RELT Is Upregulated in Breast Cancer and Induces Death in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
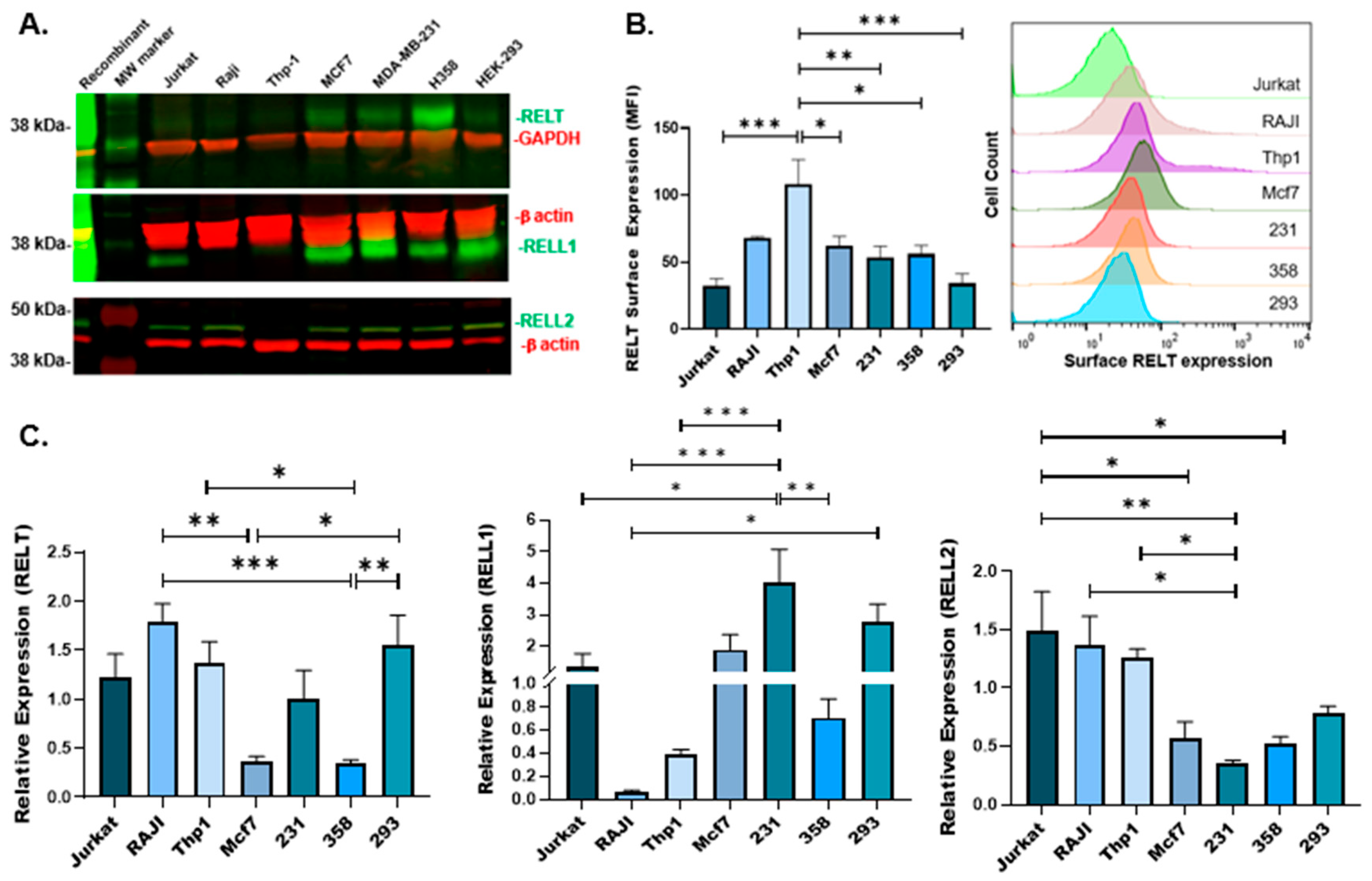
3.1. Expression of RELTfms in Different Cancer Cell Lines
3.2. Ability of RELT to Sensitize MDA-MB-231 (231) Cells to Chemotherapeutic Agents
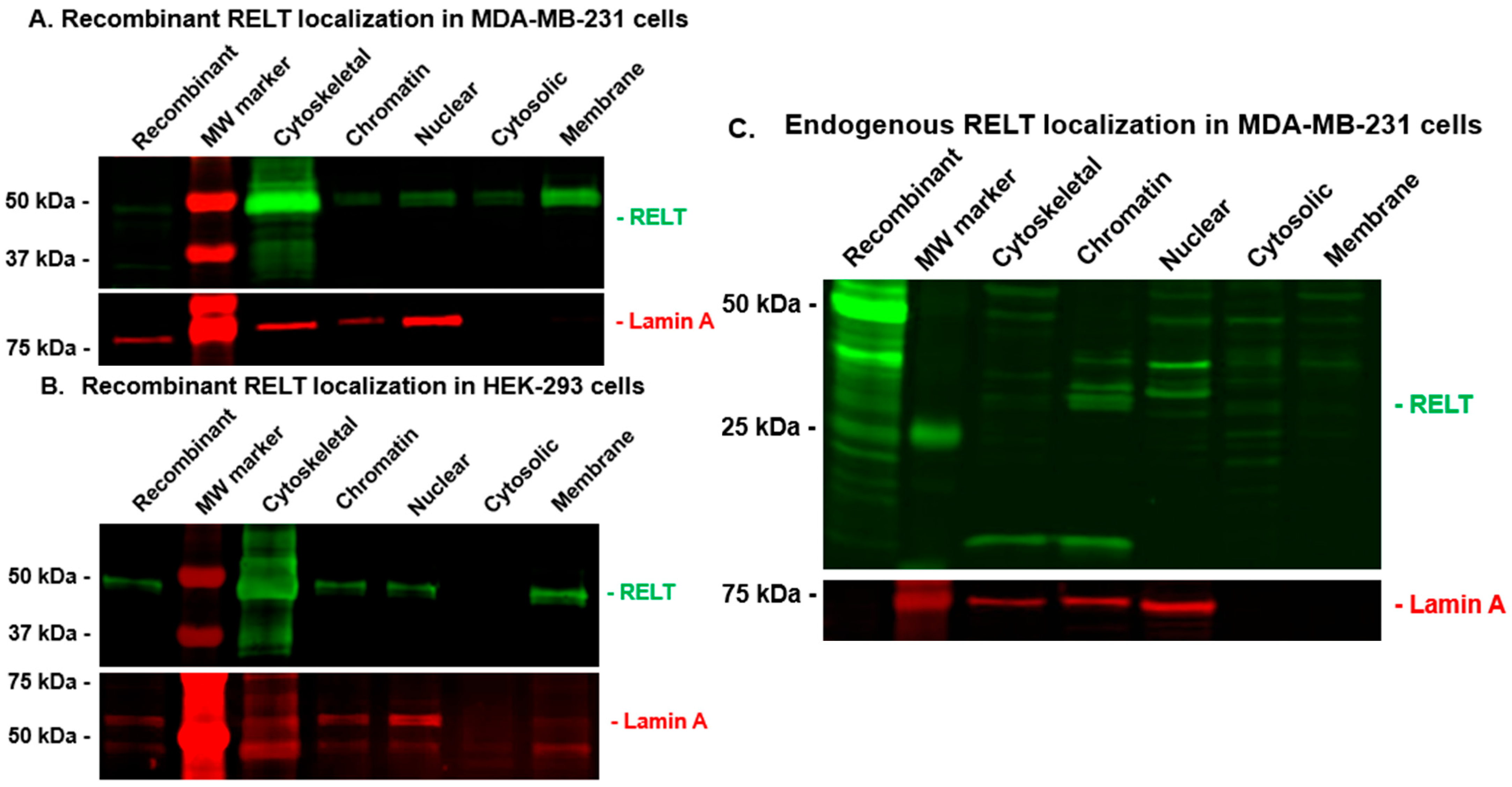
3.3. RELT Localization in MDA-MB-231 (231) Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Collette, Y.; Gilles, A.; Pontarotti, P.; Olive, D. A co-evolution perspective of the TNFSF and TNFRSF families in the immune system. Trends Immunol. 2003, 24, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cusick, J.K.; Alcaide, J.; Shi, Y. The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development. Biomedicines 2023, 11, 2695. [Google Scholar] [CrossRef]
- Sica, G.L.; Zhu, G.; Tamada, K.; Liu, D.; Ni, J.; Chen, L. RELT, a new member of the tumor necrosis factor receptor superfamily, is selectively expressed in hematopoietic tissues and activates transcription factor NF-kappaB. Blood 2001, 97, 2702–2707. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, S.H.; Kim, Y.H.; Lee, D.G.; Oh, H.S.; Han, C.; Kim, Y.I.; Jeon, Y.; Lee, H.; Kwon, B.S. RELT negatively regulates the early phase of the T-cell response in mice. Eur. J. Immunol. 2018, 48, 1739–1749. [Google Scholar] [CrossRef]
- Kim, J.W.; Zhang, H.; Seymen, F.; Koruyucu, M.; Hu, Y.; Kang, J.; Kim, Y.J.; Ikeda, A.; Kasimoglu, Y.; Bayram, M.; et al. Mutations in RELT cause autosomal recessive amelogenesis imperfecta. Clin. Genet. 2019, 95, 375–383. [Google Scholar] [CrossRef]
- Nikolopoulos, G.; Smith, C.E.L.; Brookes, S.J.; El-Asrag, M.E.; Brown, C.J.; Patel, A.; Murillo, G.; O’Connell, M.J.; Inglehearn, C.F.; Mighell, A.J. New missense variants in RELT causing hypomineralised amelogenesis imperfecta. Clin. Genet. 2020, 97, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Cusick, J.K.; Xu, L.G.; Bin, L.H.; Han, K.J.; Shu, H.B. Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem. Biophys. Res. Commun. 2006, 340, 535–543. [Google Scholar] [CrossRef]
- Cusick, J.K.; Mustian, A.; Goldberg, K.; Reyland, M.E. RELT induces cellular death in HEK 293 epithelial cells. Cell Immunol. 2010, 261, 1–8. [Google Scholar] [CrossRef]
- Moua, P.; Checketts, M.; Xu, L.G.; Shu, H.B.; Reyland, M.E.; Cusick, J.K. RELT family members activate p38 and induce apoptosis by a mechanism distinct from TNFR1. Biochem. Biophys. Res. Commun. 2017, 491, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Polek, T.C.; Talpaz, M.; Spivak-Kroizman, T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem. Biophys. Res. Commun. 2006, 343, 125–134. [Google Scholar] [CrossRef]
