Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, the Study Model and Sample Collection
2.2. Immunohistochemical Staining
2.3. Quantitative Analyses
2.4. Statistical Analyses
3. Results
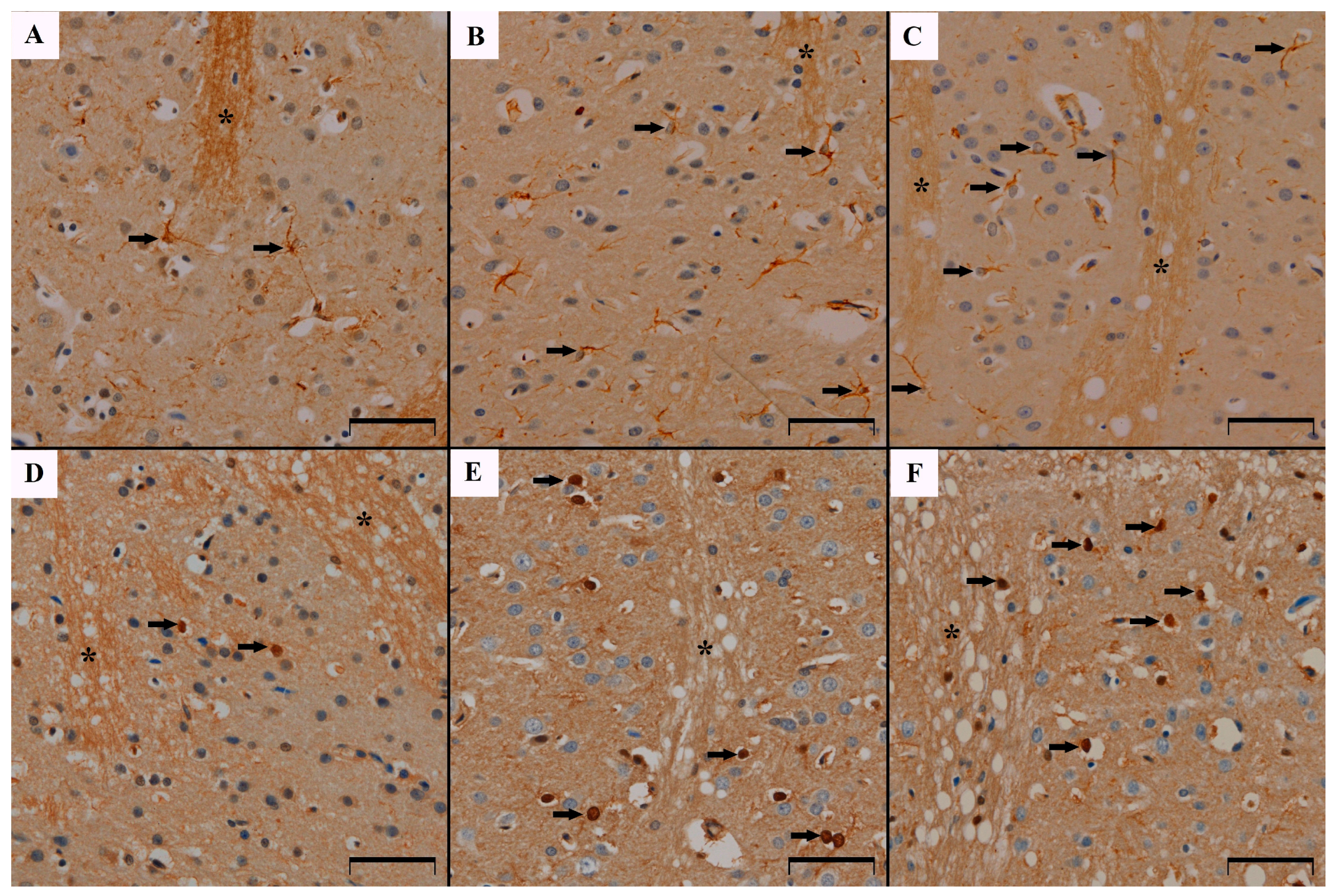
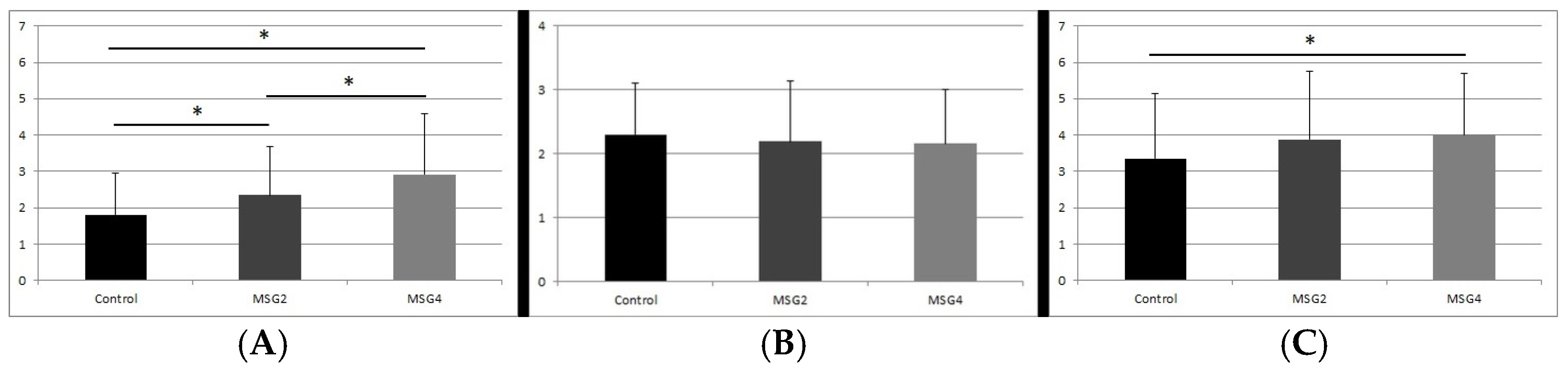
3.1. GFAP Immunoreactivity Results in the Studied Animals
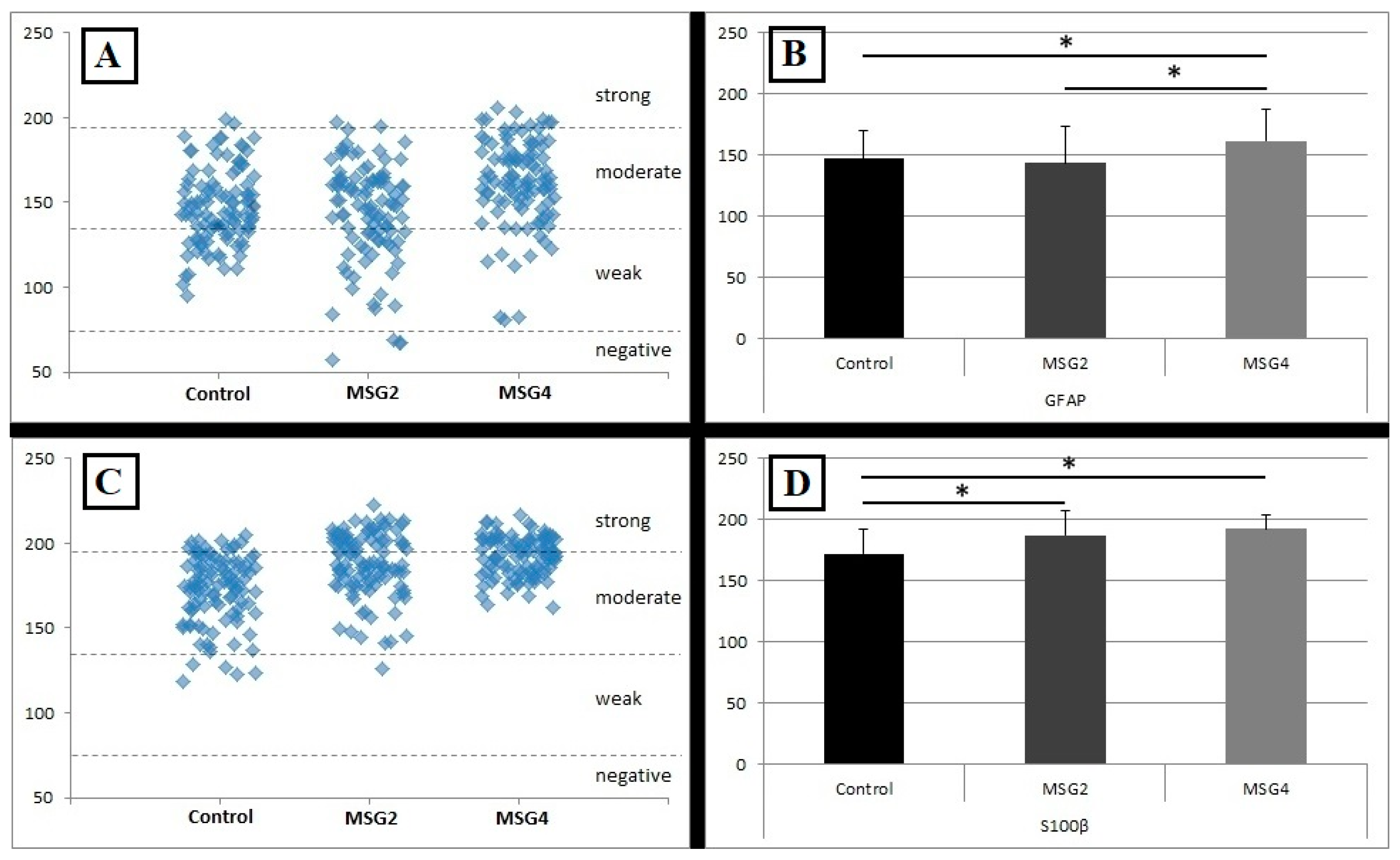
3.2. Results of GFAP Reaction Intensity
3.3. S100β Immunoreactivity Results in the Studied Animals
3.4. Results of S100β Reaction Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganesan, K.; Sukalingam, K.; Balamurali, K.; Alaudeen, S.; Ponnusamy, K.; Ariffin, I.A. A studies on Monosodium L- glutamate toxicity in animal models—A review. IJPCBS 2013, 3, 1257–1268. [Google Scholar]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Attwell, D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 2010, 11, 227–238. [Google Scholar] [CrossRef]
- Adermark, L.; Lagström, O.; Loftén, A.; Licheri, V.; Havenäng, A.; Loi, E.; Stomberg, R.; Söderpalm, B.; Domi, A.; Ericson, M. Astrocytes modulate extracellular neurotransmitter levels and excitatory neurotransmission in dorsolateral striatum via dopamine D2 receptor signaling. Neuropsychopharmacol 2022, 47, 1493–1502. [Google Scholar] [CrossRef]
- D’Ascenzo, M.; Fellin, T.; Terunuma, M.; Revilla-Sanchez, R.; Meaney, D.F.; Auberson, Y.P.; Moss, S.J.; Haydon, P.G. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2007, 104, 1995–2000. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Kawata, K.; Liu, C.Y.; Merkel, S.F.; Ramirez, S.H.; Tierney, R.T.; Langford, D. Blood biomarkers for brain injury: What are we measuring? Neurosci. Biobehav. Rev. 2016, 68, 460–473. [Google Scholar] [CrossRef]
- Krawczyk, A.; Jaworska-Adamu, J.; Rycerz, K. Immunohistochemical evaluation of hippocampal CA1 region astrocytes in 10-day-old rats after monosodium glutamate treatment. Pol. J. Vet. Sci. 2015, 18, 767–774. [Google Scholar] [CrossRef]
