Fecal Nervonic Acid as a Biomarker for Diagnosing and Monitoring Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Bristol Stool Chart
2.3. Gastrointestinal Symptom Rating Scale
2.4. Analysis of Fecal Fatty Acids
2.5. Analysis of C-Reactive Protein
2.6. Analysis of Creatinine and Calculation of Glomerular Filtration Rate
2.7. Analysis of Fecal Calprotectin
2.8. Statistical Analysis
3. Results
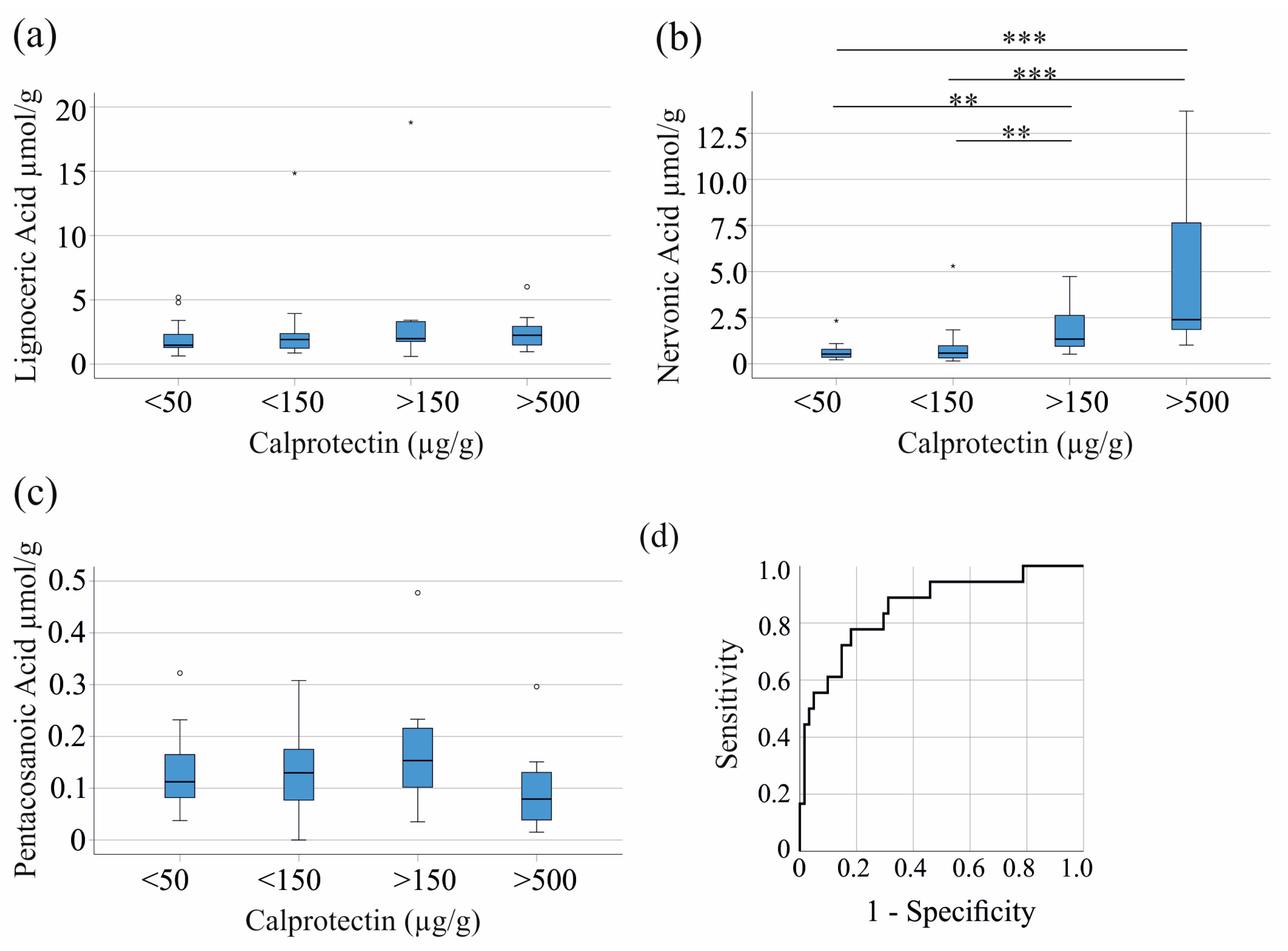
3.1. Fecal Fatty Acids in Patients with Inflammatory Bowel Disease and Healthy Controls
3.2. Fecal Nervonic Acid Levels Correlate with Clinical Markers of Inflammation in Inflammatory Bowel Disease
3.3. Relation of Fecal Fatty Acids with Stool Consistency and Gastrointestinal Symptom Rating Scale
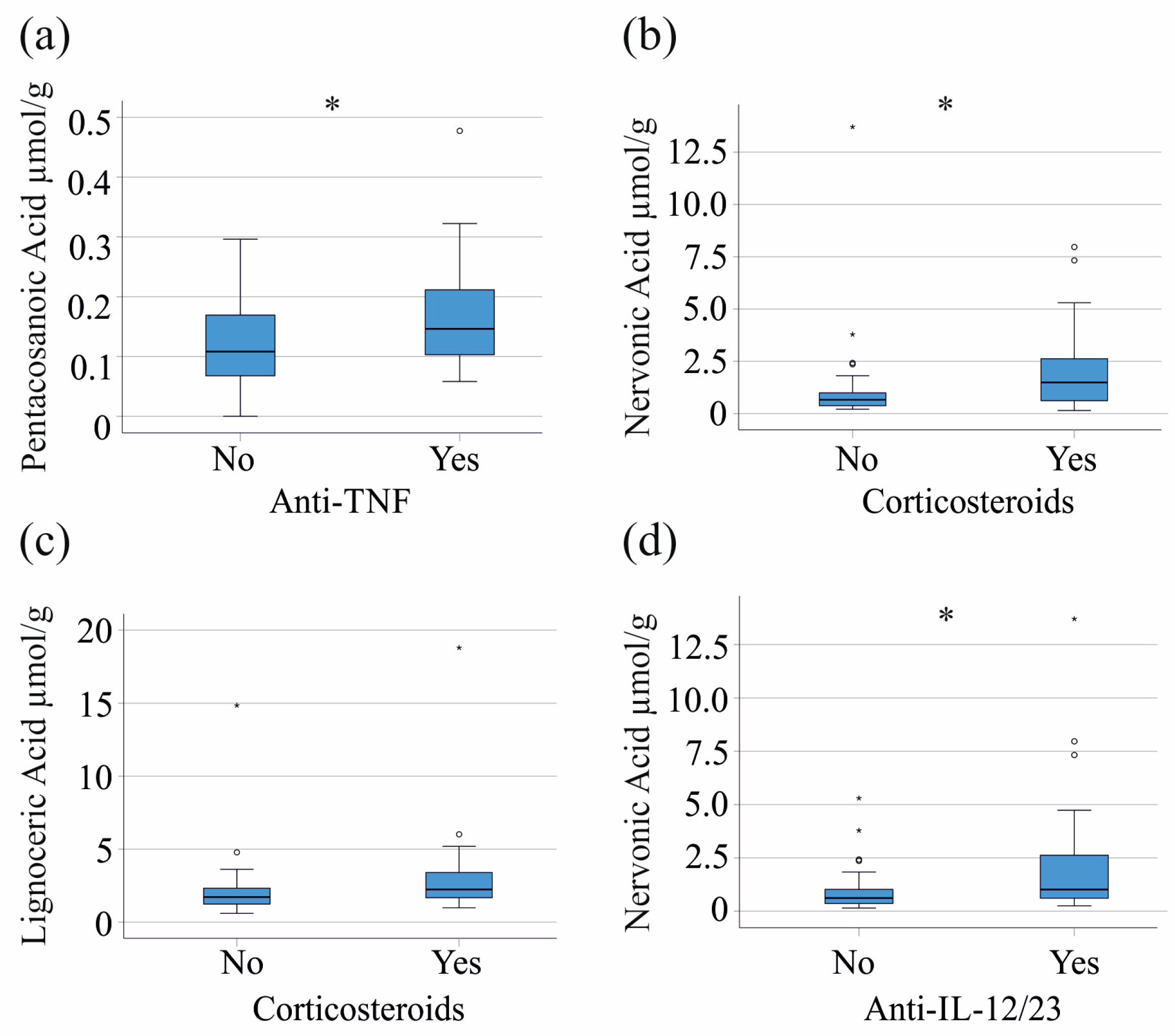
3.4. Effects of Medication on Fecal Fatty Acid Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tumen, D.; Buttenschon, J.; Muller, M.; Gulow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Obermeier, F.; Hofmann, C.; Falk, W. Inflammatory bowel diseases: When natural friends turn into enemies-the importance of CpG motifs of bacterial DNA in intestinal homeostasis and chronic intestinal inflammation. Int. J. Inflam. 2010, 2010, 641910. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Ciccocioppo, R.; Luinetti, O.; Ricevuti, L.; Morera, R.; Cifone, M.G.; Solcia, E.; Corazza, G.R. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis. Colon. Rectum 2003, 46, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, C.; Tanaka, M.; Kudo, H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J. Gastroenterol. Hepatol. 2002, 17, 758–764. [Google Scholar] [CrossRef]
- Marini, M.; Bamias, G.; Rivera-Nieves, J.; Moskaluk, C.A.; Hoang, S.B.; Ross, W.G.; Pizarro, T.T.; Cominelli, F. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8366–8371. [Google Scholar] [CrossRef]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef]
- Strater, J.; Wellisch, I.; Riedl, S.; Walczak, H.; Koretz, K.; Tandara, A.; Krammer, P.H.; Moller, P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: A possible role in ulcerative colitis. Gastroenterology 1997, 113, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Dietary modification of inflammation with lipids. Proc. Nutr. Soc. 2002, 61, 345–358. [Google Scholar] [CrossRef]
- Piotrowska, M.; Binienda, A.; Fichna, J. The role of fatty acids in Crohn’s disease pathophysiology—An overview. Mol. Cell Endocrinol. 2021, 538, 111448. [Google Scholar] [CrossRef]
