A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far
Abstract
1. Introduction
1.1. Neuropsychological Profiles of Main Neurodegenerative Diseases
1.2. Neuropsychological Profiles of Main Neurodegenerative Diseases
2. Materials and Methods
- Identified our research question as “what is known so far from the existing literature about CCT and VR studies targeting cognitive impairment in most common neurodegenerative conditions?” In particular, we aimed to point out the scientific evidence currently available in order to provide support for health professionals to consider these promising therapeutic tools when planning rehabilitative interventions.
- Identified relevant studies which would be as comprehensive as possible in answering our central research question. To this purpose, we adopted a strategy that involved searching for research evidence via different sources (electronic databases, reference lists, hand-searching of key journals). In a first step, we performed an EBSCO, Google Scholar and PubMed-based search using these specific combinations of keywords: “Cognitive Training” OR “Virtual Reality Training” OR “Augmented Reality Training” OR “telerehabilitation” AND ‘‘Alzheimer’s disease’’ OR ‘‘fronto-temporal dementia’’ OR ‘‘Parkinson’s disease’’ OR ‘‘Multiple Sclerosis’’. Since we were interested in exploring the latest evidence, we focused our literature search on articles that have been published between 2015 and 2020. However, we also included previously published articles whenever was necessary for clarifying the information which emerged from more recent studies. Successively, the review was further extended by considering all relevant articles reported in the references of each paper
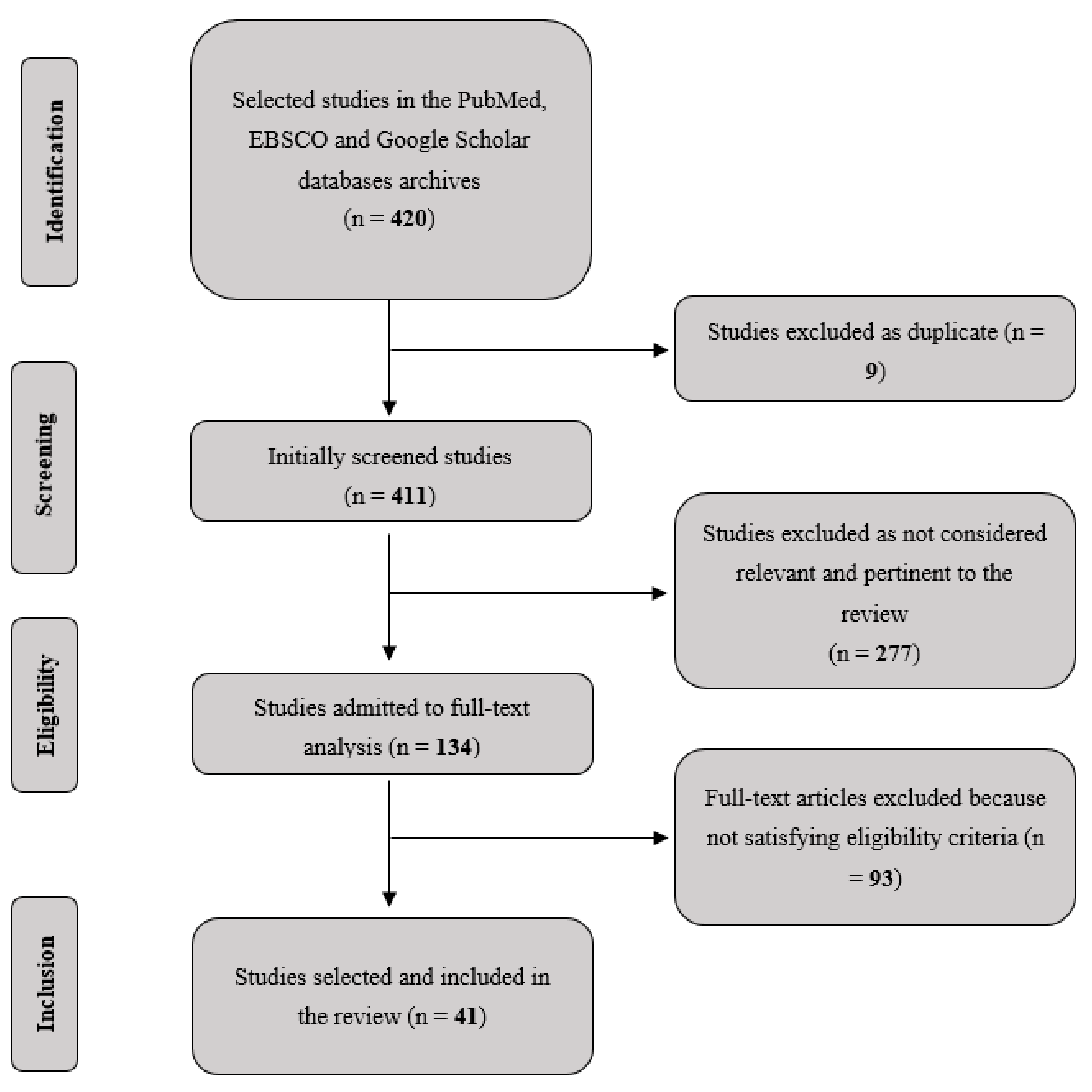
- Selected the studies adopting inclusion and exclusion criteria, based on our specific research question. Analysis has been primarily focused on studies clearly reporting details about cognitive training, patients’ characteristics, presence/absence of cognitive symptoms, study design and experimental protocols, quantification of training parameters of interest (in terms of length and frequency of training sessions) and brain imaging data, where available. We excluded research on healthy subjects and/or conducted in non-human animals. Finally, Duplicates and/or redundant resources across databases were removed. Figure 1 reports the followed flow-chart.
- Charted our data, summarizing the relevant aspects of our selected studies. We recorded information as follows: Authors, Year published, Size of the sample, Diagnosis of the clinical sample, Mean age of the sample, Duration of the intervention/training, Study type, Type of experimental control condition or group, Cognitive training used, Main results, Duration and presence of a follow-up (see Table 1 and Table 2).
- Summarized and reported our narrative account of findings, organizing the literature thematically according to a first criterion (type of neurodegenerative disorder) and an ensuring second criterion (kind CCT and VR training/intervention).
3. Results
3.1. Alzheimer’s Disease (AD)
3.1.1. Computerized Cognitive Training (CCT)
3.1.2. Virtual Reality (VR) and Augmented Reality (AR) Trainings
3.2. Fronto-Temporal Dementia (FTD)
3.2.1. Computerized Cognitive Training (CCT)
3.2.2. Virtual Reality Training (VRT)
3.3. Parkinson’s Disease (PD)
3.3.1. Computerized Cognitive Training (CCT)
3.3.2. Virtual Reality Training (VRT)
3.4. Multiple Sclerosis (MS)
3.4.1. Computerized Cognitive Training (CCT)
| Number, Authors, Published Year | Sample (n) | Diagnosis | Mean Age (Years) (SD) | Duration (Days × Weeks) | Study Type | Control | Cognitive Training Used | Main Results | Duration Post-Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Alescio-Lautier et al. (2019) [87] | 12 | AD | 81 1.68 | 15 sessions | RCT | Control group | Memory, attention and semantic tasks | Increased memory recall and verbal fluency | Not tested |
| Cavallo et al. (2016) [88] | 80 | AD | 76.5 2.88 | 3 d × 12 w | RCT | Control group | Memory, attention, EF and language tasks | Improvement in different neuropsychological domains | 6 months |
| Rodriguez-Mora et al. (2020) [89] | 39 | AD | 76.31 7.17 | 5 d × 12 months | Pilot study | None | Different cognitive trainings, ADL and motor tasks | Arrested decline in all tested functions | Not tested |
| Imbeault et al. (2018) [90] | 1 | AD | 65 | 2 d × 8 w + 23 sessions | Single-case | None | Prospective memory task on tablet (telerehabilitation) | Improved ADL and memory abilities | Not tested |
| Lizio et al. (2019) [93] | 15 | AD | 69.7 0.8 | 7 d × 2 w | Pilot study | None | Spatial abilities, EF and memory tasks on tablet (telerehabilitation) | Increased accuracy and reduced reaction times in all domains | Not tested |
| Savulich et al. (2017) [96] | 42 | aMCI | 75.2 7.4 | 8 sessions | RCT | Control group | Memory and visuospatial game on iPad | Increased episodic memory and visuospatial abilities | Not tested |
| Barban et al. (2016) [98] | 348 | AD, MCI and HE | 77 5.7 | 2 d × 3 months | Crossover RCT | Control group | Different cognitive functions + RT | Increased MMSE scoring | None |
| Newhart et al. (2009) [156] | 2 | lvFTD, svFTD | 65/60 | ~25 sessions | Proof-of-concept study | None | Cueing hierarchy naming treatment | Increased naming performances on treated items in both subjects and also in untreated items in lvFTD one | Not tested |
| Evans et al. (2016) [157] | 1 | svFTD | 72 | 24 sessions (20 months) | Single-case | None | Flashcard naming task (telerehabilitation) | Increased naming performances | Not tested |
| Croot et al. (2019) [158] | 8 | Various PPA | 64.8 5.9 | 2 w + 2 w + 26 w | Single-Case Experimental Design | None | Repetition and reading with cueing pictures (telerehabilitation) | Mixed results, 3 subjects showed increased picture naming performances | Up to 6 months |
| Henry et al. (2019) [159] | 18 | lvFTD, svFTD | 65.2 8.3 | 1 d × 4–8 w/2 d × 4–8 w | Clinical Trial | None | LRCT | Increased naming on trained and untrained items | 1 year for trained and 6 months for untrained items |
| Beeson et al. (2011) [161] | 1 | lvPPA | 77 | 6 d × 2 w | Single-case | None | Generative naming task | Improved word retreival on trained and untrained items | 6 months |
| Macoir et al. (2015) [162] | 1 | svFTD | 72 | 5 d × 2 w | Single-case | None | Video-cued action naming task | Increased naming on trained actions | 4 weeks |
| Dial et al. (2019) [165] | 31 | lvFTD, svFTD, nfvFTD | ~65 ~8 | (not clearly reported) | Clinical Trial | None | LRCT or VISTA (telerehabilitation or face-to-face) | Increased primary outcomes (word retrieval or fluency); no differences between telerehabilitation and face-to-face interventions | 12 months |
| Lavoie et al. (2019) [166] | 5 | lvFTS, svFTD | 72.2 5.4 | 4 d × 4 w | Single-case | None | Functional Vocabulary Treatment (telerehabilitation) | Increased naming for trained items and reduced anomia in natural conversation | 2 months |
| Walton et al. (2018) [175] | 65 | PD | ~68 ~8 | 2 d × 7 w | RCT | Active control group | Battery with different cognitive trainings | Reduced FoG and increased processing speed | Not tested |
| Sinforiani et al. (2004) [176] | 20 | PD | 68.9 7.1 | 2 d × 6 w | Pilot study | None | Attention, abstract reasoning and visuospatial training | Increased verbal fluency, logic memory and Raven’s matrices | 6 months |
| Petrelli et al. (2015) [177] | 47 | Non-demented PD | ~69 ~9 | 2 d × 6 w | RCT | Control group | Attention, memory and EF tasks | Reduced cognitive decline | 12 months |