- Feng, L.; Hu, J.; Zhang, W.; Dong, Y.; Xiong, S.; Dong, C. RELL1 inhibits autophagy pathway and regulates Mycobacterium tuberculosis survival in macrophages. Tuberculosis 2020, 120, 101900. [Google Scholar] [CrossRef]
- Groza, T.; Gomez, F.L.; Mashhadi, H.H.; Munoz-Fuentes, V.; Gunes, O.; Wilson, R.; Cacheiro, P.; Frost, A.; Keskivali-Bond, P.; Vardal, B.; et al. The International Mouse Phenotyping Consortium: Comprehensive knockout phenotyping underpinning the study of human disease. Nucleic Acids Res. 2023, 51, D1038–D1045. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Huang, X.Y.; Yu, H.Z.; Xue, Y.; Zhu, P.L. Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-kappaB axis in endothelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, H.; Dou, L.; Li, Y. CircRELL1 promotes osteoarthritis progression by regulating miR-200c-3p. Heliyon 2024, 10, e34251. [Google Scholar] [CrossRef]
- Sang, H.; Zhang, W.; Peng, L.; Wei, S.; Zhu, X.; Huang, K.; Yang, J.; Chen, M.; Dang, Y.; Zhang, G. Exosomal circRELL1 serves as a miR-637 sponge to modulate gastric cancer progression via regulating autophagy activation. Cell Death Dis. 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Angenard, G.; Merdrignac, A.; Louis, C.; Edeline, J.; Coulouarn, C. Expression of long non-coding RNA ANRIL predicts a poor prognosis in intrahepatic cholangiocarcinoma. Dig. Liver Dis. 2019, 51, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Chen, Q.; Li, S.; Jia, X.; Xu, L.; Wei, L. RELT promotes the growth of esophageal squamous cell carcinoma by activating the NF-kappaB pathway. Cell Cycle 2021, 20, 1231–1241. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Luan, H.; Xiao, J.; Zhao, Z.; Yu, P.; Deng, M.; Liu, Y.; Ji, S.; Ma, J.; et al. LILRB4 on multiple myeloma cells promotes bone lesion by p-SHP2/NF-kappaB/RELT signal pathway. J. Exp. Clin. Cancer Res. 2024, 43, 183. [Google Scholar] [CrossRef]
- Jung, H.; Park, S.; Gunassekaran, G.R.; Jeon, M.; Cho, Y.E.; Baek, M.C.; Park, J.Y.; Shim, G.; Oh, Y.K.; Kim, I.S.; et al. A Peptide Probe Enables Photoacoustic-Guided Imaging and Drug Delivery to Lung Tumors in K-ras(LA2) Mutant Mice. Cancer Res. 2019, 79, 4271–4282. [Google Scholar] [CrossRef]
- Cusick, J.K.; Alhomsy, Y.; Wong, S.; Talbott, G.; Uversky, V.N.; Hart, C.; Hejazi, N.; Jacobs, A.T.; Shi, Y. RELT stains prominently in B-cell lymphomas and binds the hematopoietic transcription factor MDFIC. Biochem. Biophys. Rep. 2020, 24, 100868. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Shen, T.; Xu, F.; Zhang, J.; Wang, Y.; Wu, J.; Bu, H.; Fu, D.; Fang, B.; Lv, H.; et al. KCNN4 may weaken anti-tumor immune response via raising Tregs and diminishing resting mast cells in clear cell renal cell carcinoma. Cancer Cell Int. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.K.; Wang, Z.W.; Su, W.H.; Xu, K.; Jia, H.; Chen, J. Identification of Novel Biomarkers for Predicting Prognosis and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma Based on ceRNA Network and Immune Infiltration Analysis. BioMed Res. Int. 2021, 2021, 4532438. [Google Scholar] [CrossRef]
- Ge, S.; Hua, X.; Chen, J.; Xiao, H.; Zhang, L.; Zhou, J.; Liang, C.; Tai, S. Identification of a Costimulatory Molecule-Related Signature for Predicting Prognostic Risk in Prostate Cancer. Front. Genet. 2021, 12, 666300. [Google Scholar] [CrossRef]
- Mei, W.; Dong, Y.; Gu, Y.; Kapoor, A.; Lin, X.; Su, Y.; Vega Neira, S.; Tang, D. IQGAP3 is relevant to prostate cancer: A detailed presentation of potential pathomechanisms. J. Adv. Res. 2023, 54, 195–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, P.; Zhang, C.; Luo, Y.; Zhang, G.; Zeng, Q.; Wang, L.; Yang, Z.; Sun, N.; He, J. Tumor Necrosis Factor Family Member Profile Predicts Prognosis and Adjuvant Chemotherapy Benefit for Patients with Small-Cell Lung Cancer. Front. Immunol. 2021, 12, 745769. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xie, H.; Liu, X.; Shen, Q.; Wang, Z.; Hao, H.; Gu, Y. RELL1, a novel oncogene, accelerates tumor progression and regulates immune infiltrates in glioma. Int. Immunopharmacol. 2020, 87, 106707. [Google Scholar] [CrossRef]
- Kadkhoda, S.; Darbeheshti, F.; Rezaei, N.; Azizi-Tabesh, G.; Zolfaghari, F.; Tavakolibazaz, S.; Taslimi, R.; Tavakkoly-Bazzaz, J. Investigation of circRNA-miRNA-mRNA network in colorectal cancer using an integrative bioinformatics approach. Gastroenterol. Hepatol. Bed Bench. 2021, 14, 141–153. [Google Scholar]
- Musha, K.; Ge, X.; Ablikim, N.; Lu, B.; Chen, C.; Huang, J. Comprehensive Analysis of RELL2 as a Potential Biomarker Associated with Tumor Immune Infiltrating Cells in a Pan-Cancer Analysis. Dis. Markers 2022, 2022, 5009512. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, L.T.; Hu, L.J.; Lv, M. Comprehensive analysis of ECHDC3 as a potential biomarker and therapeutic target for acute myeloid leukemia: Bioinformatic analysis and experimental verification. Front. Oncol. 2022, 12, 947492. [Google Scholar] [CrossRef]
- Tang, S.J.; Shen, H.; An, O.; Hong, H.; Li, J.; Song, Y.; Han, J.; Tay, D.J.T.; Ng, V.H.E.; Bellido Molias, F.; et al. Cis- and trans-regulations of pre-mRNA splicing by RNA editing enzymes influence cancer development. Nat. Commun. 2020, 11, 799. [Google Scholar] [CrossRef]