- Seki, H.; Tani, Y.; Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009, 89, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty acids and lipid mediators in inflammatory bowel disease: From mechanism to treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Sartini, A.; Bellanova, M.; Campieri, M.; Festi, D.; Bazzoli, F.; Belluzzi, A. Eicosapentaenoic Acid Reduces Fecal Levels of Calprotectin and Prevents Relapse in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1268–1275.e2. [Google Scholar] [CrossRef]
- Alhouayek, M.; Ameraoui, H.; Muccioli, G.G. Bioactive lipids in inflammatory bowel diseases—From pathophysiological alterations to therapeutic opportunities. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158854. [Google Scholar] [CrossRef]
- Espinoza, K.S.; Snider, A.J. Therapeutic Potential for Sphingolipids in Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 789. [Google Scholar] [CrossRef]
- Keppley, L.J.W.; Walker, S.J.; Gademsey, A.N.; Smith, J.P.; Keller, S.R.; Kester, M.; Fox, T.E. Nervonic acid limits weight gain in a mouse model of diet-induced obesity. FASEB J. 2020, 34, 15314–15326. [Google Scholar] [CrossRef]
- Namiecinska, M.; Piatek, P.; Lewkowicz, P. Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. Int. J. Mol. Sci. 2024, 25, 3792. [Google Scholar] [CrossRef]
- Phung, N.V.; Rong, F.; Xia, W.Y.; Fan, Y.; Li, X.Y.; Wang, S.A.; Li, F.L. Nervonic acid and its sphingolipids: Biological functions and potential food applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 8766–8785. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.N.; Wang, M.X.; Han, J.L.; Feng, C.Y.; Wang, M.; Wang, M.; Sun, J.Y.; Li, N.Y.; Simal-Gandara, J.; Liu, C. Improved colonic inflammation by nervonic acid via inhibition of NF-kappaB signaling pathway of DSS-induced colitis mice. Phytomedicine 2023, 112, 154702. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, J.; La, Y.; Liu, X.; Yuan, Y.; Ye, J.; Zhang, Z.; Chen, H.; Ma, Y.; Zhong, Z.; et al. Very long-chain fatty acids control peroxisome dynamics via a feedback loop in intestinal stem cells during gut regeneration. Dev. Cell 2024, 59, 3008–3024.e8. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.K.; Weidner, K.J.; Hoppner, J.; Becker, N.; Friedrich, D.; Stokes, C.S.; Lammert, F.; Kollner, V. Design and validation of a German version of the GSRS-IBS—An analysis of its psychometric quality and factorial structure. BMC Gastroenterol. 2017, 17, 139. [Google Scholar] [CrossRef]