| Diez-Cirarda et al. (2017) [180] | 15 | PD | 66.07 4.8 | 3 d × 13 w | Clinical Trial | None | Attention, memory and EF tasks | Increased cognitive performances and increased functional connectivity | 18 months |
| Perez-Martin et al. (2017) [196] | 62 | MS | 44.9 9.8 | 12 sessions | RCT | Control group | Training of several cognitive domains (telerehabilitation) | Increased memory, verbal fluency and reduced anxiety | Not tested |
| Charvet et al. (2017) [197] | 135 | MS | 50 12 | 5 d × 12 w | RCT | Active control group | Training of several cognitive domains (telerehabilitation) | Increased cognitive functions | Not tested |
| Brissart et al. (2013) [198] | 20 | MS | 42.5 5.1 | 13 sessions | RCT | Control group | Training of several cognitive domains | Increased verbal, working memory and verbal fluency performances | Not tested |
| Mattioli et al. (2010) [199] | 150 | MS | 41–53 | 3 d × 12 w | RCT | Control group | Attention, Information Processing, EF trainings | Increase in all cognitive functions and reduced depression | Not tested |
| Fink et al. (2010) [200] | 50 | MS | 44.8 8.2 | 4–5 d × 6 w | RCT | Control group | EF or visual CCT trainings | Increased EF and verbal learning | 12 months |
| Cerasa et al. (2013) [206] | 23 | MS | 31.7 9.2 | 2 d × 6 w | RCT | Control group | Different attentional trainings | Increased attentional abilities and SPL activity | Not tested |
| Filippi et al. (2012) [207] | 20 | MS | 46.7 | 3 d × 12 w | RCT | Control group | Attention, Information Processing, EF trainings | Increased cognitive functions and increased activity in fronto-parietal regions | Not tested |
| Parisi et al. (2014) [208] | 18 | MS | 43.6 | 12 weeks | RCT | Control group | Attention, Information Processing, EF trainings | Increased cognitive functions and changes in FC | 6 months |
| Sandroff et al. (2017) [209] | 8 | MS | 43.5 10 | 3 d × 12 w | Pilot RCT | Control group | Treadmill walking | Increased learning and memory abilities and related changes in hippocampal viscoelastic properties | Not tested |
3.4.2. Virtual Reality Training (VRT)
4. Conclusions
| Number, Authors, Published Year | Sample (n) | Diagnosis | Mean Age (Years) (SD) | Duration (Days × Weeks) | Study Type | Control | Virtual-Reality Training Used | Main Results | Duration Post-Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Manera et al. (2016) [126] | 57 | MCI/AD | 75.6 7 | 1 session | Feasibility study | VR task vs. Paper-pencil task | Attentional task (Selective and sustained attention) | Increased satisfaction and preference to VR-task | Not tested |
| White and Moussavi (2016) [130] | 1 | AD | 74 | 1 d × 7 w | Case study | None | Virtual Reality Navigation environment | Improved navigation skill | 5 weeks/28 weeks |
| Serino et al. (2017) [136] | 20 | AD | 87.6 4.8 | 3 d × 3/4 w | Development-of-Concept Trial | Control Group | VR-training for spatial abilities | Improved long-term Spatial memory | Not tested |
| Caggianese et al. (2018) [145] | - | - | - | - | Project study | - | VR for realistic enviroment | - | Not tested |
| Quintana and Favela (2012) [146] | 6 | Healthy subjects | 28 | 1 session | Project study | None | Ambient aNnotation System (ANS) | Improved recognition of tags with audio notifications | Not tested |
| Rohrbach et al. (2019) [148] | 10 | AD | 71.8 11.1 | 1 session | Crossover study | AR condition vs. Natural condition | Therapy Lens (Microsoft HololensTM) | Trend in diminished sequencing errors | Not tested |
| Aruanno and Garzotto (2019) [149] | 11 | MCI | 84.1 7.2 | 1 session | Feasibility study | None | MemHolo (Mixed Reality HoloLensTM) | Positive evaluation of MemHolo | Not tested |
| Burdea et al. (2015) [164] | 1 | PPA | 51 | 2 d × 8 w | Single-case study | None | BrightBrainerTM | Improved verbal skills | Not tested |
| Robles-Garcia et al. (2016) [183] | 16 | PD | 66.6 9.5 | 4 w | Randomized controlled pilot-study | Active-control group | VR-Motor imitation | Decreased hypometria | Not tested |
| de Melo et al. (2018) [185] | 37 | PD | 62.2 10.6 | 3 d × 4 w | Randomized, controlled clinical study | Control group, Treadmill group | VR-Gait training | Improved gait | 30 days |
| Janeh et al. (2019) [190] | 15 | PD | 67.6 7 | 1 session | Pilot study | Natural gait vs. VR-gait | VR-Gait training (GAITRiteTM; CIR Systems, Inc., Franklin, NJ, USA) | Improved gait | Not tested |
| de Menezes Sanguinet et al. (2016) [191] | 14 | PD | 64 9 | 6 m | Uncontrolled clinical study | None | Non-immersive virtual reality games with KinectTM (One Microsoft Way, Redmond, WA, USA) | Improved PDQ-39 scores and mobility/cognitive skills | Not tested |
| Maggio et al. (2018) [192] | 20 | PD | 69.4 8.2 | 3 d × 8 w | Randomized Clinical study | Control group | BTS Nirvana (BTS-N) | Improved cognitive function | Not tested |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Erkkinen, M.; Kim, M.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar]
- Hill, N.T.M.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar]
- De la Monte, S.M. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989, 25, 450–459. [Google Scholar]
- Boublay, N.; Schott, A.M.; Krolak-Salmon, P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: A review of 20 years of research. Eur. J. Neurol. 2016, 23, 1500–1509. [Google Scholar]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar]
- Squire, L.R.; Zola-Morgan, S. The medial temporal lobe memory system. Science 1991, 253, 1380–1386. [Google Scholar]
- Levy, D.A.; Bayley, P.J.; Squire, L.R. The anatomy of semantic knowledge: Medial vs. lateral temporal lobe. Proc. Natl. Acad. Sci. USA 2004, 101, 6710–6715. [Google Scholar]
- O’keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Oxford Clarendon Press: Oxford, UK, 1978. [Google Scholar]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar]
- Kálmán, J.; Maglóczky, E.; Janka, Z. Disturbed visuo-spatial orientation in the early stage of Alzheimer’s dementia. Arch. Gerontol. Geriatr. 1995, 21, 27–34. [Google Scholar]
- Yew, B.; Alladi, S.; Shailaja, M.; Hodges, J.R.; Hornberger, M. Lost and forgotten? Orientation versus memory in Alzheimer’s disease and frontotemporal dementia. J. Alzheimer’s Dis. 2013, 33, 473–481. [Google Scholar]
- Smits, L.L.; Pijnenburg, Y.A.; Koedam, E.L.; van der Vlies, A.E.; Reuling, I.E.; Koene, T.; Teunissen, C.E.c.; Scheltens, P.; van der Flier, W.M. Early onset Alzheimer’s disease is associated with a distinct neuropsychological profile. J. Alzheimer’s Dis. 2012, 30, 101–108. [Google Scholar]
- Braak, H.; Braak, E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 2012, 94, 3–12. [Google Scholar]
- Weintraub, S.; Wicklund, A.H.; Salmon, D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006171. [Google Scholar]
- Stopford, C.L.; Thompson, J.C.; Neary, D.; Richardson, A.M.; Snowden, J.S. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex 2012, 48, 429–446. [Google Scholar]
- Perry, R.J.; Hodges, J.R. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology 2000, 54, 2277–2284. [Google Scholar]
- Koeppe, R.A.; Gilman, S.; Junck, L.; Wernette, K.; Frey, K.A. Differentiating Alzheimer’s disease from dementia with Lewy bodies and Parkinson’s disease with (+)-[11C] dihydrotetrabenazine positron emission tomography. Alzheimer’s Dement. 2008, 4, S67–S76. [Google Scholar]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Quality of life in Alzheimer’s disease: Patient and caregiver reports. J. Ment. Health Aging 1999, 5, 21–32. [Google Scholar]
- Bell, C.M.; Araki, S.S.; Neumann, P.J. The association between caregiver burden and caregiver health-related quality of life in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2001, 15, 129–136. [Google Scholar]
