying Motives in Survey Responses
4. Discussion
4.1. Neuropeptide and ACE Scores
4.2. Altruism and Biological Sex
4.3. Neuropeptides in Saliva
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEs | Adverse childhood experiences |
| CLS-H | Compassionate Love Scale for Humanity |
| α-MSH | Alpha-melanocyte stimulating hormone |
| NT | Neurotensin |
Appendix A
Appendix A.1. The Compassionate Love Scale for Humanity
- Scoring: An average score is calculated for all 21 items.
Appendix A.2. The Adverse Childhood Experiences Questionnaire
Appendix A.3. The Perceived Stress Scale
- ________ l. In the last month, how often have you been upset because of something that happened unexpectedly?
- ________ 2. In the last month, how often have you felt that you were unable to control the important things in your life?
- ________ 3. In the last month, how often have you felt nervous and stressed?
- ________ 4. In the last month, how often have you felt confident about your ability to handle your personal problems?
- ________ 5. In the last month, how often have you felt that things were going your way?
- ________ 6. In the last month, how often have you found that you could not cope with all the things that you had to do?
- ________ 7. In the last month, how often have you been able to control irritations in your life?
- ________ 8. In the last month, how often have you felt that you were on top of things?
- ________ 9. In the last month, how often have you been angered because of things that happened that were outside of your control?
- ________ 10. In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?
- First, reverse your scores for questions 4, 5, 7, and 8. On these 4 questions, change the scores like this: 0 = 4, 1 = 3, 2 = 2, 3 = 1, 4 = 0.
- Now add up your scores for each item to get a total. My total score is ___________.
- Individual scores on the PSS can range from 0 to 40 with higher scores indicating higher perceived stress.
- ►
- Scores ranging from 0 to 13 would be considered low stress.
- ►
- Scores ranging from 14 to 26 would be considered moderate stress.
- ►
- Scores ranging from 27 to 40 would be considered high perceived stress.
References
- Hook, V.; Lietz, C.B.; Podvin, S.; Cajka, T.; Fiehn, O. Diversity of neuropeptide cell-cell signaling molecules generated by proteolytic processing revealed by neuropeptidomics mass spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 807–816. [Google Scholar] [CrossRef]
- Burbach, J.P. Neuropeptides from concept to online database www.neuropeptides.nl. Eur. J. Pharmacol. 2010, 626, 27–48. [Google Scholar] [CrossRef]
- Bali, A.; Singh, N.; Jaggi, A.S. Neuropeptides as therapeutic targets to combat stress-associated behavioral and neuroendocrinological effects. CNS Neurol. Disord. Drug Targets. 2014, 13, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Danielli, J.F. Altruism and the internal reward system or the opium of the people. J. Soc. Biol. Syst. 1980, 3, 87–94. [Google Scholar] [CrossRef]
- Love, T.M. Oxytocin, motivation, and the role of dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef]
- López-Arjona, M.; Botía, M.; Martínez-Subiela, S.; Joaquín Cerón, J. Oxytocin measurements in saliva: An analytical perspective. BMC Vet. Res. 2023, 19, 96. [Google Scholar] [CrossRef]
- Donadon, M.F.; Martin-Santos, R.; de Lima Osório, F. The associations between oxytocin and trauma in humans: A systematic review. Front. Pharmacol. 2018, 9, 154. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Liu, L.; Lin, W. Gender differences of empathy. Adv. Psychol. Sci. 2014, 22, 1423. [Google Scholar] [CrossRef]