- Sommersberger, S.; Gunawan, S.; Elger, T.; Fererberger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Muller, M.; Liebisch, G.; et al. Altered fecal bile acid composition in active ulcerative colitis. Lipids Health Dis. 2023, 22, 199. [Google Scholar] [CrossRef]
- Ecker, J.; Scherer, M.; Schmitz, G.; Liebisch, G. A rapid GC-MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J. Chromatogr. B 2012, 897, 98–104. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Aldeguer, X.; Kruis, W.; Lasson, A.; Mittmann, U.; Nally, K.; Peyrin-Biroulet, L.; Schoepfer, A.; Vatn, M.; Vavricka, S.; et al. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: Expert clinical opinion. J. Crohns Colitis 2013, 7, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Ukashi, O.; Kopylov, U.; Ungar, B.; Talan Asher, A.; Shachar, E.; Engel, T.; Albshesh, A.; Yablecovitch, D.; Lahat, A.; Eliakim, R.; et al. Fecal calprotectin diagnostic level gradient along the small bowel in patients with Crohn’s disease. J. Crohns Colitis 2024, jjae123. [Google Scholar] [CrossRef]
- Glassock, R.J.; Winearls, C. Ageing and the glomerular filtration rate: Truths and consequences. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 419–428. [Google Scholar]
- Brown, S.J.; Mayer, L. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 2007, 102, 2058–2069. [Google Scholar] [CrossRef]
- Freeman, K.; Taylor-Phillips, S.; Willis, B.H.; Ryan, R.; Clarke, A. Test accuracy of faecal calprotectin for inflammatory bowel disease in UK primary care: A retrospective cohort study of the IMRD-UK data. BMJ Open 2021, 11, e044177. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Alrefai, W.A.; Dudeja, P.K. Pathophysiology of IBD associated diarrhea. Tissue Barriers 2018, 6, e1463897. [Google Scholar] [CrossRef]
- Fox, T.E.; Bewley, M.C.; Unrath, K.A.; Pedersen, M.M.; Anderson, R.E.; Jung, D.Y.; Jefferson, L.S.; Kim, J.K.; Bronson, S.K.; Flanagan, J.M.; et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. J. Lipid Res. 2011, 52, 509–517. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef]
- Demarquoy, J.; Le Borgne, F. Crosstalk between mitochondria and peroxisomes. World J. Biol. Chem. 2015, 6, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, M.; Makdissi, S.; Scur, M.; Parsons, B.D.; Baker, K.; Otley, A.; MacIntyre, B.; Nguyen, H.D.; Kim, P.K.; Stadnyk, A.W.; et al. Peroxisomal cholesterol metabolism regulates yap-signaling, which maintains intestinal epithelial barrier function and is altered in Crohn’s disease. Cell Death Dis. 2024, 15, 536. [Google Scholar] [CrossRef]