- Woods, B.; Thorgrimsen, L.; Spector, A.; Royan, L.; Orrell, M. Improved quality of life and cognitive stimulation therapy in dementia. Aging Ment. Health 2006, 10, 219–226. [Google Scholar]
- Cummings, J.L.; Vinters, H.V.; Cole, G.M.; Khachaturian, Z.S. Alzheimer’s disease: Etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 1998, 51 (Suppl. S1), S2–S17. [Google Scholar]
- McLellan, D.L. Functional recovery and the principles of disability medicine. Clin. Neurol. 1991, 1, 768–790. [Google Scholar]
- Wilson, B.A. Towards a comprehensive model of cognitive rehabilitation. Neuropsychol. Rehabil. 2002, 12, 97–110. [Google Scholar]
- Weder, N.D.; Aziz, R.; Wilkins, K.; Tampi, R.R. Frontotemporal dementia: A review. Ann. Gen. Psychiatry 2007, 6, 15. [Google Scholar]
- Miller, B.L. Clinical advances in degenerative dementias. Br. J. Psychiatry 1997, 171, 1–3. [Google Scholar]
- Poletti, M.; Cavallo, M.; Adenzato, M. Detecting dysexecutive syndrome in neurodegenerative diseases: Are we using an appropriate approach and effective diagnostic tools? J. Neurol. Neurosurg. Psychiatry 2017, 88, 195. [Google Scholar]
- Frisoni, G.B.; Laakso, M.P.; Beltramello, A.; Geroldi, C.; Bianchetti, A.; Soininen, H.; Trabucchi, M. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology 1999, 52, 91. [Google Scholar]
- Huang, H.C.; Jiang, Z.F. Accumulated amyloid-β peptide and hyperphosphorylated tau protein: Relationship and links in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 15–27. [Google Scholar]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar]
- Talbot, P.R.; Snowden, J.S.; Lloyd, J.J.; Neary, D.; Testa, H.J. The contribution of single-photon emission tomography to the clinical differentiation of degenerative cortical brain disorders. J. Neurol. 1995, 242, 579–586. [Google Scholar]
- Roberson, E.D.; Hesse, J.H.; Rose, K.D.; Slama, H.; Johnson, J.K.; Yaffe, K.; Forman, M.S.; Miller, C.A.; Trojanowski, J.Q.; Kramer, J.H. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology 2005, 65, 719–725. [Google Scholar]
- Boxer, A.L.; Miller, B.L. Clinical features of frontotemporal dementia. Alzheimer Dis. Assoc. Disord. 2005, 19, S3–S6. [Google Scholar]
- Snowden, J.S.; Bathgate, D.; Varma, A.; Blackshaw, A.; Gibbons, Z.C.; Neary, D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 323–332. [Google Scholar]
- Adenzato, M.; Cavallo, M.; Enrici, I. Theory of mind ability in the behavioural variant of frontotemporal dementia: An analysis of the neural, cognitive, and social levels. Neuropsychologia 2010, 48, 2–12. [Google Scholar]
- Williams, G.B.; Nestor, P.J.; Hodges, J.R. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage 2005, 24, 1042–1051. [Google Scholar]
- Snowden, J.S.; Pickering-Brown, S.M.; Mackenzie, I.R.; Richardson, A.M.T.; Varma, A.; Neary, D.; Mann, D.M.A. Progranulin gene mutations associated with frontotemporal dementia and progressive nonfluent aphasia. Brain 2006, 129, 3091–3102. [Google Scholar]
- Kuca, K.; Klimova, B.; Novotny, M. Semantic Dementia: Mini-Review. Mini-Rev. Med. Chem. 2017, 18, 3–8. [Google Scholar]
- Gorno-Tempini, M.L.; Murray, R.C.; Rankin, K.P.; Weiner, M.W.; Miller, B.L. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: A case report. Neurocase 2004, 10, 426–436. [Google Scholar]
- Hodges, J.R.; Patterson, K. Nonfluent progressive aphasia and semantic dementia: A comparative neuropsychological study. J. Int. Neuropsychol. Soc. 1996, 2, 511–524. [Google Scholar]
- Aarsland, D.; Kurz, M.W. The epidemiology of dementia associated with Parkinson disease. J. Neurol. Sci. 2010, 289, 18–22. [Google Scholar]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar]
- Enrici, I.; Adenzato, M.; Ardito, R.B.; Mitkova, A.; Cavallo, M.; Zibetti, M.; Lopiano, L.; Castelli, L. Emotion processing in Parkinson’s disease: A three-level study on recognition, representation, and regulation. PLoS ONE 2015, 10, e0131470. [Google Scholar]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1689–1707. [Google Scholar]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.; Poewe, W.; Rascol, O.; Goetz, C.G. The Movement Disorder Society evidence-based medicine review update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26, S42–S80. [Google Scholar]
- Rolinski, M.; Fox, C.; Maidment, I.; McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Marson, F.; Lasaponara, S.; Cavallo, M. A scoping review of neuromodulation techniques in neurodegenerative diseases: A useful tool for clinical practice? Medicina 2021, 57, 215. [Google Scholar]
- Amato, M.P.; Zipoli, V.; Portaccio, E. Multiple sclerosis-related cognitive changes: A review of cross-sectional and longitudinal studies. J. Neurol. Sci. 2006, 245, 41–46. [Google Scholar]
- Borghi, M.; Cavallo, M.; Carletto, S.; Ostacoli, L.; Zuffranieri, M.; Picci, R.L.; Scavelli, F.; Johnston, H.; Furlan, P.M.; Bertolotto, A.; et al. Presence and significant determinants of cognitive impairment in a large sample of patients with multiple sclerosis. PLoS ONE 2013, 8, e69820. [Google Scholar]
- Borghi, M.; Carletto, S.; Ostacoli, L.; Scavelli, F.; Pia, L.; Pagani, M.; Bertolotto, A.; Malucchi, S.; Signori, A.; Cavallo, M.; et al. Decline of neuropsychological abilities in a large sample of patients with Multiple Sclerosis: A two-year longitudinal study. Front. Hum. Neurosci. 2016, 10, 282. [Google Scholar]
- Carletto, S.; Borghi, M.; Scavelli, F.; Francone, D.; Perucchini, M.L.; Cavallo, M.; Pagnini, F.; Bertolotto, A.; Oliva, F.; Ostacoli, L. Prevalence of posttraumatic stress disorder in patients with multiple sclerosis. J. Ment. Nerv. Dis. 2018, 206, 149–151. [Google Scholar]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar]
- Sokolov, A.A.; Collignon, A.; Bieler-Aeschlimann, M. Serious video games and virtual reality for prevention and neurorehabilitation of cognitive decline because of aging and neurodegeneration. Curr. Opin. Neurol. 2020, 33, 239–248. [Google Scholar]
- Van Den Berg, S.; Shapiro, D.A.; Bickerstaffe, D.; Cavanagh, K. Computerized cognitive–behaviour therapy for anxiety and depression: A practical solution to the shortage of trained therapists. J. Psychiatr. Ment. Health Nurs. 2004, 11, 508–513. [Google Scholar]
- Kueider, A.M.; Parisi, J.M.; Gross, A.L.; Rebok, G.W. Computerized cognitive training with older adults: A systematic review. PLoS ONE 2012, 7, e40588. [Google Scholar]
- Bakker, D.; Kazantzis, N.; Rickwood, D.; Rickard, N. Mental health smartphone apps: Review and evidence-based recommendations for future developments. JMIR Ment. Health 2016, 3, e7. [Google Scholar]
- Torous, J.; Staples, P.; Fenstermacher, E.; Dean, J.; Keshavan, M. Barriers, benefits, and beliefs of brain training smartphone apps: An internet survey of younger US consumers. Front. Hum. Neurosci. 2016, 10, 180. [Google Scholar]
- Bohil, C.J.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752–762. [Google Scholar]
- Baus, O.; Bouchard, S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: A review. Front. Hum. Neurosci. 2014, 8, 112. [Google Scholar]
- Clay, F.; Howett, D.; FitzGerald, J.; Fletcher, P.; Chan, D.; Price, A. Use of Immersive Virtual Reality in the Assessment and Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 75, 23–43. [Google Scholar]
- Manjrekar, S.; Sandilya, S.; Bhosale, D.; Kanchi, S.; Pitkar, A.; Gondhalekar, M. CAVE: An Emerging Immersive Technology—A Review. In Proceedings of the 2014 UKSim-AMSS 16th International Conference on Computer Modelling and Simulation IEEE, Cambridge, UK, 26–28 March 2014; pp. 131–136. [Google Scholar]
- Paszkiel, S. Using BCI and VR technology in neurogaming. In Analysis and Classification of EEG Signals for Brain–Computer Interfaces; Springer: Cham, Switzerland, 2020; pp. 93–99. [Google Scholar]