- Rezaei, O.; Pakdaman, H.; Gharehgozli, K.; Simani, L.; Vahedian-Azimi, A.; Asaadi, S.; Sahraei, Z.; Hajiesmaeili, M. S100 B: A new concept in neurocritical care. Iran. J. Neurol. 2017, 16, 83–89. [Google Scholar]
- Aboul Fotouh, G.; Eldin Sayed, S.; Altayeb, Z.; Zaher, E. The Possible Neuroprotective Effect of Astaxanthin on Monosodium Glutamate and Aspartame Induced Hippocampal Changes in Albino Rats: (Histological and Immuno-histochemical Study). EJH 2020, 43, 684–701. [Google Scholar]
- Hazzaa, S.M.; Abdelaziz, S.A.M.; Abd Eldaim, M.A.; Abdel-Daim, M.M.; Elgarawany, G.E. Neuroprotective Potential of Allium sativum against Monosodium Glutamate-Induced Excitotoxicity: Impact on Short-Term Memory, Gliosis, and Oxidative Stress. Nutrients 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Atef, R.M.; Abdel Fattah, I.O.; Mahmoud, O.M.; Abdel-Rahman, G.M.; Salem, N.A. Protective effects of Rosemary extract and/or Fluoxetine on Monosodium Glutamate-induced hippocampal neurotoxicity in rat. Rom. J. Morphol. Embryol. 2021, 62, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, A.F.K.; Mussulini, B.H.; Zenki, K.C.; Baggio, S.; Pasqualotto, A.; Rosemberg, D.B.; Bogo, M.R.; de Oliveira, D.L.; Rico, E.P. Prolonged ethanol exposure alters glutamate uptake leading to astrogliosis and neuroinflammation in adult zebrafish brain. Neurotoxicology 2022, 88, 57–64. [Google Scholar] [CrossRef]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. 2023, 328, 21814. [Google Scholar] [CrossRef]
- Zhang, H.; Sulzer, D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J. Neurosci. 2003, 23, 10585–10592. [Google Scholar] [CrossRef]
- Onaolapo, O.J.; Onaolapo, A.Y.; Akanmu, M.A.; Gbola, O. Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology 2016, 23, 147–156. [Google Scholar] [CrossRef]
- Dief, A.E.; Kamha, E.S.; Baraka, A.M.; Elshorbagy, A.K. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: A potential role for cyclic AMP protein kinase. Neurotoxicology 2014, 42, 76–82. [Google Scholar] [CrossRef]