- Chiesi, F.; Lau, C.; Saklofske, D.H. A revised short version of the compassionate love scale for humanity (CLS-H-SF): Evidence from item response theory analyses and validity testing. BMC Psychol. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int. J. Mol. Sci. 2021, 22, 338. [Google Scholar] [CrossRef]
- Boyadjieva, N.I.; Ortigüela, M.; Arjona, A.; Cheng, X.; Sarkar, D.K. Beta-endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol. Clin. Exp. Res. 2009, 33, 931–937. [Google Scholar] [CrossRef] [PubMed]
- DeVane, C.L. Substance P: A new era, a new role. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 1061–1069. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance, P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Roviš, D.; Vasiljev, V.; Jenko-Pražnikar, Z.; Petelin, A.; Drevenšek, G.; Peruč, D.; Černelič-Bizjak, M. Mental health and drug use severity: The role of substance P, neuropeptide Y, self-reported childhood history of trauma, parental bonding and current resiliency. J. Ment. Health 2019, 30, 88–96. [Google Scholar] [CrossRef]
- Humes, C.; Sic, A.; Knezevic, N.N. Substance P’s Impact on Chronic Pain and Psychiatric Conditions-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 5905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groh, A.; Rhein, M.; Roy, M.; Gessner, C.; Lichtinghagen, R.; Heberlein, A.; Hillemacher, T.; Bleich, S.; Walter, M.; Frieling, H. Trauma severity in early childhood correlates with stress and satiety hormone levels in a pilot cohort receiving diamorphine maintenance treatment. Eur. Addict. Res. 2020, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Charney, D.S. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am. J. Psychiatry 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Ryznar, R.; Andrews, N.; Emery, K.; Snow, M.; Payton, M.; Towne, F.; Gubler, D. Specific salivary neuropeptides shift synchronously during acute stress in fire recruits. Brain Sci. 2004, 14, 492. [Google Scholar] [CrossRef]
- Normandeau, C.P.; Ventura-Silva, A.P.; Hawken, E.R.; Angelis, S.; Sjaarda, C.; Liu, X.; Pêgo, J.M.; Dumont, É.C. A Key Role for Neurotensin in Chronic-Stress-Induced Anxiety-Like Behavior in Rats. Neuropsychopharmacology 2018, 43, 285–293. [Google Scholar] [CrossRef]
- Pittenger, S.T.; Swalve, N.; Chou, S.; Smith, M.D.; Hoonakker, A.; Pudiak, C.M.; Fleckenstein, A.E.; Hanson, G.R.; Bevins, R.A. Sex differences in neurotensin and substance P following nicotine self-administration in rats. Synapse 2016, 70, 336–346. [Google Scholar] [CrossRef]
- Chapman, D.P.; Whitfield, C.L.; Felitti, V.J.; Dube, S.R.; Edwards, V.J.; Anda, R.F. Adverse childhood experiences and the risk of depressive disorders in adulthood. J. Affect. Disord. 2004, 82, 217–225. [Google Scholar] [CrossRef]
- Jackson, D.B.; Testa, A.; Woodward, K.P.; Qureshi, F.; Ganson, K.T.; Nagata, J.M. Adverse childhood experiences and cardiovascular risk among young adults: Findings from the 2019 behavioral risk factor surveillance system. Int. J. Environ. Res. Public Health 2019, 19, 11710. [Google Scholar] [CrossRef] [PubMed]
- Thumfart, K.M.; Jawaid, A.; Bright, K.; Flachsmann, M.; Mansuy, I.M. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci. Biobehav. Rev. 2021, 132, 1049–1066. [Google Scholar] [CrossRef]
- Ramo-Fernández, L.; Schneider, A.; Wilker, S.; Kolassa, I. Epigenetic alterations associated with war trauma and childhood maltreatment. Behav. Sci. Law. 2015, 33, 701–721. [Google Scholar] [CrossRef]
- Music, G. Selfless genes, altruism, and trauma: Research and clinical implications. Br. J. Psychother. 2012, 28, 154–171. [Google Scholar] [CrossRef]
- Staub, E.; Vollhardt, J.R. Altruism born of suffering: The roots of caring and helping after victimization and other trauma. Am. J. Orthopsychiatry 2008, 78, 267–280. [Google Scholar] [CrossRef]