- Li, Z.; Qin, C.; Zhao, B.; Wang, Y.; Li, T.; Zhao, Y.; Wang, W. DHX38 restricts chemoresistance by regulating the alternative pre-mRNA splicing of RELL2 in pancreatic ductal adenocarcinoma. PLoS Genet. 2023, 19, e1010847. [Google Scholar] [CrossRef]
- Cusick, J.K.; Mustian, A.; Jacobs, A.T.; Reyland, M.E. Identification of PLSCR1 as a protein that interacts with RELT family members. Mol. Cell. Biochem. 2012, 362, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Maines, L.W.; Fitzpatrick, L.R.; French, K.J.; Zhuang, Y.; Xia, Z.; Keller, S.N.; Upson, J.J.; Smith, C.D. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 2008, 53, 997–1012. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic bodies: Bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J. Nanobiotechnol. 2023, 21, 218. [Google Scholar] [CrossRef]
- Delpire, E.; Gagnon, K.B. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol. Genom. 2007, 28, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chang, H.Y.; Yang, X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef]
- Li, W.H.; Wang, F.; Song, G.Y.; Yu, Q.H.; Du, R.P.; Xu, P. PARP-1: A critical regulator in radioprotection and radiotherapy-mechanisms, challenges, and therapeutic opportunities. Front. Pharmacol. 2023, 14, 1198948. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Xu, S.; Murtagh, S.; Han, Y.; Wan, F.; Toriola, A.T. Breast Cancer Incidence Among US Women Aged 20 to 49 Years by Race, Stage, and Hormone Receptor Status. JAMA Netw. Open 2024, 7, e2353331. [Google Scholar] [CrossRef]
- Johansson, J.; Tabor, V.; Wikell, A.; Jalkanen, S.; Fuxe, J. TGF-β1-Induced Epithelial-Mesenchymal Transition Promotes Monocyte/Macrophage Properties in Breast Cancer Cells. Front. Oncol. 2015, 5, 3. [Google Scholar] [CrossRef]
- Zhong, L.; Ge, K.; Zu, J.C.; Zhao, L.H.; Shen, W.K.; Wang, J.F.; Zhang, X.G.; Gao, X.; Hu, W.; Yen, Y.; et al. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008, 10, R40. [Google Scholar] [CrossRef]
- Soussi, T. p53 Antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar]
- Chen, Y.T.; Scanlan, M.J.; Sahin, U.; Tureci, O.; Gure, A.O.; Tsang, S.; Williamson, B.; Stockert, E.; Pfreundschuh, M.; Old, L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 1997, 94, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Goodell, V.; Waisman, J.; Salazar, L.G.; de la Rosa, C.; Link, J.; Coveler, A.L.; Childs, J.S.; Fintak, P.A.; Higgins, D.M.; Disis, M.L. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol. Cancer Ther. 2008, 7, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wandall, H.H.; Blixt, O.; Tarp, M.A.; Pedersen, J.W.; Bennett, E.P.; Mandel, U.; Ragupathi, G.; Livingston, P.O.; Hollingsworth, M.A.; Taylor-Papadimitriou, J.; et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010, 70, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Ulanet, D.B.; Torbenson, M.; Dang, C.V.; Casciola-Rosen, L.; Rosen, A. Unique conformation of cancer autoantigen B23 in hepatoma: A mechanism for specificity in the autoimmune response. Proc. Natl. Acad. Sci. USA 2003, 100, 12361–12366. [Google Scholar] [CrossRef]