- Eweka, A.O.; Eweka, A.; Om’iniabohs, F.A. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. N. Am. J. Med. Sci. 2010, 2, 146–149. [Google Scholar] [CrossRef]
- Hashem, H.E.; El-Din Safwat, M.D.; Algaidi, S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Hist. 2012, 43, 179–186. [Google Scholar] [CrossRef]
- Farhat, F. Monosodium glutamate safety, neurotoxicity and some recent studies. AJPS 2021, 64, 222–243. [Google Scholar] [CrossRef]
- Jaworska-adamu, J.; Krawczyk, A.; Rycerz, K. Influence of monosodium glutamate on calretinin immunoreactivity in the dorsal raphe nucleus in adult rats. Med. Weter. 2019, 75, 426–430. [Google Scholar] [CrossRef]
- Rycerz, K.; Krawczyk, A.; Jaworska-Adamu, J.; Krawczyk-Marc, I. Effects of monosodium glutamate treatment on calretinin-immunoreactive neurons in hippocampus of postnatal rats. Folia. Histochem. Cytobiol. 2014, 52, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Jaworska-Adamu, J.; Rycerz, K. Immunohistochemical Evaluation of Calretinin in the Periaqueductal Gray Matter of Rats Treated with Monosodium Glutamate. Pak. Vet. J. 2020, 40, 93–97. [Google Scholar]
- Yoo, J.H.; Chun, J.W.; Choi, M.R.; Cho, H.; Kim, J.Y.; Choi, J.; Kim, D.J. Caudate nucleus volume mediates the link between glutamatergic neurotransmission and problematic smartphone use in youth. J. Behav. Addict. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Brovelli, A.; Nazarian, B.; Meunier, M.; Boussaoud, D. Differential roles of caudate nucleus and putamen during instrumental learning. Neuroimage 2011, 57, 1580–1590. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008, 86, 141–155. [Google Scholar] [CrossRef]
- Brown, T.G.; Thayer, M.N.; VanTreeck, J.G.; Zarate, N.; Hart, D.W.; Heilbronner, S.; Gomez-Pastor, R. Striatal spatial heterogeneity, clustering, and white matter association of GFAP+ astrocytes in a mouse model of Huntington’s disease. Front. Cell. Neurosci. 2023, 17, 1094503. [Google Scholar] [CrossRef]
