Outcomes after Flow Diverter Treatment in Subarachnoid Hemorrhage: A Meta-Analysis and Development of a Clinical Prediction Model (OUTFLOW)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Article Selection
2.2. Outcomes
2.3. Data Collection
2.4. Bias Evaluation
2.5. Statistics
3. Results
3.1. Study Population and Patient Characteristics
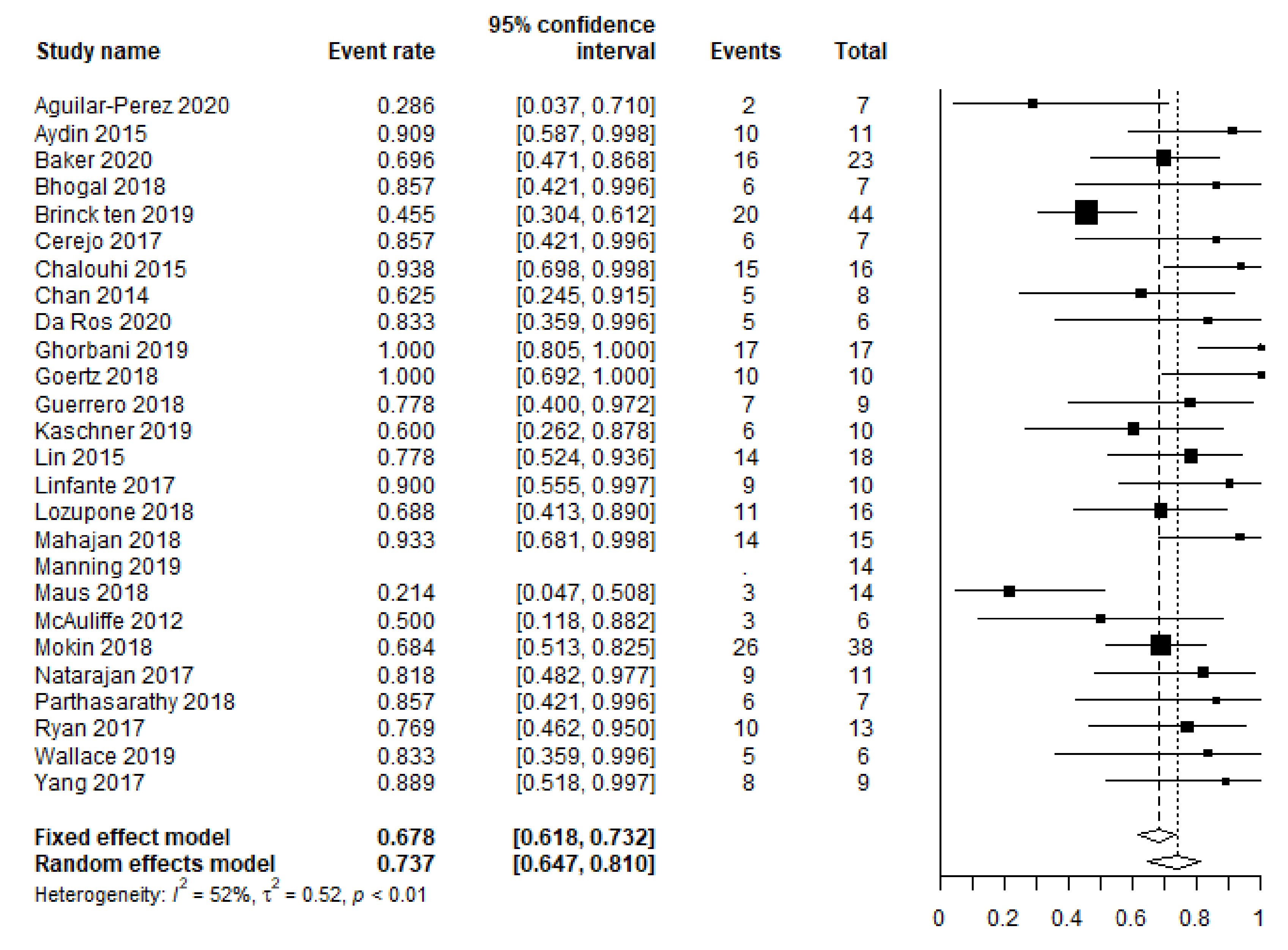
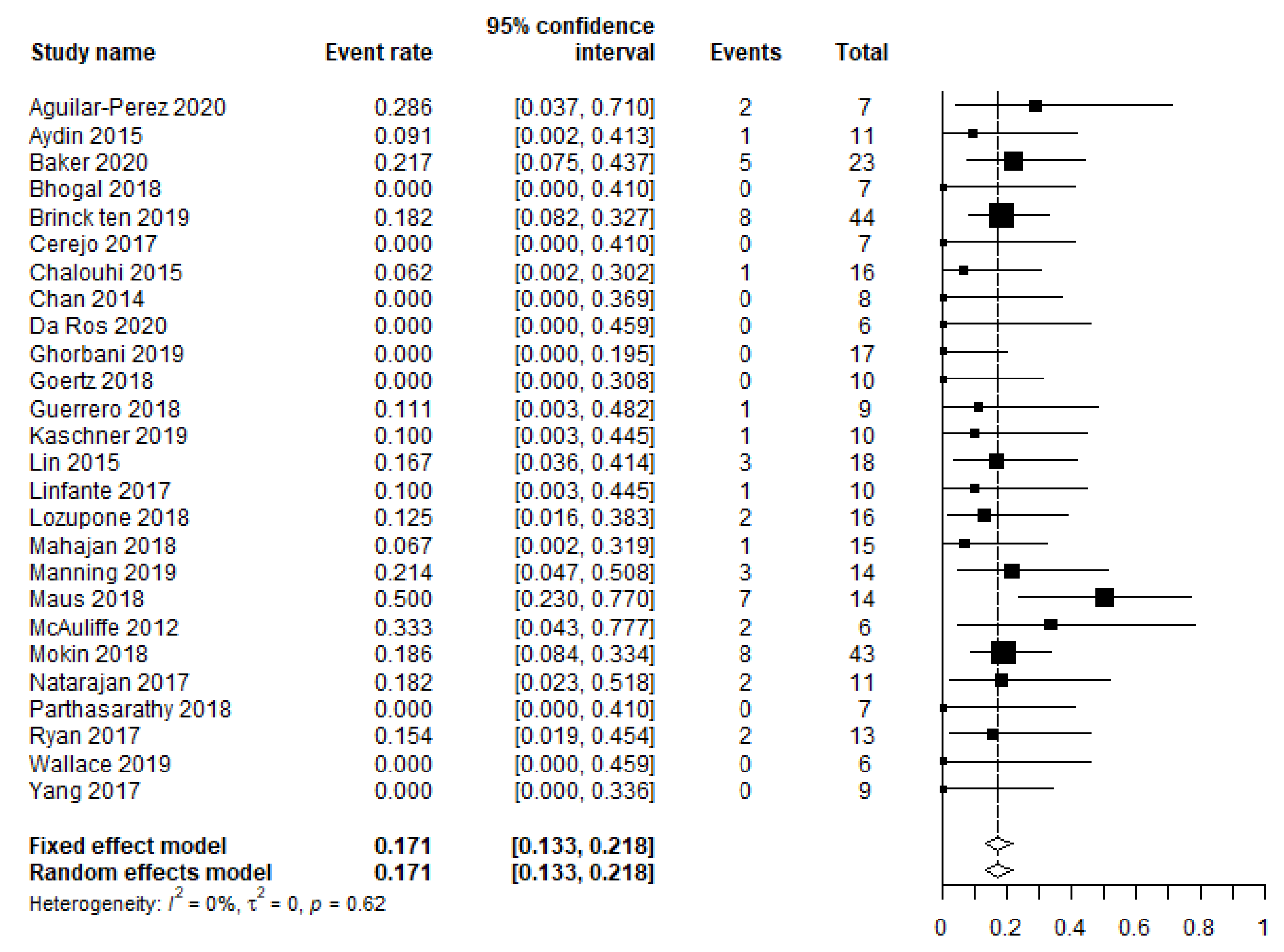
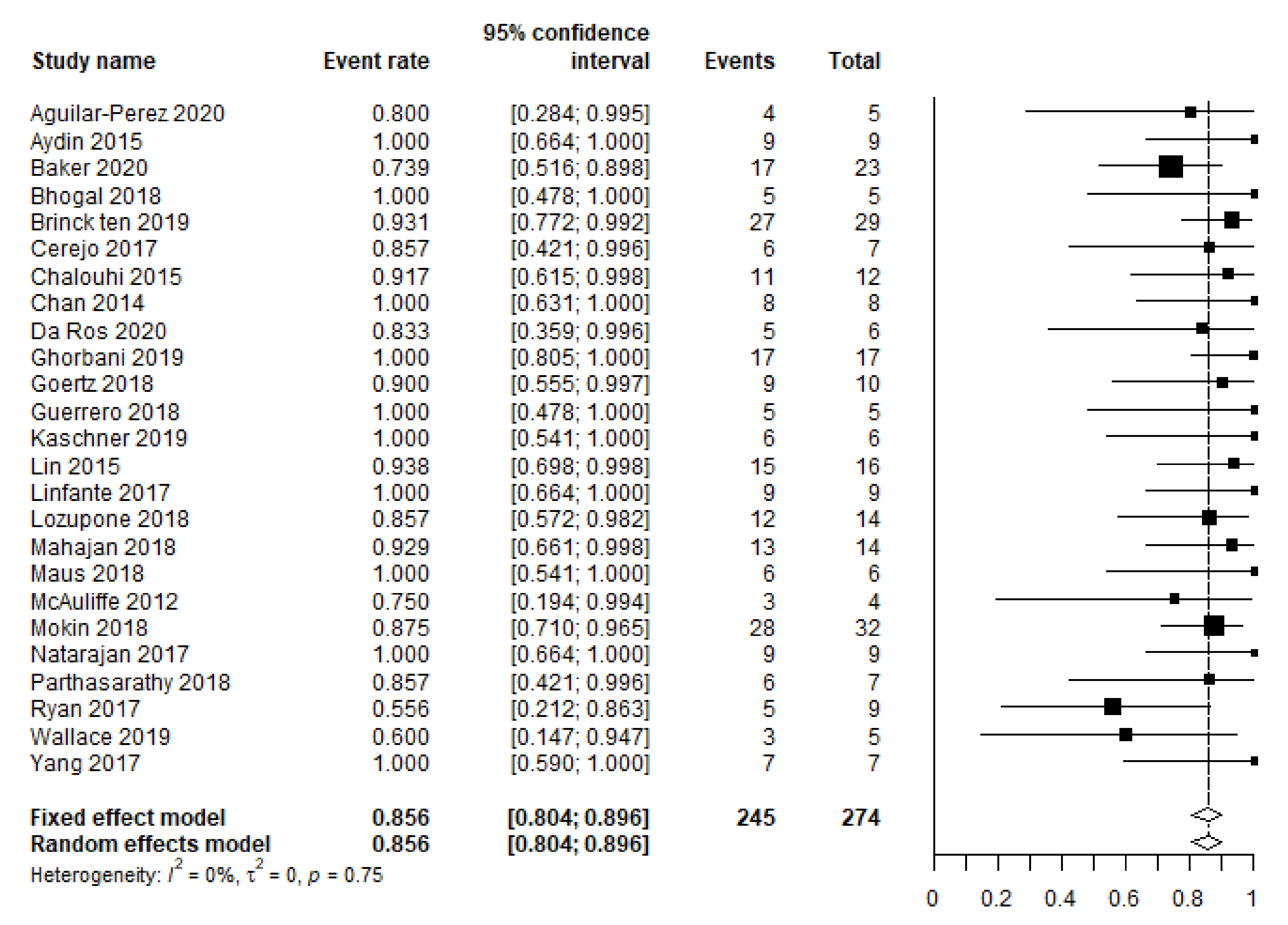
3.2. Primary and Secondary Outcomes
3.3. Quality of Evidence
3.4. Prediction Model
4. Discussion
4.1. Prediction Model
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Syntaxes
PubMed
Embase
Appendix B. Data Collection—Baseline Characteristics and Outcome
Registered Baseline Items
Registered Outcome Parameters
References
- Walcott, B.P.; Koch, M.J.; Stapleton, C.J.; Patel, A.B. Blood Flow Diversion as a Primary Treatment Method for Ruptured Brain Aneurysms-Concerns, Controversy, and Future Directions. Neurocritical Care 2017, 26, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Cagnazzo, F.; di Carlo, D.T.; Cappucci, M.; Lefevre, P.H.; Costalat, V.; Perrini, P. Acutely Ruptured Intracranial Aneurysms Treated with Flow-Diverter Stents: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2018, 39, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Dossani, R.H.; Patra, D.P.; Kosty, J.; Jumah, F.; Kuybu, O.; Mohammed, N.; Waqas, M.; Riaz, M.; Cuellar, H. Early Versus Delayed Flow Diversion for Ruptured Intracranial Aneurysms: A Meta-Analysis. World Neurosurg. 2019, 126, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Madaelil, T.P.; Moran, C.J.; Cross, D.T., 3rd; Kansagra, A.P. Flow Diversion in Ruptured Intracranial Aneurysms: A Meta-Analysis. AJNR Am. J. Neuroradiol. 