- Teo, W.P.; Muthalib, M.; Yamin, S.; Hendy, A.M.; Bramstedt, K.; Kotsopoulos, E.; Perrey, S.; Ayaz, H. Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation?—A narrative review of the literature. Front. Hum. Neurosci. 2016, 10, 284. [Google Scholar]
- Paszkiel, S. Augmented Reality (AR) Technology in Correlation with Brain–Computer Interface Technology. In Analysis and Classification of EEG Signals for Brain–Computer Interfaces; Springer: Cham, Switzerland, 2020; pp. 87–91. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar]
- Clare, L.; Woods, R.T. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: A review. Neuropsychol. Rehabil. 2004, 14, 385–401. [Google Scholar]
- Buschert, V.; Bokde, A.L.; Hampel, H. Cognitive intervention in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 508. [Google Scholar]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer’s or vascular type: A review. Alzheimer’s Res. Ther. 2013, 5, 35. [Google Scholar]
- Alves, J.; Magalhaes, R.; Thomas, R.E.; Goncalves, O.F.; Petrosyan, A.; Sampaio, A. Is there evidence for cognitive intervention in Alzheimer disease? A systematic review of efficacy, feasibility, and cost-effectiveness. Alzheimer Dis. Assoc. Disord. 2013, 27, 195–203. [Google Scholar]
- Choi, J.; Twamley, E.W. Cognitive rehabilitation therapies for Alzheimer’s disease: A review of methods to improve treatment engagement and self-efficacy. Neuropsychol. Rev. 2013, 23, 48–62. [Google Scholar]
- Passaro, A.; Soavi, C.; Marusic, U.; Rejc, E.; Sanz, J.M.; Morieri, M.L.; Nora, E.D.; Kavcic, V.; Narici, M.V.; Reggiani, C.; et al. Computerized cognitive training and brain derived neurotrophic factor during bed rest: Mechanisms to protect individual during acute stress. Aging 2017, 9, 393. [Google Scholar]
- Bodner, K.A.; Goldberg, T.E.; Devanand, D.P.; Doraiswamy, P.M. Advancing computerized cognitive training for early Alzheimer’s disease in a pandemic and post-pandemic world. arXiv 2020, arXiv:2004.14344. [Google Scholar]
- Simons, D.J.; Boot, W.R.; Charness, N.; Gathercole, S.E.; Chabris, C.F.; Hambrick, D.Z.; Stine-Morrow, E.A. Do “brain-training” programs work? Psychol. Sci. Public Interest 2016, 17, 103–186. [Google Scholar]
- García-Betances, R.I.; Cabrera-Umpiérrez, M.F.; Arredondo, M.T. Computerized neurocognitive interventions in the context of the brain training controversy. Rev. Neurosci. 2017, 29, 55–69. [Google Scholar]
- Tetlow, A.M.; Edwards, J.D. Systematic literature review and meta-analysis of commercially available computerized cognitive training among older adults. J. Cogn. Enhanc. 2017, 1, 559–575. [Google Scholar]
- Harvey, P.D.; McGurk, S.R.; Mahncke, H.; Wykes, T. Controversies in computerized cognitive training. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 907–915. [Google Scholar]
- Gates, N.J.; Vernooij, R.W.; Di Nisio, M.; Karim, S.; March, E.; Martinez, G.; Rutjes, A.W. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Yong, E.; The Weak Evidence behind Brain-Training Games. The Atlantic. 2016. Available online: http://www.theatlantic.com/science/archive/2016/10/the-weak-evidence-behind-braintraining-games/502559/ (accessed on 5 June 2020).
- Lampit, A.; Hallock, H.; Valenzuela, M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med. 2014, 11, e1001756. [Google Scholar]
- Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Shah, P. Short-and long-term benefits of cognitive training. Proc. Natl. Acad. Sci. USA 2011, 108, 10081–10086. [Google Scholar]
- Cavallo, M.; Angilletta, C. Long-Lasting Neuropsychological Effects of a Computerized Cognitive Training in Patients Affected by Early Stage Alzheimer’s Disease: Are They Stable Over Time? J. Appl. Gerontol. 2019, 38, 1035–1044. [Google Scholar]
- Hu, M.; Wu, X.; Shu, X.; Hu, H.; Chen, Q.; Peng, L.; Feng, H. Effects of computerised cognitive training on cognitive impairment: A meta-analysis. J. Neurol. 2019, 1–9. [Google Scholar] [CrossRef]
- Leung, N.T.; Tam, H.M.; Chu, L.W.; Kwok, T.C.; Chan, F.; Lam, L.C.; Woo, J.; Lee, T. Neural plastic effects of cognitive training on aging brain. Neural Plast. 2015. [Google Scholar] [CrossRef]
- Koepsell, T.D.; Monsell, S.E. Reversion from mild cognitive impairment to normal or near-normal cognition: Risk factors and prognosis. Neurology 2012, 79, 1591–1598. [Google Scholar]
- Alescio-Lautier, B.; Sambucchi, N.; Michel, B.F.; Chambon, C. Multifactorial Cognitive Training can Slow Down the Cognitive Decline in Early Alzheimer Patients. J. Alzheimer’s Dis. Parkinsonism 2019, 9, 1000470. [Google Scholar]
- Cavallo, M.; Hunter, E.M.; van der Hiele, K.; Angilletta, C. Computerized structured cognitive training in patients affected by early-stage Alzheimer’s disease is feasible and effective: A randomized controlled study. Arch. Clin. Neuropsychol. 2016, 31, 868–876. [Google Scholar]
- Rodríguez-Mora, Á.; Cordón, J.R.; de la Torre, G.G.; Mestre, J.M. The Impact of a Twelve-Month Comprehensive Program of Cognitive Training for Alzheimer Patients: A Pilot Study. Psychiatry Int. 2020, 1, 83–97. [Google Scholar]
- Imbeault, H.; Langlois, F.; Bocti, C.; Gagnon, L.; Bier, N. Can people with Alzheimer’s disease improve their day-to-day functioning with a tablet computer? Neuropsychol. Rehabil. 2018, 28, 779–796. [Google Scholar]
- De Leo, G.; Brivio, E.; Sautter, S.W. Supporting autobiographical memory in patients with Alzheimer’s disease using smart phones. Appl. Neuropsychol. 2011, 18, 69–76. [Google Scholar]
- Zmily, A.; Abu-Saymeh, D. Alzheimer’s Disease rehabilitation using smartphones to improve patients’ quality of life. In Proceedings of the 2013 7th International Conference on Pervasive Computing Technologies for Healthcare and Workshops IEEE, Venice, Italy, 5–8 May 2013; pp. 393–396. [Google Scholar]
- Lizio, R.; Del Percio, C.; Noce, G.; Janson, J.; Barulli, M.R.; Logroscino, G.; Musarò, C.; Scianatico, G.; Rossini, P.M.; Lacido, G.; et al. Two weeks of a computerized cognitive training may produce beneficial effects in Alzheimer’s disease patients. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019; pp. 1276–1279. [Google Scholar]
- Vallejo, V.; Wyss, P.; Rampa, L.; Mitache, A.V.; Müri, R.M.; Mosimann, U.P.; Nef, T. Evaluation of a novel Serious Game based assessment tool for patients with Alzheimer’s disease. PLoS ONE 2017, 12, e0175999. [Google Scholar]
- Ben-Sadoun, G.; Manera, V.; Alvarez, J.; Sacco, G.; Robert, P. Recommendations for the design of serious games in neurodegenerative diseases. Front. Aging Neurosci. 2018, 10, 13. [Google Scholar]
- Savulich, G.; Piercy, T.; Fox, C.; Suckling, J.; Rowe, J.B.; O’Brien, J.T.; Sahakian, B.J. Cognitive training using a novel memory game on an iPad in patients with amnestic mild cognitive impairment (aMCI). Int. J. Neuropsychopharmacol. 2017, 20, 624–633. [Google Scholar]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer’s disease and frontotemporal dementia: A systematic review. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar]
- Barban, F.; Annicchiarico, R.; Pantelopoulos, S.; Federici, A.; Perri, R.; Fadda, L.; Carlesimo, G.A.; Ricci, C.; Giuli, S.; Scalici, F.; et al. Protecting cognition from aging and Alzheimer’s disease: A computerized cognitive training combined with reminiscence therapy. Int. J. Geriatr. Psychiatry 2016, 31, 340–348. [Google Scholar]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; D’Amico, F.; Ferlisi, G.; Floriana Denitto, F.d.; Belardinelli, M.O. Patients with moderate Alzheimer’s disease engage in verbal reminiscence with the support of a computer-aided program: A pilot study. Front. Aging Neurosci. 2015, 7, 109. [Google Scholar]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Petesi, M.; Rosini, S.; Ferrari, C.; Zanetti, O.; Miniussi, C. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front. Aging Neurosci. 2014, 6, 38. [Google Scholar]