- Kobold, S.; Lutkens, T.; Cao, Y.; Bokemeyer, C.; Atanackovic, D. Autoantibodies against tumor-related antigens: Incidence and biologic significance. Hum. Immunol. 2010, 71, 643–651. [Google Scholar] [CrossRef]
- Lu, H.; Ladd, J.; Feng, Z.; Wu, M.; Goodell, V.; Pitteri, S.J.; Li, C.I.; Prentice, R.; Hanash, S.M.; Disis, M.L. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prev. Res. 2012, 5, 1036–1043. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Q.; Du, X.; Chen, Y.; Zhang, T. Targeted regulation of Rell2 by microRNA-18a is implicated in the anti-metastatic effect of polyphyllin VI in breast cancer cells. Eur. J. Pharmacol. 2019, 851, 161–173. [Google Scholar] [CrossRef]
- Chen, W.; Yazicioglu, M.; Cobb, M.H. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J. Biol. Chem. 2004, 279, 11129–11136. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Cobb, M.H.; Goldsmith, E.J. Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci. 2009, 18, 304–313. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Wu, J.; Dai, X.; Xu, J. High expression of OSR1 as a predictive biomarker for poor prognosis and lymph node metastasis in breast cancer. Breast Cancer Res. Treat. 2020, 182, 35–46. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Qin, J.; Wu, J.; Dai, X.; Xu, J. OSR1 phosphorylates the Smad2/3 linker region and induces TGF-β1 autocrine to promote EMT and metastasis in breast cancer. Oncogene 2021, 40, 68–84. [Google Scholar] [CrossRef]
- Lee, K.J.; Piett, C.G.; Andrews, J.F.; Mann, E.; Nagel, Z.D.; Gassman, N.R. Defective base excision repair in the response to DNA damaging agents in triple negative breast cancer. PLoS ONE 2019, 14, e0223725. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; Dixit, V.; Ashkenazi, A. Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 2009, 10, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; O’Rourke, K.; Yu, G.L.; Lyons, R.H.; Garg, M.; Duan, D.R.; Xing, L.; Gentz, R.; Ni, J.; Dixit, V.M. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 1996, 274, 990–992. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Rose, M.; Cardon, T.; Aboulouard, S.; Hajjaji, N.; Kobeissy, F.; Duhamel, M.; Fournier, I.; Salzet, M. Surfaceome Proteomic of Glioblastoma Revealed Potential Targets for Immunotherapy. Front. Immunol. 2021, 12, 746168. [Google Scholar] [CrossRef]