2017, 38, 590–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Brinck, M.F.M.; Vries, J.D. Subarachnoid Hemorrhage Due to Ruptured Intracranial Aneurysms: The Scientific Base for Flow Diverters. In Evidence for Neurosurgery; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Foreman, P.M.; Ilyas, A.; Cress, M.C.; Vachhani, J.A.; Hirschl, R.A.; Agee, B.; Griessenauer, C.J. Ruptured Intracranial Aneurysms Treated with the Pipeline Embolization Device: A Systematic Review and Pooled Analysis of Individual Patient Data. AJNR Am. J. Neuroradiol. 2021, 42, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Chinea, A.; Primiani, C.T.; Ren, Z.; Kan, P.; Srinivasan, V.M.; Hanel, R.; Aguilar-Salinas, P.; Turk, A.S.; Turner, R.D.; et al. Treatment of blood blister aneurysms of the internal carotid artery with flow diversion. J. Neurointerventional Surg. 2018, 10, 1074–1078. [Google Scholar] [CrossRef]
- Ten Brinck, M.F.M.; Jäger, M.; de Vries, J.; Grotenhuis, J.A.; Aquarius, R.; Mørkve, S.H.; Rautio, R.; Numminen, J.; Raj, R.; Wakhloo, A.K.; et al. Flow diversion treatment for acutely ruptured aneurysms. J. Neurointerventional Surg. 2020, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- van Donkelaar, C.E.; Bakker, N.A.; Birks, J.; Veeger, N.; Metzemaekers, J.D.M.; Molyneux, A.J.; Groen, R.J.M.; van Dijk, J.M.C. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage. Stroke 2019, 50, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.; Cusimano, M.D.; Etminan, N.; Hanggi, D.; Hasan, D.; Ilodigwe, D.; Lantigua, H.; Le Roux, P.; Lo, B.; Louffat-Olivares, A.; et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: A systematic review. Neurocritical Care 2013, 18, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Andrews, P. Advances in the understanding of delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. F1000Res 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Br. J. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Perez, M.; Hellstern, V.; AlMatter, M.; Wendl, C.; Bäzner, H.; Ganslandt, O.; Henkes, H. The p48 Flow Modulation Device with Hydrophilic Polymer Coating (HPC) for the Treatment of Acutely Ruptured Aneurysms: Early Clinical Experience Using Single Antiplatelet Therapy. Cardiovasc. Interv. Radiol. 2020, 43, 740–748. [Google Scholar] [CrossRef] [Green Version]