- Bentwich, J.; Dobronevsky, E.; Aichenbaum, S.; Shorer, R.; Peretz, R.; Khaigrekht, M.; Marton, R.G.; Rabey, J.M. Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease: A proof of concept study. J. Neural Transm. 2011, 118, 463–471. [Google Scholar]
- Tárraga, L.; Boada, M.; Modinos, G.; Espinosa, A.; Diego, S.; Morera, A.; M Guitart, J.B.; Becker, J.T. A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1116–1121. [Google Scholar]
- Buschert, V.C.; Teipel, S.J.; Hampel, H.; Bürger, K. Current status of cognition-based interventions in Alzheimer’s disease. Der Nervenarzt 2009, 80, 273–287. [Google Scholar]
- Faucounau, V.; Wu, Y.H.; Boulay, M.; De Rotrou, J.; Rigaud, A.S. Cognitive intervention programmes on patients affected by mild cognitive impairment: A promising intervention tool for MCI? J. Nutr. Health Aging 2010, 14, 31–35. [Google Scholar]
- Peretz, C.; Korczyn, A.D.; Shatil, E.; Aharonson, V.; Birnboim, S.; Giladi, N. Computer-based, personalized cognitive training versus classical computer games: A randomized double-blind prospective trial of cognitive stimulation. Neuroepidemiology 2011, 36, 91–99. [Google Scholar]
- Smith, G.E.; Housen, P.; Yaffe, K.; Ruff, R.; Kennison, R.F.; Mahncke, H.W.; Zelinski, E.M. A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J. Am. Geriatr. Soc. 2009, 57, 594–603. [Google Scholar]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: An eLORETA controlled study on resting states. Neural Plast. 2015. [Google Scholar] [CrossRef]
- Barban, F.; Mancini, M.; Cercignani, M.; Adriano, F.; Perri, R.; Annicchiarico, R.; Carlesimo, G.A.; Ricci, C.; Lombardi, M.G.; Teodonno, V.; et al. A pilot study on brain plasticity of functional connectivity modulated by cognitive training in mild Alzheimer’s disease and mild cognitive impairment. Brain Sci. 2017, 7, 50. [Google Scholar]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Sekiguchi, A.; Kotozaki, Y.; Nakagawa, S.; Miyauchi, C.M.; Sassa, Y.; Kawashima, R. Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Mansouri, F.A.; Tanaka, K.; Buckley, M.J. Conflict-induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat. Rev. Neurosci. 2009, 10, 141–152. [Google Scholar]
- Donoso, M.; Collins, A.G.; Koechlin, E. Foundations of human reasoning in the prefrontal cortex. Science 2014, 344, 1481–1486. [Google Scholar]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar]
- Sescousse, G.; Redouté, J.; Dreher, J.C. The architecture of reward value coding in the human orbitofrontal cortex. J. Neurosci. 2010, 30, 13095–13104. [Google Scholar]
- Grady, C.L.; McIntosh, A.R.; Beig, S.; Keightley, M.L.; Burian, H.; Black, S.E. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J. Neurosci. 2003, 23, 986–993. [Google Scholar]
- Holthoff, V.A.; Beuthien-Baumann, B.; Kalbe, E.; Lüdecke, S.; Lenz, O.; Zündorf, G.; Spirling, S.; Schierz, K.; Winiecki, P.; Sorbi, S.; et al. Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biol. Psychiatry 2005, 57, 412–421. [Google Scholar]
- García-Betances, R.I.; Arredondo Waldmeyer, M.T.; Fico, G.; Cabrera-Umpiérrez, M.F. A succinct overview of virtual reality technology use in Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 80. [Google Scholar]
- Edler, D.; Kühne, O.; Keil, J.; Dickmann, F. Audiovisual cartography: Established and new multimedia approaches to represent soundscapes. KN-J. Cartogr. Geogr. Inf. 2019, 69, 5–17. [Google Scholar]
- Hruby, F. The sound of being there: Audiovisual cartography with immersive virtual environments. KN-J. Cartogr. Geogr. Inf. 2019, 69, 19–28. [Google Scholar]
- Mancini, M.; Cherubino, P.; Cartocci, G.; Martinez, A.; Borghini, G.; Guastamacchia, E.; di Flumeri, G.; Rossi, D.; Modica, E.; Menicocci, S.; et al. Forefront Users’ Experience Evaluation by Employing Together Virtual Reality and Electroencephalography: A Case Study on Cognitive Effects of Scents. Brain Sci. 2021, 11, 256. [Google Scholar]
- Castelvecchi, D. Low-cost headsets boost virtual reality’s lab appeal. Nature 2016, 533, 153. [Google Scholar]
- Lee, G.H. Effects of a Virtual Reality Exercise Program (Wii) on Cognitive Function of Elderly People with Alzheimer Dementia. Off. J. Korean Acad. Kinesiol. 2017, 19, 35–44. [Google Scholar]
- Westwood, J.D. Real-time 3D avatars for tele-rehabilitation in virtual reality. Med. Meets Virtual Real. 18 NextMed 2011, 163, 290. [Google Scholar]
- Lloréns, R.; Noé, E.; Naranjo, V.; Borrego, A.; Latorre, J.; Alcañiz, M. Tracking systems for virtual rehabilitation: Objective performance vs. subjective experience. A practical scenario. Sensors 2015, 15, 6586–6606. [Google Scholar]
- Schröder, J.; van Criekinge, T.; Embrechts, E.; Celis, X.; Van Schuppen, J.; Truijen, S.; Saeys, W. Combining the benefits of tele-rehabilitation and virtual reality-based balance training: A systematic review on feasibility and effectiveness. Disabil. Rehabil. Assist. Technol. 2019, 14, 2–11. [Google Scholar]
- Vilalta-Franch, J.; Calvó-Perxas, L.; Garre-Olmo, J.; Turró-Garriga, O.; López-Pousa, S. Apathy syndrome in Alzheimer’s disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. J. Alzheimer’s Dis. 2013, 33, 535–543. [Google Scholar]
- Manera, V.; Chapoulie, E.; Bourgeois, J.; Guerchouche, R.; David, R.; Ondrej, J.; Drettakis, G.; Robert, P. A feasibility study with image-based rendered virtual reality in patients with mild cognitive impairment and dementia. PLoS ONE 2016, 11, e0151487. [Google Scholar]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.M.; Hornberger, M. Spatial navigation deficits—Overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar]
- Kober, S.E.; Wood, G.; Hofer, D.; Kreuzig, W.; Kiefer, M.; Neuper, C. Virtual reality in neurologic rehabilitation of spatial disorientation. J. Neuroeng. Rehabil. 2013, 10, 17. [Google Scholar]
- Cogné, M.; Taillade, M.; N’Kaoua, B.; Tarruella, A.; Klinger, E.; Larrue, F.; Sauzéon, H.; Joseph, P.A.; Sorita, E. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: A systematic literature review. Ann. Phys. Rehabil. Med. 2017, 60, 164–176. [Google Scholar]
- White, P.J.; Moussavi, Z. Neurocognitive treatment for a patient with Alzheimer’s disease using a virtual reality navigational environment. J. Exp. Neurosci. 2016, 10, JEN-S40827. [Google Scholar]
- Byagowi, A.; Mohaddes, D.; Moussavi, Z. Design and application of a novel virtual reality navigational technology (VRNChair). J. Exp. Neurosci. 2014, 8, JEN-S13448. [Google Scholar]
- Riva, G.; Mantovani, F.; Capideville, C.S.; Preziosa, A.; Morganti, F.; Villani, D.; Gaggioli, A.; Botella, C.; Alcañiz, M. Affective interactions using virtual reality: The link between presence and emotions. CyberPsychology Behav. 2007, 10, 45–56. [Google Scholar]
- Klatzky, R.L. Allocentric and egocentric spatial representations: Definitions, distinctions, and interconnections. In Spatial Cognition; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–17. [Google Scholar]
- Serino, S.; Riva, G. Getting lost in Alzheimer’s disease: A break in the mental frame syncing. Med. Hypotheses 2013, 80, 416–421. [Google Scholar]
- Serino, S.; Cipresso, P.; Morganti, F.; Riva, G. The role of egocentric and allocentric abilities in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2014, 16, 32–44. [Google Scholar]