- Li, J.; Jia, J.; Zhu, W.; Chen, J.; Zheng, Q.; Li, D. Therapeutic effects on cancer of the active ingredients in rhizoma paridis. Front. Pharmacol. 2023, 14, 1095786. [Google Scholar] [CrossRef]
- Bateman, A.; Finn, R.D.; Sims, P.J.; Wiedmer, T.; Biegert, A.; Soding, J. Phospholipid scramblases and Tubby-like proteins belong to a new superfamily of membrane tethered transcription factors. Bioinformatics 2009, 25, 159–162. [Google Scholar] [CrossRef]
- Lin-Lee, Y.C.; Pham, L.V.; Tamayo, A.T.; Fu, L.; Zhou, H.J.; Yoshimura, L.C.; Decker, G.L.; Ford, R.J. Nuclear localization in the biology of the CD40 receptor in normal and neoplastic human B lymphocytes. J. Biol. Chem. 2006, 281, 18878–18887. [Google Scholar] [CrossRef]
- Kojima, T.; Morikawa, Y.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Senba, E.; Kitamura, T. TROY, a newly identified member of the tumor necrosis factor receptor superfamily, exhibits a homology with Edar and is expressed in embryonic skin and hair follicles. J. Biol. Chem. 2000, 275, 20742–20747. [Google Scholar] [CrossRef]
- Haselmann, V.; Kurz, A.; Bertsch, U.; Hubner, S.; Olempska-Muller, M.; Fritsch, J.; Hasler, R.; Pickl, A.; Fritsche, H.; Annewanter, F.; et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014, 146, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, U.; Roder, C.; Kalthoff, H.; Trauzold, A. Compartmentalization of TNF-related apoptosis-inducing ligand (TRAIL) death receptor functions: Emerging role of nuclear TRAIL-R2. Cell Death Dis. 2014, 5, e1390. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Shen, H.C.; Rivera Rosado, L.A.; Zhang, Y.; Di, X.; Zhang, B. Mislocalization of death receptors correlates with cellular resistance to their cognate ligands in human breast cancer cells. Oncotarget 2012, 3, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Liao, R.; Chen, X.; Wu, X.; Li, X.; Wang, Y.; Cao, Q.; Dong, C. Nuclear translocation of PLSCR1 activates STAT1 signaling in basal-like breast cancer. Theranostics 2020, 10, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Young, T.M.; Wang, Q.; Pe’ery, T.; Mathews, M.B. The human I-mfa domain-containing protein, HIC, interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol. Cell Biol. 2003, 23, 6373–6384. [Google Scholar] [CrossRef]
- Thebault, S.; Mesnard, J.M. How the sequestration of a protein interferes with its mechanism of action: Example of a new family of proteins characterized by a particular cysteine-rich carboxy-terminal domain involved in gene expression regulation. Curr. Protein Pept. Sci. 2001, 2, 155–167. [Google Scholar] [CrossRef]