- Aydin, K.; Arat, A.; Sencer, S.; Hakyemez, B.; Barburoglu, M.; Sencer, A.; Izgi, N. Treatment of ruptured blood blister-like aneurysms with flow diverter SILK stents. J. Neurointerventional Surg. 2015, 7, 202–209. [Google Scholar] [CrossRef]
- Baker, C.; Grandhi, R.; Griessenauer, C.J.; Dmytriw, A.A.; Kapadia, A.; Yang, V.X.D.; Ghorbani, M.; Chen, K.; Aziz-Sultan, M.A.; Rinaldo, L.; et al. Pipeline Embolization in Patients with Posterior Circulation Subarachnoid Hemorrhages: Is Takotsubo Cardiomyopathy a Limiting Factor? World Neurosurg. 2020, 143, e523–e528. [Google Scholar] [CrossRef]
- Bhogal, P.; Henkes, E.; Schob, S.; AlMatter, M.; Hellstern, V.; Bäzner, H.; Ganslandt, O.; Henkes, H.; Pérez, M.A. The use of flow diverters to treat small (≤5 mm) ruptured, saccular aneurysms. Surg. Neurol. Int. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Cerejo, R.; Bain, M.; John, S.; Hardman, J.; Moore, N.; Hussain, M.S.; Toth, G. Flow diverter treatment of cerebral blister aneurysms. Neuroradiology 2017, 59, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Zanaty, M.; Whiting, A.; Tjoumakaris, S.; Hasan, D.; Ajiboye, N.; Hann, S.; Rosenwasser, R.H.; Jabbour, P. Treatment of ruptured intracranial aneurysms with the pipeline embolization device. Neurosurgery 2015, 76, 165–172; discussion 172. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.S.; Mak, C.H.; Wong, A.K.; Chan, K.Y.; Leung, K.M. Use of the pipeline embolization device to treat recently ruptured dissecting cerebral aneurysms. Interv. Neuroradiol. 2014, 20, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Ros, V.; Diana, F.; Sabuzi, F.; Malatesta, E.; Sanna, A.; Scaggiante, J.; Di Giuliano, F.; Gandini, R.; Floris, R.; Ruggiero, M. Flow diverters for ruptured posterior circulation perforator aneurysms: Multicenter experience and literature review. J. Neurointerventional Surg. 2020, 12, 688–694. [Google Scholar] [CrossRef]