- Serino, S.; Pedroli, E.; Tuena, C.; De Leo, G.; Stramba-Badiale, M.; Goulene, K.; Mariotti, N.G.; Riva, G. A novel Virtual Reality-based training protocol for the enhancement of the “mental frame syncing” in individuals with Alzheimer’s Disease: A development-of-concept trial. Front. Aging Neurosci. 2017, 9, 240. [Google Scholar]
- Doniger, G.M.; Beeri, M.S.; Bahar-Fuchs, A.; Gottlieb, A.; Tkachov, A.; Kenan, H.; Livny, A.; Bahat, Y.; Sharon, H.; Ben-Gal, O.; et al. Virtual reality-based cognitive-motor training for middle-aged adults at high Alzheimer’s disease risk: A randomized controlled trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 118–129. [Google Scholar]
- Al-shammari, M.K.M.; Han, G.T. Improve Memory for Alzheimer Patient by Employing Mind Wave on Virtual Reality with Deep Learning. In Innovative Mobile and Internet Services in Ubiquitous Computing, Proceedings of the 12th International Conference on Innovative Mobile, and Internet Services in Ubiquitous Computing (IMIS-2018), Matsue, Japan, 4–6 July 2018; Springer: Cham, Switzerland, 2018; pp. 412–419. [Google Scholar]
- Lee, J.H.; Ku, J.; Cho, W.; Hahn, W.Y.; Kim, I.Y.; Lee, S.M.; Kang, Y.; Kim, D.Y.; Yu, T.; Wiederhold, B.K.; et al. A virtual reality system for the assessment and rehabilitation of the activities of daily living. CyberPsychology Behav. 2003, 6, 383–388. [Google Scholar]
- Yamaguchi, T.; Foloppe, D.A.; Richard, P.; Richard, E.; Allain, P. A dual-modal virtual reality kitchen for (re) learning of everyday cooking activities in alzheimer’s disease. Presence Teleoperators Virtual Environ. 2012, 21, 43–57. [Google Scholar]
- Foloppe, D.A.; Richard, P.; Yamaguchi, T.; Etcharry-Bouyx, F.; Allain, P. The potential of virtual reality-based training to enhance the functional autonomy of Alzheimer’s disease patients in cooking activities: A single case study. Neuropsychol. Rehabil. 2015, 28, 709–733. [Google Scholar]
- Hurley, A.C.; Gauthier, M.A.; Horvath, K.J.; Harvey, R.; Smith, S.J.; Trudeau, S.; Cipolloni, P.B.; Hendricks, A.; Duffy, M. Promoting safer home environments for persons with Alzheimer’s disease: The home safety/injury model. J. Gerontol. Nurs. 2004, 30, 43–51. [Google Scholar]
- Hargrave, R.; Reed, B.; Mungas, D. Depressive syndromes and functional disability in dementia. J. Geriatr. Psychiatry Neurol. 2000, 13, 72–77. [Google Scholar]
- Tsuno, N.; Homma, A. What is the association between depression and Alzheimer’s disease? Expert Rev. Neurother. 2009, 9, 1667–1676. [Google Scholar]
- Caggianese, G.; Chirico, A.; De Pietro, G.; Gallo, L.; Giordano, A.; Predazzi, M.; Neroni, P. Towards a virtual reality cognitive training system for mild cognitive impairment and Alzheimer’s disease patients. In Proceedings of the 2018 32nd International Conference on Advanced Information Networking and Applications Workshops (WAINA), Cracow, Poland, 16–18 May 2018; pp. 663–667. [Google Scholar]
- Quintana, E.; Favela, J. Augmented reality annotations to assist persons with Alzheimers and their caregivers. Pers. Ubiquitous Comput. 2013, 17, 1105–1116. [Google Scholar]
- Kanno, K.M.; Lamounier, E.A.; Cardoso, A.; Lopes, E.J.; de Lima, G.F.M. Augmented Reality System for Aiding Mild Alzheimer Patients and Caregivers. In Proceedings of the 2018 IEEE Conference on Virtual Reality and 3D User Interfaces (VR), Reutlingen, Germany, 18–22 March 2018; pp. 593–594. [Google Scholar]
- Rohrbach, N.; Gulde, P.; Armstrong, A.R.; Hartig, L.; Abdelrazeq, A.; Schröder, S.; Neuse, J.; Grimmer, T.; Diehl-Schmid, J.; Hermsdörfer, J. An augmented reality approach for ADL support in Alzheimer’s disease: A crossover trial. J. Neuroeng. Rehabil. 2019, 16, 1–11. [Google Scholar]
- Aruanno, B.; Garzotto, F. MemHolo: Mixed reality experiences for subjects with Alzheimer’s disease. Multimed. Tools Appl. 2019, 78, 13517–13537. [Google Scholar]
- Shinagawa, S.; Nakajima, S.; Plitman, E.; Graff-Guerrero, A.; Mimura, M.; Nakayama, K.; Miller, B.L. Non-pharmacological management for patients with frontotemporal dementia: A systematic review. J. Alzheimer’s Dis. 2015, 45, 283–293. [Google Scholar]
- Boxer, A.L.; Boeve, B.F. Frontotemporal dementia treatment: Current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis. Assoc. Disord. 2007, 21, S79–S87. [Google Scholar]
- Manoochehri, M.; Huey, E.D. Diagnosis and management of behavioral issues in frontotemporal dementia. Curr. Neurol. Neurosci. Rep. 2012, 12, 528–536. [Google Scholar]
- Carthery-Goulart, M.T.; Silveira, A.D.C.D.; Machado, T.H.; Mansur, L.L.; Parente, M.A.D.M.P.; Senaha, M.L.H.; Brucki, S.M.D.; Nitrini, R. Non-pharmacological interventions for cognitive impairments following primary progressive aphasia: A systematic review of the literature. Dement. Neuropsychol. 2013, 7, 122–131. [Google Scholar]
- Jokel, R.; Graham, N.L.; Rochon, E.; Leonard, C. Word retrieval therapies in primary progressive aphasia. Aphasiology 2014, 28, 1038–1068. [Google Scholar]
- Croot, K. Treatment for lexical retrieval impairments in primary progressive aphasia: A research update with implications for clinical practice. In Seminars in Speech and Language; Thieme Medical Publishers: New York, NY, USA, 2018; Volume 39, pp. 242–256. [Google Scholar]
- Newhart, M.; Davis, C.; Kannan, V.; Heidler-Gary, J.; Cloutman, L.; Hillis, A.E. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology 2009, 23, 823–834. [Google Scholar]
- Evans, W.S.; Quimby, M.; Dickey, M.W.; Dickerson, B.C. Relearning and retaining personally-relevant words using computer-based flashcard software in primary progressive aphasia. Front. Hum. Neurosci. 2016, 10, 561. [Google Scholar]
- Croot, K.; Raiser, T.; Taylor-Rubin, C.; Ruggero, L.; Ackl, N.; Wlasich, E.; Danek, A.; Scharfenberg, A.; Foxe, D.; Hodges, J.R.; et al. Lexical retrieval treatment in primary progressive aphasia: An investigation of treatment duration in a heterogeneous case series. Cortex 2019, 115, 133–158. [Google Scholar]
- Henry, M.L.; Hubbard, H.I.; Grasso, S.M.; Dial, H.R.; Beeson, P.M.; Miller, B.L.; Gorno-Tempini, M.L. Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. J. Speech Lang. Hear. Res. 2019, 62, 2723–2749. [Google Scholar]
- Cadório, I.; Lousada, M.; Martins, P.; Figueiredo, D. Generalization and maintenance of treatment gains in primary progressive aphasia (PPA): A systematic review. Int. J. Lang. Commun. Disord. 2017, 52, 543–560. [Google Scholar]
- Beeson, P.M.; King, R.M.; Bonakdarpour, B.; Henry, M.L.; Cho, H.; Rapcsak, S.Z. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. J. Mol. Neurosci. 2011, 45, 724–736. [Google Scholar]
- Macoir, J.; Leroy, M.; Routhier, S.; Auclair-Ouellet, N.; Houde, M.; Laforce Jr, R. Improving verb anomia in the semantic variant of primary progressive aphasia: The effectiveness of a semantic-phonological cueing treatment. Neurocase 2015, 21, 448–456. [Google Scholar]
- Conroy, P.; Sage, K.; Lambon Ralph, M.A. The effects of decreasing and increasing cue therapy on improving naming speed and accuracy for verbs and nouns in aphasia. Aphasiology 2009, 23, 707–730. [Google Scholar]
- Coelho, C.A.; McHugh, R.E.; Boyle, M. Semantic feature analysis as a treatment for aphasic dysnomia: A replication. Aphasiology 2000, 14, 133–142. [Google Scholar]
- Dial, H.R.; Hinshelwood, H.A.; Grasso, S.M.; Hubbard, H.I.; Gorno-Tempini, M.L.; Henry, M.L. Investigating the utility of teletherapy in individuals with primary progressive aphasia. Clin. Interv. Aging 2019, 14, 453. [Google Scholar]
- Lavoie, M.; Bier, N.; Laforce, R., Jr.; Macoir, J. Improvement in functional vocabulary and generalization to conversation following a self-administered treatment using a smart tablet in primary progressive aphasia. Neuropsychol. Rehabil. 2019, 30, 1224–1254. [Google Scholar]