- Ikeda, A.; Shahid, S.; Blumberg, B.R.; Suzuki, M.; Bartlett, J.D. ADAM10 is Expressed by Ameloblasts, Cleaves the RELT TNF Receptor Extracellular Domain and Facilitates Enamel Development. Sci. Rep. 2019, 9, 14086. [Google Scholar] [CrossRef]
- Bossen, C.; Ingold, K.; Tardivel, A.; Bodmer, J.L.; Gaide, O.; Hertig, S.; Ambrose, C.; Tschopp, J.; Schneider, P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 2006, 281, 13964–13971. [Google Scholar] [CrossRef]
- Vandevoorde, V.; Haegeman, G.; Fiers, W. Induced expression of trimerized intracellular domains of the human tumor necrosis factor (TNF) p55 receptor elicits TNF effects. J. Cell Biol. 1997, 137, 1627–1638. [Google Scholar] [CrossRef]
- He, X.L.; Garcia, K.C. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 2004, 304, 870–875. [Google Scholar] [CrossRef]
- Geerts, D.; Cusick, J.K.; Connelly, L. Editorial: The Tumor Necrosis Factor Superfamily: An Increasing Role in Breast Cancer. Front. Oncol. 2020, 10, 622588. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.A.; Carnevale, R.P.; Proietti, C.J.; Rosemblit, C.; Beguelin, W.; Salatino, M.; Charreau, E.H.; Frahm, I.; Sapia, S.; Brouckaert, P.; et al. TNF α acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp. Cell Res. 2008, 314, 509–529. [Google Scholar] [CrossRef] [PubMed]






| Sample | RELT Staining Intensity (Mean ± SEM) |
|---|---|
| Normal (N = 6) | 44.2 ± 17.2 |
| Malignant (N = 10) | 109.5 ± 16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batiste, M.; Joy, B.; Yee, C.K.; Cho, L.; Christensen, A.; Abed, I.; Nguyen, K.; Yanumula, A.; Chang, H.; Cho, E.D.; et al. RELT Is Upregulated in Breast Cancer and Induces Death in Breast Cancer Cells. Biomedicines 2024, 12, 2667. https://doi.org/10.3390/biomedicines12122667
Batiste M, Joy B, Yee CK, Cho L, Christensen A, Abed I, Nguyen K, Yanumula A, Chang H, Cho ED, et al. RELT Is Upregulated in Breast Cancer and Induces Death in Breast Cancer Cells. Biomedicines. 2024; 12(12):2667. https://doi.org/10.3390/biomedicines12122667
Chicago/Turabian StyleBatiste, Maryann, Bethany Joy, Cara K. Yee, Luke Cho, Ashley Christensen, Ihab Abed, Kailey Nguyen, Anusri Yanumula, Hannah Chang, Evan D. Cho, and et al. 2024. "RELT Is Upregulated in Breast Cancer and Induces Death in Breast Cancer Cells" Biomedicines 12, no. 12: 2667. https://doi.org/10.3390/biomedicines12122667
APA StyleBatiste, M., Joy, B., Yee, C. K., Cho, L., Christensen, A., Abed, I., Nguyen, K., Yanumula, A., Chang, H., Cho, E. D., Wang, W., Chou, E., Chang, E. H., Shyu, Y. L., Abram, A., Alcaide, J., Zhou, J., Gillespie, B., Senderovich, M., ... Cusick, J. K. (2024). RELT Is Upregulated in Breast Cancer and Induces Death in Breast Cancer Cells. Biomedicines, 12(12), 2667. https://doi.org/10.3390/biomedicines12122667