- Ghorbani, M.; Griessenauer, C.J.; Wipplinger, C.; Azar, M.; Shojaei, H.; Bavand, K.; Khosravi, D. Flow diverter embolization device for endovascular treatment of ruptured blister and wide necked very small aneurysms. Heliyon 2019, 5, e02241. [Google Scholar] [CrossRef] [Green Version]
- Goertz, L.; Dorn, F.; Kraus, B.; Borggrefe, J.; Schlamann, M.; Forbrig, R.; Turowski, B.; Kabbasch, C. Safety and efficacy of the Derivo Embolization Device for the treatment of ruptured intracranial aneurysms. J. Neurointerventional Surg. 2018, 11, 290–295. [Google Scholar] [CrossRef]
- Guerrero, W.R.; Ortega-Gutierrez, S.; Hayakawa, M.; Derdeyn, C.P.; Rossen, J.D.; Hasan, D.; Samaniego, E.A. Endovascular Treatment of Ruptured Vertebrobasilar Dissecting Aneurysms Using Flow Diversion Embolization Devices: Single-Institution Experience. World Neurosurg. 2018, 109, e164–e169. [Google Scholar] [CrossRef] [PubMed]
- Kaschner, M.G.; Petridis, A.; Turowski, B. Single-center experience with the new generation Derivo Embolization Device in ruptured dissecting and blister aneurysms. Acta Radiol. 2020, 61, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Brouillard, A.M.; Keigher, K.M.; Lopes, D.K.; Binning, M.J.; Liebman, K.M.; Veznedaroglu, E.; Magarik, J.A.; Mocco, J.; Duckworth, E.A.; et al. Utilization of Pipeline embolization device for treatment of ruptured intracranial aneurysms: US multicenter experience. J. Neurointerventional Surg. 2015, 7, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Linfante, I.; Mayich, M.; Sonig, A.; Fujimoto, J.; Siddiqui, A.; Dabus, G. Flow diversion with Pipeline Embolic Device as treatment of subarachnoid hemorrhage secondary to blister aneurysms: Dual-center experience and review of the literature. J. Neurointerventional Surg. 2017, 9, 29–33. [Google Scholar] [CrossRef]
- Lozupone, E.; Piano, M.; Valvassori, L.; Quilici, L.; Pero, G.; Visconti, E.; Boccardi, E. Flow diverter devices in ruptured intracranial aneurysms: A single-center experience. J. Neurosurg. 2018, 128, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Das, B.; Narang, K.S.; Jha, A.N.; Singh, V.P.; Sapra, H.; Goel, G. Surpass Flow Diverter in the Treatment of Ruptured Intracranial Aneurysms-A Single-Center Experience. World Neurosurg. 2018, 120, e1061–e1070. [Google Scholar] [CrossRef]