- Meyer, A.M.; Getz, H.R.; Brennan, D.M.; Hu, T.M.; Friedman, R.B. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology 2016, 30, 483–507. [Google Scholar]
- Moyle, W.; Jones, C.; Dwan, T.; Petrovich, T. Effectiveness of a virtual reality forest on people with dementia: A mixed methods pilot study. Gerontologist 2018, 58, 478–487. [Google Scholar]
- Tarnanas, I.; Schlee, W.; Tsolaki, M.; Müri, R.; Mosimann, U.; Nef, T. Ecological validity of virtual reality daily living activities screening for early dementia: Longitudinal study. J. Med. Internet Res. Serious Games 2013, 1, e1. [Google Scholar]
- Hodge, J.; Balaam, M.; Hastings, S.; Morrissey, K. Exploring the design of tailored virtual reality experiences for people with dementia. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21–26 April 2018; pp. 1–13. [Google Scholar]
- Burdea, G.C.; Polistico, K.; House, G.P.; Liu, R.R.; Muniz, R.; Macaro, N.A.; Slater, L.M. Novel integrative virtual rehabilitation reduces symptomatology of primary progressive aphasia-a case report. Int. J. Neurosci. 2015, 125, 949–958. [Google Scholar]
- Leung, I.H.; Walton, C.C.; Hallock, H.; Lewis, S.J.; Valenzuela, M.; Lampit, A. Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 2015, 85, 1843–1851. [Google Scholar]
- Vemuri, P.; Fields, J.; Peter, J.; Klöppel, S. Cognitive interventions in Alzheimer’s and Parkinson’s diseases: Emerging mechanisms and role of imaging. Curr. Opin. Neurol. 2016, 29, 405. [Google Scholar]
- Walton, C.C.; Naismith, S.L.; Lampit, A.; Mowszowski, L.; Lewis, S.J. Cognitive training in Parkinson’s disease: A theoretical perspective. Neurorehabilit. Neural Repair 2017, 31, 207–216. [Google Scholar]
- Walton, C.C.; Mowszowski, L.; Gilat, M.; Hall, J.M.; O’Callaghan, C.; Muller, A.J.; Georgiades, M.; Szeto, J.Y.Y.; Ehgoetz Martens, K.A.; Shine, J.M.; et al. Cognitive training for freezing of gait in Parkinson’s disease: A randomized controlled trial. NPJ Parkinson’s Dis. 2018, 4, 15. [Google Scholar]
- Sinforiani, E.; Banchieri, L.; Zucchella, C.; Pacchetti, C.; Sandrini, G. Cognitive rehabilitation in Parkinson’s disease. Arch. Gerontol. Geriatr. 2004, 387–391. [Google Scholar] [CrossRef]
- Petrelli, A.; Kaesberg, S.; Barbe, M.T.; Timmermann, L.; Rosen, J.B.; Fink, G.R.; Kessler, J.; Kalbe, E. Cognitive training in Parkinson’s disease reduces cognitive decline in the long term. Eur. J. Neurol. 2015, 22, 640–647. [Google Scholar]
- Nombela, C.; Bustillo, P.J.; Castell, P.; Medina, V.; Herrero, M.T. Cognitive rehabilitation in Parkinson’s disease: Evidence from neuroimaging. Front. Neurol. 2011, 2, 82. [Google Scholar]
- Cerasa, A.; Gioia, M.C.; Salsone, M.; Donzuso, G.; Chiriaco, C.; Realmuto, S.; Nicoletti, A.; Bellavia, G.; Banco, A.; D’Amelio, M.; et al. Neurofunctional correlates of attention rehabilitation in Parkinson’s disease: An explorative study. Neurol. Sci. 2014, 35, 1173–1180. [Google Scholar]
- Díez-Cirarda, M.; Ojeda, N.; Peña, J.; Cabrera-Zubizarreta, A.; Lucas-Jiménez, O.; Gómez-Esteban, J.C.; Gomez-Beldarrain, M.A.; Ibarretxe-Bilbao, N. Long-term effects of cognitive rehabilitation on brain, functional outcome and cognition in Parkinson’s disease. Eur. J. Neurol. 2017, 25, 5–12. [Google Scholar]
- Angelucci, F.; Peppe, A.; Carlesimo, G.A.; Serafini, F.; Zabberoni, S.; Barban, F.; Shofany, J.; Caltagirone, C.; Costa, A. A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson’s disease. Front. Hum. Neurosci. 2015, 9, 130. [Google Scholar]
- Brehmer, Y.; Westerberg, H.; Bellander, M.; Fürth, D.; Karlsson, S.; Bäckman, L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett. 2009, 467, 117–120. [Google Scholar]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; García-Sancho, C.; Sanmartín, G.; Flores, J.; Cudeiro, J.; Arias, P. Effects of movement imitation training in Parkinson’s disease: A virtual reality pilot study. Parkinsonism Relat. Disord. 2016, 26, 17–23. [Google Scholar]
- Cantello, R.; Tarletti, R.; Civardi, C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res. Rev. 2002, 38, 309–327. [Google Scholar]
- De Melo, G.E.L.; Kleiner, A.F.R.; Lopes, J.B.P.; Dumont, A.J.L.; Lazzari, R.D.; Galli, M.; Oliveira, C.S. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. NeuroRehabilitation 2018, 42, 473–480. [Google Scholar]
- Lazzari, R.D.; Politti, F.; Belina, S.F.; Collange Grecco, L.A.; Santos, C.A.; Dumont, A.J.L.; Palma Lopes, J.B.; Cimolin, V.; Galli, M.; Santos Oliveira, C. Effect of transcranial direct current stimulation combined with virtual reality training on balance in children with cerebral palsy: A randomized, controlled, double-blind, clinical trial. J. Mot. Behav. 2017, 49, 329–336. [Google Scholar]
- Boletsis, C.; Cedergren, J.E. VR locomotion in the new era of virtual reality: An empirical comparison of prevalent techniques. Adv. Hum.-Comput. Interact. 2019, 2019, 7420781. [Google Scholar]
- Keil, J.; Edler, D.; O’Meara, D.; Korte, A.; Dickmann, F. Effects of Virtual Reality Locomotion Techniques on Distance Estimations. ISPRS Int. J. Geo-Inf. 2021, 10, 150. [Google Scholar]
- Cherep, L.A.; Lim, A.F.; Kelly, J.W.; Acharya, D.; Velasco, A.; Bustamante, E.; Ostrander, A.G.; Gilbert, S.B. Spatial cognitive implications of teleporting through virtual environments. J. Exp. Psychol. Appl. 2020, 26, 480–492. [Google Scholar]
- Janeh, O.; Fründt, O.; Schönwald, B.; Gulberti, A.; Buhmann, C.; Gerloff, C.; Steinicke, F.; Pötter-Nerger, M. Gait Training in Virtual Reality: Short-Term Effects of Different Virtual Manipulation Techniques in Parkinson’s Disease. Cells 2019, 8, 419. [Google Scholar]
- De Menezes Sanguinet, D.C.; de Sales, M.D.G.W.; de Santana, C.M.F.; de Albuquerque Ângelo, T.D.; de Araújo Silva, J.P.; Câmara, S.B.; Asano, A.G.; Lins, O.G. Quality of life of people with Parkinson’s disease after treatment with non-immersive virtual reality. Acta Fisiátrica 2016, 23, 85–88. [Google Scholar]
- Maggio, M.G.; De Cola, M.C.; Latella, D.; Maresca, G.; Finocchiaro, C.; La Rosa, G.; Cimino, V.; Sorbera, C.; Bramanti, P.; De Luca, R.; et al. What About the Role of Virtual Reality in Parkinson Disease’s Cognitive Rehabilitation? Preliminary Findings from a Randomized Clinical Trial. J. Geriatr. Psychiatry Neurol. 2018, 31, 312–318. [Google Scholar]
- Mitolo, M.; Venneri, A.; Wilkinson, I.D.; Sharrack, B. Cognitive rehabilitation in multiple sclerosis: A systematic review. J. Neurol. Sci. 2015, 354, 1–9. [Google Scholar]
- Goverover, Y.; Chiaravalloti, N.D.; O’Brien, A.R.; DeLuca, J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: An updated review of the literature from 2007 to 2016. Arch. Phys. Med. Rehabil. 2018, 99, 390–407. [Google Scholar]
- O’Brien, A.R.; Chiaravalloti, N.; Goverover, Y.; DeLuca, J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: A review of the literature. Arch. Phys. Med. Rehabil. 2008, 89, 761–769. [Google Scholar]
- Pérez-Martín, M.Y.; González-Platas, M.; Eguía-del Río, P.; Croissier-Elías, C.; Sosa, A.J. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 245. [Google Scholar]
- Charvet, L.E.; Yang, J.; Shaw, M.T.; Sherman, K.; Haider, L.; Xu, J.; Krupp, L.B. Cognitive function in multiple sclerosis improves with telerehabilitation: Results from a randomized controlled trial. PLoS ONE 2017, 12, e0177177. [Google Scholar]
- Brissart, H.; Leroy, M.; Morele, E.; Baumann, C.; Spitz, E.; Debouverie, M. Cognitive rehabilitation in multiple sclerosis. Neurocase 2013, 19, 553–565. [Google Scholar]
- Mattioli, F.; Stampatori, C.; Zanotti, D.; Parrinello, G.; Capra, R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J. Neurol. Sci. 2010, 288, 101–105. [Google Scholar]