- Rudilosso, S.; Rodríguez-Vázquez, A.; Urra, X.; Arboix, A. The Potential Impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int. J. Mol. Sci. 2022, 23, 1497. [Google Scholar] [CrossRef]
- Ye, N.; Yang, Q.; Chen, Z.; Teng, C.; Liu, P.; Liu, X.; Xiong, Y.; Lin, X.; Li, S.; Li, X. Classification of Gliomas and Germinomas of the Basal Ganglia by Transfer Learning. Front. Oncol. 2022, 12, 844197. [Google Scholar] [CrossRef]
- Duan, W.W.; Yang, L.T.; Liu, J.; Dai, Z.Y.; Wang, Z.Y.; Zhang, H.; Zhang, X.; Liang, X.S.; Luo, P.; Zhang, J.; et al. A TGF-β signaling-related lncRNA signature for prediction of glioma prognosis, immune microenvironment, and immunotherapy response. CNS Neurosci. Ther. 2024, 30, e14489. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.W.; Roberts, R.C.; Melendez-Ferro, M.; Perez-Costas, E. Neurochemical characterization of the tree shrew dorsal striatum. Front. Neuroanat. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Beron, C.C.; Zimmer, J.A.; Wen, M.J.; Wang, W.; Sabatini, B.L. Dopamine and glutamate regulate striatal acetylcholine in decision-making. Nature 2023, 621, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. Corticostriatal circuitry. Dialogues. Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The ins and outs of the striatum: Role in drug addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef]
- König, J.F.R.; Klippel, R.A. A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem; Williams and Wilkins: Baltimore, MD, USA, 1963. [Google Scholar]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta. Histochem. Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Matos, L.L.; Stabenow, E.; Tavares, M.R.; Ferraz, A.R.; Capelozzi, V.L.; Pinhal, M.A. Immunohistochemistry quantification by a digital computer-assisted method compared to semiquantitative analysis. Clinics 2006, 61, 417–424. [Google Scholar] [CrossRef]