- Ali, H.; Morito, K.; Hasi, R.Y.; Aihara, M.; Hayashi, J.; Kawakami, R.; Kanemaru, K.; Tsuchiya, K.; Sango, K.; Tanaka, T. Characterization of uptake and metabolism of very long-chain fatty acids in peroxisome-deficient CHO cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159088. [Google Scholar] [CrossRef]
- Drover, V.A.; Nguyen, D.V.; Bastie, C.C.; Darlington, Y.F.; Abumrad, N.A.; Pessin, J.E.; London, E.; Sahoo, D.; Phillips, M.C. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 2008, 283, 13108–13115. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Masia, D.; Diez, I.; Calatayud, S.; Hernandez, C.; Cosin-Roger, J.; Hinojosa, J.; Esplugues, J.V.; Barrachina, M.D. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PLoS ONE 2012, 7, e48535. [Google Scholar] [CrossRef]
- Peters, V.; Tigchelaar-Feenstra, E.F.; Imhann, F.; Dekens, J.A.M.; Swertz, M.A.; Franke, L.H.; Wijmenga, C.; Weersma, R.K.; Alizadeh, B.Z.; Dijkstra, G.; et al. Habitual dietary intake of IBD patients differs from population controls: A case-control study. Eur. J. Nutr. 2021, 60, 345–356. [Google Scholar] [CrossRef]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef]
- Suseno, S.H.; Nurjanah, N.; Yoshiara, Y.; Saraswati, S. Determination of extraction temperature and period of fish oil from tilapia (Oreochromis niloticus) by product using wet rendering method. KnE Life Sci. 2013, 1, 125–135. [Google Scholar] [CrossRef]
- Terluk, M.R.; Tieu, J.; Sahasrabudhe, S.A.; Moser, A.; Watkins, P.A.; Raymond, G.V.; Kartha, R.V. Nervonic Acid Attenuates Accumulation of Very Long-Chain Fatty Acids and is a Potential Therapy for Adrenoleukodystrophy. Neurotherapeutics 2022, 19, 1007–1017. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | IBD | Controls |
|---|---|---|
| Number (female/male) | 62 (28/34) | 17 (10/7) |
| Age (years) | 42 (19–78) | 48 (23–78) |
| Body Mass Index (kg/m2) | 24 (16–44) | not determined |
| C-reactive protein (mg/L) | 3 (0–144) | not determined |
| Creatinine (mg/dL) | 0.85 (0.51–1.25) | not determined |
| Glomerular filtration rate (mL/min) | 99 (61–136) | not determined |
| Fecal calprotectin (µg/g) | 62 (17–1616) | not determined |
| Characteristics | ||||||
|---|---|---|---|---|---|---|
| Age (years) | 20–30 | 31–40 | 41–50 | 51–60 | >61 | p-value |
| Number (female/male) | 14 (6/8) | 14 (8/6) | 9 (2/7) | 18 (10/8) | 7(2/5) | 0.360 |