- Warttig, S.L.; Forshaw, M.J.; South, J.; White, A.K. New, normative, english-sample data for the short form perceived stress scale (PSS-4). J. Health Psychol. 2013, 18, 1617–1628. [Google Scholar] [CrossRef]
- Baik, S.H.; Fox, R.S.; Mills, S.D.; Roesch, S.C.; Sadler, G.R.; Klonoff, E.A.; Malcarne, V.L. Reliability and validity of the perceived stress scale-10 in hispanic americans with english or spanish language preference. J. Health Psychol. 2019, 24, 628–639. [Google Scholar] [CrossRef]
- Furuhashi, N.; Takahashi, T.; Kono, H.; Shinkawa, O.; Fukaya, T.; Suzuki, M. Sex difference in the human peripheral plasma beta-endorphin and betalipotropin levels. Gynecol. Obstet. Investig. 1984, 17, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Baroni, S.; Mucci, F.; Piccinni, A.; Moroni, I.; Giannaccini, G.; Carmassi, C.; Massimetti, E.; Dell’Osso, L. Sex-related differences in plasma oxytocin levels in humans. Clin. Prac. Epidemiol. Ment. Health 2019, 15, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, N.; Caquineau, C.; Dayanithi, G.; Bull, P.M.; Douglas, A.J.; Guan, X.; Jiang, M.M.; van der Ploeg, L.H.; Leng, G. α-Melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 2003, 23, 10351–10358. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Sippel, L.M.; Santa Maria, M.M.; Hartwell, K.J.; Brady, K.T.; Joseph, J.E. Impact of oxytocin on the neural correlates of fearful face processing in PTSD related to childhood trauma. Eur. J. Psychotraumatol. 2019, 10, 1606626. [Google Scholar] [CrossRef]
- Klein, K.J.K.; Hodges, S.D. Gender Differences, Motivation, and Empathic Accuracy: When it Pays to Understand. Personal. Soc. Psychol. Bull. 2001, 27, 720–730. [Google Scholar] [CrossRef]
- Kupcova, I.; Danisovic, L.; Bernatova, S.; Harsanyi, S. Analysis of salivary neuropeptides in anxiety and depression using the LUMINEX MAGPIX® System. Cureus 2024, 16, e67984. [Google Scholar] [CrossRef]
- Ryznar, R.; Wong, C.; Onat, E.; Towne, F.; LaPorta, A.; Payton, M. Principal component analysis of salivary cytokines and hormones in the acute stress response. Front. Psychiatry 2022, 13, 957545. [Google Scholar] [CrossRef] [PubMed]
- Teixeira de Almeida, M.; Quattrocchi, L.; Perroud, N.; Aboulafia-Brakha, T. Circadian Variation of Salivary Oxytocin in Young Adult Women. Psychophysiology 2025, 62, e70139. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Gabay, A.S.; Mehta, M.; Paloyelis, Y. Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. eLife 2020, 9, e62456. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khong, J.; Bennett, L.; Felix Rivera, J.; Andrews, N.; Vuong, V.; Zapata, D.; Khong, P.; Ryznar, R. Neuropeptides, Altruism, and Adverse Childhood Experiences: Investigating Biological and Behavioral Correlations in Medical Students. Brain Sci. 2025, 15, 1128. https://doi.org/10.3390/brainsci15101128
Khong J, Bennett L, Felix Rivera J, Andrews N, Vuong V, Zapata D, Khong P, Ryznar R. Neuropeptides, Altruism, and Adverse Childhood Experiences: Investigating Biological and Behavioral Correlations in Medical Students. Brain Sciences. 2025; 15(10):1128. https://doi.org/10.3390/brainsci15101128
Chicago/Turabian StyleKhong, Jennifer, Lauren Bennett, Johanna Felix Rivera, Nathan Andrews, Veronica Vuong, Demi Zapata, Phillip Khong, and Rebecca Ryznar. 2025. "Neuropeptides, Altruism, and Adverse Childhood Experiences: Investigating Biological and Behavioral Correlations in Medical Students" Brain Sciences 15, no. 10: 1128. https://doi.org/10.3390/brainsci15101128
APA StyleKhong, J., Bennett, L., Felix Rivera, J., Andrews, N., Vuong, V., Zapata, D., Khong, P., & Ryznar, R. (2025). Neuropeptides, Altruism, and Adverse Childhood Experiences: Investigating Biological and Behavioral Correlations in Medical Students. Brain Sciences, 15(10), 1128. https://doi.org/10.3390/brainsci15101128