- Skowrońska, K.; Obara-Michlewska, M.; Czarnecka, A.; Dąbrowska, K.; Zielińska, M.; Albrecht, J. Persistent Overexposure to N-Methyl-D-Aspartate (NMDA) Calcium-Dependently Downregulates Glutamine Synthetase, Aquaporin 4, and Kir4.1 Channel in Mouse Cortical Astrocytes. Neurotox. Res. 2019, 35, 271–280. [Google Scholar] [CrossRef]
- Bagga, P.; Crescenzi, R.; Krishnamoorthy, G.; Verma, G.; Nanga, R.P.; Reddy, D.; Greenberg, J.; Detre, J.A.; Hariharan, H.; Reddy, R. Mapping the alterations in glutamate with GluCEST MRI in a mouse model of dopamine deficiency. J. Neurochem. 2016, 139, 432–439. [Google Scholar] [CrossRef]
- Sahu, S.; Nag, D.S.; Swain, A.; Samaddar, D.P. Biochemical changes in the injured brain. World J. Biol. Chem. 2017, 8, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Abdel-Sattar, S.A.; Abbas, A.N.; Mahran, Y.F.; Alshanwani, A.R.; Hamdan, A.M.E.; Atwa, A.M.; Reda, E.; Ahmed, Y.M.; Zaghlool, S.S.; et al. The protective effect of thymoquinone or/and thymol against monosodium glutamate-induced attention-deficit/hyperactivity disorder (ADHD)-like behavior in rats: Modulation of Nrf2/HO-1, TLR4/NF-κB/NLRP3/caspase-1 and Wnt/β-Catenin signaling pathways in rat model. Biomed. Pharmacother. 2022, 155, 113799. [Google Scholar]
- Abdelhamid, W.G.; Mowaad, N.A.; Asaad, G.F.; Galal, A.F.; Mohammed, S.S.; Mostafa, O.E.; Sadek, D.R.; Elkhateb, L.A. The potential protective effect of Camellia Sinensis in mitigating monosodium glutamate-induced neurotoxicity: Biochemical and histological study in male albino rats. Metab. Brain Dis. 2024, 39, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Tramontina, F.; Tramontina, A.C.; Souza, D.F.; Leite, M.C.; Gottfried, C.; Souza, D.O.; Wofchuk, S.T.; Gonçalves, C.A. Glutamate uptake is stimulated by extracellular S100B in hippocampal astrocytes. Cell. Mol. Neurobiol. 2006, 26, 81–86. [Google Scholar]