- Manning, N.W.; Cheung, A.; Phillips, T.J.; Wenderoth, J.D. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: Multicentre experience. J. Neurointerventional Surg. 2019, 11, 694–698. [Google Scholar] [CrossRef]
- Maus, V.; Mpotsaris, A.; Dorn, F.; Mohlenbruch, M.; Borggrefe, J.; Stavrinou, P.; Abdullayev, N.; Barnikol, U.B.; Liebig, T.; Kabbasch, C. The Use of Flow Diverter in Ruptured, Dissecting Intracranial Aneurysms of the Posterior Circulation. World Neurosurg. 2018, 111, e424–e433. [Google Scholar] [CrossRef]
- McAuliffe, W.; Wenderoth, J.D. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. AJNR Am. J. Neuroradiol. 2012, 33, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.K.; Shallwani, H.; Fennell, V.S.; Beecher, J.S.; Shakir, H.J.; Davies, J.M.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I. Flow Diversion after Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Clin. N. Am. 2017, 28, 375–388. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Gupta, V.; Gupta, A. Safety of Prasugrel loading in ruptured blister like aneurysm treated with a Pipeline device. Br. J. Radiol. 2018, 91, 20170476. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.W.; Khan, A.S.; Barco, R.; Choulakian, A. Pipeline flow diversion of ruptured blister aneurysms of the supraclinoid carotid artery using a single-device strategy. Neurosurg. Focus 2017, 42, E11. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.N.; Madaelil, T.P.; Kamran, M.; Miller, T.R.; Delgado Almandoz, J.E.; Grossberg, J.A.; Kansagra, A.P.; Gandhi, D.; Kayan, Y.; Cawley, C.M.; et al. Pipeline Embolization of Vertebrobasilar Aneurysms-A Multicenter Case Series. World Neurosurg. 2019, 124, e460–e469. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Vadasz, A.; Szikora, I. Treatment of ruptured blood blister aneurysms using primary flow-diverter stenting with considerations for adjunctive coiling: A single-centre experience and literature review. Interv. Neuroradiol. 2017, 23, 465–476. [Google Scholar] [CrossRef]