- Fink, F.; Rischkau, E.; Butt, M.; Klein, J.; Eling, P.; Hildebrandt, H. Efficacy of an executive function intervention programme in MS: A placebo-controlled and pseudo-randomized trial. Mult. Scler. J. 2010, 16, 1148–1151. [Google Scholar]
- Sastre-Garriga, J.; Alonso, J.; Renom, M.; Arévalo, M.J.; González, I.; Galán, I.; Montalban, X.; Rovira, A. A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Mult. Scler. J. 2011, 17, 457–467. [Google Scholar]
- Chiaravalloti, N.D.; Wylie, G.; Leavitt, V.; DeLuca, J. Increased cerebral activation after behavioral treatment for memory deficits in MS. J. Neurol. 2012, 259, 1337–1346. [Google Scholar]
- Staffen, W.; Mair, A.; Zauner, H.; Unterrainer, J.; Niederhofer, H.; Kutzelnigg, A.; Ritter, S.; Golaszewski, B.; Iglseder, B.; Ladurner, G. Cognitive function and fMRI in patients with multiple sclerosis: Evidence for compensatory cortical activation during an attention task. Brain 2002, 125, 1275–1282. [Google Scholar]
- Forn, C.; Barros-Loscertales, A.; Escudero, J.; Belloch, V.; Campos, S.; Parcet, M.A.; Ávila, C. Cortical reorganization during PASAT task in MS patients with preserved working memory functions. Neuroimage 2006, 31, 686–691. [Google Scholar]
- Mainero, C.; Caramia, F.; Pozzilli, C.; Pisani, A.; Pestalozza, I.; Borriello, G.; Bozzao, L.; Pantano, P. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 2004, 21, 858–867. [Google Scholar]
- Cerasa, A.; Gioia, M.C.; Valentino, P.; Nisticò, R.; Chiriaco, C.; Pirritano, D.; Tomaiuolo, F.; Mangone, G.; Trotta, M.; Talarico, T.; et al. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: A randomized trial with fMRI correlates. Neurorehabilit. Neural Repair 2013, 27, 284–295. [Google Scholar]
- Filippi, M.; Riccitelli, G.; Mattioli, F.; Capra, R.; Stampatori, C.; Pagani, E.; Valsasina, P.; Copetti, M.; Falini, A.; Comi, G.; et al. Multiple sclerosis: Effects of cognitive rehabilitation on structural and functional MR imaging measures—An explorative study. Radiology 2012, 262, 932–940. [Google Scholar]
- Parisi, L.; Rocca, M.A.; Mattioli, F.; Copetti, M.; Capra, R.; Valsasina, P.; Stampatori, C.; Filippi, M. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult. Scler. J. 2014, 20, 686–694. [Google Scholar]
- Sandroff, B.M.; Johnson, C.L.; Motl, R.W. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: A novel application of magnetic resonance elastography. Neuroradiology 2017, 59, 61–67. [Google Scholar]
- Muthupillai, R.; Lomas, D.J.; Rossman, P.J.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 1995, 269, 1854–1857. [Google Scholar]
- Johnson, C.L.; McGarry, M.D.J.; Gharibans, A.A.; Weaver, J.B.; Paulsen, K.D.; Wang, H.; Olivero, W.C.; Sutton, B.P.; Georgiadis, J.G. Local mechanical properties of white matter structures in the human brain. Neuroimage 2013, 79, 145–152. [Google Scholar]
- Johnson, C.L.; Schwarb, H.; McGarry, M.D.J.; Anderson, A.T.; Huesmann, G.R.; Sutton, B.P.; Cohen, N.J. Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp. 2016, 37, 4221–4233. [Google Scholar] [CrossRef]
- Schwarb, H.; Johnson, C.L.; McGarry, M.D.J.; Cohen, N.J. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage 2016, 132, 534–541. [Google Scholar]
- Leocani, L.; Comi, E.; Annovazzi, P.; Rovaris, M.; Rossi, P.; Cursi, M.; Comola, M.; Martinelli, V.; Comi, G. Impaired short-term motor learning in multiple sclerosis: Evidence from virtual reality. Neurorehabilit. Neural Repair 2007, 21, 273–278. [Google Scholar]
- Laver, K.; George, S.; Ratcliffe, J.; Crotty, M. Virtual reality stroke rehabilitation–hype or hope? Aust. Occup. Ther. J. 2011, 58, 215–219. [Google Scholar]
- Lozano-Quilis, J.A.; Gil-Gómez, H.; Gil-Gómez, J.A.; Albiol-Pérez, S.; Palacios-Navarro, G.; Fardoun, H.M.; Mashat, A.S. Virtual rehabilitation for multiple sclerosis using a kinect-based system: Randomized controlled trial. JMIR Serious Games 2014, 2, e12. [Google Scholar]
- Massetti, T.; Trevizan, I.L.; Arab, C.; Favero, F.M.; Ribeiro-Papa, D.C.; de Mello Monteiro, C.B. Virtual reality in multiple sclerosis–a systematic review. Mult. Scler. Relat. Disord. 2016, 8, 107–112. [Google Scholar]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar]
- Eftekharsadat, B.; Babaei-Ghazani, A.; Mohammadzadeh, M.; Talebi, M.; Eslamian, F.; Azari, E. Effect of virtual reality-based balance training in multiple sclerosis. Neurol. Res. 2015, 37, 539–544. [Google Scholar]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 1–10. [Google Scholar]
- Gutiérrez, R.O.; Galan del Rio, F.; Cano de la Cuerda, R.; Alguacil Diego, I.M.; González, R.A.; Page, J.C.M. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation 2013, 33, 545–554. [Google Scholar]
- Ortiz-Gutiérrez, R.; Cano-de-la-Cuerda, R.; Galán-del-Río, F.; Alguacil-Diego, I.M.; Palacios-Ceña, D.; Miangolarra-Page, J.C. A telerehabilitation program improves postural control in multiple sclerosis patients: A Spanish preliminary study. Int. J. Environ. Res. Public Health 2013, 10, 5697–5710. [Google Scholar]
- Baram, Y.; Miller, A. Virtual reality cues for improvement of gait in patients with multiple sclerosis. Neurology 2006, 66, 178–181. [Google Scholar]
- Peruzzi, A.; Zarbo, I.R.; Cereatti, A.; Della Croce, U.; Mirelman, A. An innovative training program based on virtual reality and treadmill: Effects on gait of persons with multiple sclerosis. Disabil. Rehabil. 2017, 39, 1557–1563. [Google Scholar]
- Peruzzi, A.; Cereatti, A.; Della Croce, U.; Mirelman, A. Effects of a virtual reality and treadmill training on gait of subjects with multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2016, 5, 91–96. [Google Scholar]
- Sampson, P.; Freeman, C.; Coote, S.; Demain, S.; Feys, P.; Meadmore, K.; Hughes, A.M. Using functional electrical stimulation mediated by iterative learning control and robotics to improve arm movement for people with multiple sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 235–248. [Google Scholar]
- Jonsdottir, J.; Perini, G.; Ascolese, A.; Bowman, T.; Montesano, A.; Lawo, M.; Bertoni, R. Unilateral arm rehabilitation for persons with Multiple Sclerosis using Serious games in a virtual reality approach: Bilateral treatment effect? Mult. Scler. Relat. Disord. 2019, 35, 76–82. [Google Scholar]
- Coyle, H.; Traynor, V.; Solowij, N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: Systematic review of the literature. Am. J. Geriatr. Psychiatry 2015, 23, 335–359. [Google Scholar]
- Gates, N.J.; Sachdev, P.S.; Singh, M.A.F.; Valenzuela, M. Cognitive and memory training in adults at risk of dementia: A systematic review. BMC Geriatr. 2011, 11, 55. [Google Scholar]
- Buschert, V.C.; Giegling, I.; Teipel, S.J.; Jolk, S.; Hampel, H.; Rujescu, D.; Buerger, K. Long-term observation of a multicomponent cognitive intervention in mild cognitive impairment. J. Clin. Psychiatry 2012, 73, 1492–1498. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasaponara, S.; Marson, F.; Doricchi, F.; Cavallo, M. A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far. Brain Sci. 2021, 11, 528. https://doi.org/10.3390/brainsci11050528
Lasaponara S, Marson F, Doricchi F, Cavallo M. A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far. Brain Sciences. 2021; 11(5):528. https://doi.org/10.3390/brainsci11050528
Chicago/Turabian StyleLasaponara, Stefano, Fabio Marson, Fabrizio Doricchi, and Marco Cavallo. 2021. "A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far" Brain Sciences 11, no. 5: 528. https://doi.org/10.3390/brainsci11050528
APA StyleLasaponara, S., Marson, F., Doricchi, F., & Cavallo, M. (2021). A Scoping Review of Cognitive Training in Neurodegenerative Diseases via Computerized and Virtual Reality Tools: What We Know So Far. Brain Sciences, 11(5), 528. https://doi.org/10.3390/brainsci11050528