- Swamy, A.H.; Patel, N.L.; Gadad, P.C.; Koti, B.C.; Patel, U.M.; Thippeswamy, A.H.; Manjula, D.V. Neuroprotective Activity of Pongamia pinnata in Monosodium Glutamate-induced Neurotoxicity in Rats. Indian J. Pharm. Sci. 2013, 75, 657–663. [Google Scholar]
- Izumi, Y.; Yamamoto, N.; Matsuo, T.; Wakita, S.; Takeuchi, H.; Kume, T.; Katsuki, H.; Sawada, H.; Akaike, A. Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. J. Neurochem. 2009, 110, 745–755. [Google Scholar] [CrossRef]
- Morales, I.; Sanchez, A.; Rodriguez-Sabate, C.; Rodriguez, M. The astrocytic response to the dopaminergic denervation of the striatum. J. Neurochem. 2016, 139, 81–95. [Google Scholar] [CrossRef]
- Kögel, D.; Peters, M.; König, H.G.; Hashemi, S.M.; Bui, N.T.; Arolt, V.; Rothermundt, M.; Prehn, J.H. S100B potently activates p65/c-Rel transcriptional complexes in hippocampal neurons: Clinical implications for the role of S100B in excitotoxic brain injury. Neuroscience 2004, 127, 913–920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rycerz, K.; Krawczyk, A.; Jaworska-Adamu, J.; Arciszewski, M.B. Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats. Biomedicines 2024, 12, 2763. https://doi.org/10.3390/biomedicines12122763
Rycerz K, Krawczyk A, Jaworska-Adamu J, Arciszewski MB. Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats. Biomedicines. 2024; 12(12):2763. https://doi.org/10.3390/biomedicines12122763
Chicago/Turabian StyleRycerz, Karol, Aleksandra Krawczyk, Jadwiga Jaworska-Adamu, and Marcin B. Arciszewski. 2024. "Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats" Biomedicines 12, no. 12: 2763. https://doi.org/10.3390/biomedicines12122763
APA StyleRycerz, K., Krawczyk, A., Jaworska-Adamu, J., & Arciszewski, M. B. (2024). Monosodium Glutamate Treatment Elevates the Immunoreactivity of GFAP and S100β in Caudate Nucleus of the Striatum in Rats. Biomedicines, 12(12), 2763. https://doi.org/10.3390/biomedicines12122763