- Jartti, P.; Isokangas, J.M.; Karttunen, A.; Jartti, A.; Haapea, M.; Koskelainen, T.; Tervonen, O. Early rebleeding after coiling of ruptured intracranial aneurysms. Acta Radiol. 2010, 51, 1043–1049. [Google Scholar] [CrossRef]
- Bsat, S.; Bsat, A.; Tamim, H.; Chanbour, H.; Alomari, S.O.; Houshiemy, M.N.E.; Moussalem, C.; Omeis, I. Safety of stent-assisted coiling for the treatment of wide-necked ruptured aneurysm: A systematic literature review and meta-analysis of prevalence. Interv. Neuroradiol. 2020, 26, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Lindsay, P.; McQuiggan, J.; Zagorski, B.; Hill, M.D.; O’Kelly, C. Declining Admission and Mortality Rates for Subarachnoid Hemorrhage in Canada Between 2004 and 2015. Stroke 2018, 50, 181–184. [Google Scholar] [CrossRef]
- Zhou, G.; Su, M.; Yin, Y.L.; Li, M.H. Complications associated with the use of flow-diverting devices for cerebral aneurysms: A systematic review and meta-analysis. Neurosurg. Focus 2017, 42, E17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pooled Variables | Number (%) | Number of Articles |
|---|---|---|
| Total population | ||
| N. eligible patients | 357 | 26 |
| N. eligible aneurysms | 368 | 26 |
| Proportion of unfavorable HH/WFNS grades at presentation | 96/348 (28) | 25 a |
| 26 | ||
| Aneurysm type | ||
| Blood blister-like | 161 (44) | |
| Saccular | 81 (22) | |
| Fusiform | 32 (9) | |
| Dissecting | 90 (24) | |
| Pseudoaneurysm | 3 (1) | |
| Mycotic | 1 (0.2) | |
| Aneurysms located in posterior circulation | 235/368 (64) | 26 |
| Aneurysms additionally coiled | 56/307 (18) | 24 b |
| Favorable clinical outcome (mRS 0–2, GOS 4–5) | 243/338 (72) | 25 c |
| Complete occlusion | 253/290 (87) | 25 d |
| Complications | 87/356 (24) | 26 |
| Leading to a permanent neurological deficit in N patients | 32/265 (12) | 21 e |
| Rebleeding | 11/368 (3) | 26 |