| Lignoceric acid µmol/g | 1.95 (0.60–3.62) | 1.47 (0.64–6.02) | 1.86 (1.00–14.84) | 1.67 (0.69–18.80) | 2.24 (1.14–3.40) | 0.827 |
| Nervonic acid µmol/g | 1.42 (0.27–13.70) | 0.56 (0.24–2.07) | 0.77 (0.27–3.78) | 0.61 (0.10–2.62) | 0.68 (0.25–7.32) | 0.250 |
| Pentacosanoic acid µmol/g | 0.11 (0–0.22) | 0.10 (0.04–0.31) | 0.13 (0.06–0.25) | 0.10 (0.04–0.48) | 0.15 (0.05–0.32) | 0.544 |
| Body Mass Index (kg/m2) | 23 (16–28) | 26 (17–35) | 23 (20–25) | 24 (21–44) | 24 (18–35) | 0.553 |
| C-reactive protein (mg/L) | 10 (1–57) | 4 (0–18) | 2 (1–11) | 1 (0–144) | 2 (1–55) | 0.264 |
| Creatinine (mg/dL) | 0.83 (0.51–1.12) | 0.82 (0.59–0.99) | 0.83 (0.76–1.02) | 0.85 (0.70–1.06) | 0.89 (0.74–1.25) | 0.389 |
| Glomerular filtration rate (mL/min) | 112 (91–136) | 112 (84–122) | 106 (87–110) | 92 (62–103) | 83 (61–97) | <0.001 |
| Fecal calprotectin (µg/g) | 121 (17–1616) | 65 (17–639) | 55 (33–538) | 45 (18–1543) | 34 (18–883) | 0.126 |
| Lignoceric Acid | Nervonic Acid | Pentacosanoic Acid | |
|---|---|---|---|
| C-reactive protein | 0.042 | 0.376 ** | −0.217 |
| Fecal Calprotectin | 0.175 | 0.575 *** | −0.017 |
| Creatinine | −0.035 | −0.171 | 0.080 |
| Glomerular filtration rate | −0.021 | 0.267 * | −0.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunst, C.; Elger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Müller, M.; Liebisch, G.; Tews, H.C.; Buechler, C. Fecal Nervonic Acid as a Biomarker for Diagnosing and Monitoring Inflammatory Bowel Disease. Biomedicines 2024, 12, 2764. https://doi.org/10.3390/biomedicines12122764
Kunst C, Elger T, Loibl J, Huss M, Kandulski A, Krautbauer S, Müller M, Liebisch G, Tews HC, Buechler C. Fecal Nervonic Acid as a Biomarker for Diagnosing and Monitoring Inflammatory Bowel Disease. Biomedicines. 2024; 12(12):2764. https://doi.org/10.3390/biomedicines12122764
Chicago/Turabian StyleKunst, Claudia, Tanja Elger, Johanna Loibl, Muriel Huss, Arne Kandulski, Sabrina Krautbauer, Martina Müller, Gerhard Liebisch, Hauke Christian Tews, and Christa Buechler. 2024. "Fecal Nervonic Acid as a Biomarker for Diagnosing and Monitoring Inflammatory Bowel Disease" Biomedicines 12, no. 12: 2764. https://doi.org/10.3390/biomedicines12122764
APA StyleKunst, C., Elger, T., Loibl, J., Huss, M., Kandulski, A., Krautbauer, S., Müller, M., Liebisch, G., Tews, H. C., & Buechler, C. (2024). Fecal Nervonic Acid as a Biomarker for Diagnosing and Monitoring Inflammatory Bowel Disease. Biomedicines, 12(12), 2764. https://doi.org/10.3390/biomedicines12122764