| All-cause mortality | 50/357 (14) | 26 |
| Predictor Variable | OR (95% CI) | p-Value |
|---|---|---|
| Unfavorable presentation (WFNS and HH 4–5) | 0.156 (0.064–0.382) | <0.01 |
| Saccular aneurysm | 2.142 (0.781–5.870) | 0.14 |
| Aneurysm location (posterior circulation) | 0.763 (0.331–1.763) | 0.53 |
| Aneurysm size (in mm) | 0.883 (0.826–0.944) | <0.01 |
| Treatment delay (in days) | 1.053 (0.945–1.174) | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ten Brinck, M.F.M.; Shimanskaya, V.E.; Aquarius, R.; Bartels, R.H.M.A.; Meijer, F.J.A.; Koopmans, P.C.; de Jong, G.; Wakhloo, A.K.; de Vries, J.; Boogaarts, H.D. Outcomes after Flow Diverter Treatment in Subarachnoid Hemorrhage: A Meta-Analysis and Development of a Clinical Prediction Model (OUTFLOW). Brain Sci. 2022, 12, 394. https://doi.org/10.3390/brainsci12030394
ten Brinck MFM, Shimanskaya VE, Aquarius R, Bartels RHMA, Meijer FJA, Koopmans PC, de Jong G, Wakhloo AK, de Vries J, Boogaarts HD. Outcomes after Flow Diverter Treatment in Subarachnoid Hemorrhage: A Meta-Analysis and Development of a Clinical Prediction Model (OUTFLOW). Brain Sciences. 2022; 12(3):394. https://doi.org/10.3390/brainsci12030394
Chicago/Turabian Styleten Brinck, Michelle F. M., Viktoria E. Shimanskaya, René Aquarius, Ronald H. M. A. Bartels, Frederick J. A. Meijer, Petra C. Koopmans, Guido de Jong, Ajay K. Wakhloo, Joost de Vries, and Hieronymus D. Boogaarts. 2022. "Outcomes after Flow Diverter Treatment in Subarachnoid Hemorrhage: A Meta-Analysis and Development of a Clinical Prediction Model (OUTFLOW)" Brain Sciences 12, no. 3: 394. https://doi.org/10.3390/brainsci12030394
APA Styleten Brinck, M. F. M., Shimanskaya, V. E., Aquarius, R., Bartels, R. H. M. A., Meijer, F. J. A., Koopmans, P. C., de Jong, G., Wakhloo, A. K., de Vries, J., & Boogaarts, H. D. (2022). Outcomes after Flow Diverter Treatment in Subarachnoid Hemorrhage: A Meta-Analysis and Development of a Clinical Prediction Model (OUTFLOW). Brain Sciences, 12(3), 394. https://doi.org/10.3390/brainsci12030394






