Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Slice Electrophysiology
2.3. Chemicals and Drugs
2.4. Statistical Analysis
3. Results
3.1. Intrinsic Properties of SNr GABAergic Neurons from Middle-Aged WT and G2019S Mice
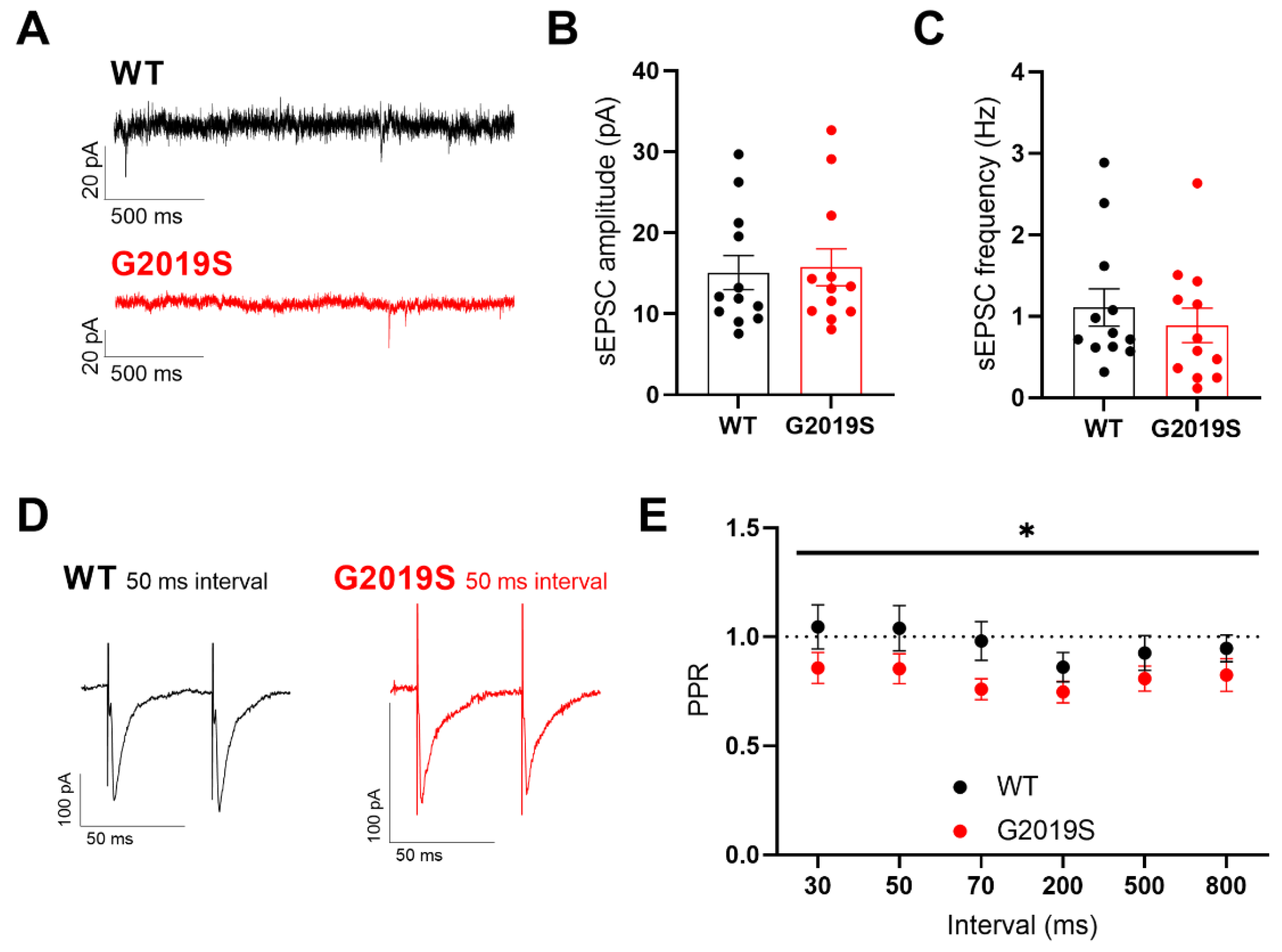
3.2. Glutamatergic Synaptic Transmission in SNr Neurons from Middle-Aged WT and G2019S Mice
3.3. NMDARs in SNr Neurons from Middle-Aged G2019S and WT Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Rodriguez, P.; Zampese, E.; Surmeier, D.J. Selective neuronal vulnerability in Parkinson’s disease. Prog. Brain Res. 2020, 252, 61–89. [Google Scholar] [PubMed]
- Pirkevi, C.; Lesage, S.; Brice, A.; Basak, A.N. From genes to proteins in mendelian Parkinson’s disease: An overview. Anat. Rec. 2009, 292, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Hinkle, K.M.; Davies, P.; Trushina, E.; Fiesel, F.C.; Christenson, T.A.; Schroeder, A.S.; Zhang, L.; Bowles, E.; Behrouz, B.; et al. Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 2015, 78, 172–195. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.G.; Falchi, M.; O’Sullivan, S.S.; Bonifati, V.; Durr, A.; Bressman, S.; Brice, A.; Aasly, J.; Zabetian, C.P.; Goldwurm, S.; et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008, 7, 583–590. [Google Scholar] [CrossRef]
- Di Maio, R.; Hoffman, E.K.; Rocha, E.M.; Keeney, M.T.; Sanders, L.H.; De Miranda, B.R.; Zharikov, A.; Van Laar, A.; Stepan, A.F. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaar5429. [Google Scholar] [CrossRef]
- Mallet, N.; Delgado, L.; Chazalon, M.; Miguelez, C.; Baufreton, J. Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping out of the Striatum. Cells 2019, 8, 1005. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- McElvain, L.E.; Chen, Y.; Moore, J.D.; Brigidi, G.S.; Bloodgood, B.L.; Lim, B.K.; Costa, R.M.; Kleinfeld, D. Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron 2021, 109, 1721–1738.e4. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Hou, X.; Zheng, B.; Melrose, H.L.; Mosley, T.; Wang, J.M. Human LRRK2 G2019S mutation represses post-synaptic protein PSD95 and causes cognitive impairment in transgenic mice. Neurobiol. Learn Mem. 2017, 142, 182–189. [Google Scholar] [CrossRef]
- Melrose, H.L.; Dachsel, J.C.; Behrouz, B.; Lincoln, S.J.; Yue, M.; Hinkle, K.M.; Kent, C.B.; Korvatska, E.; Taylor, J.P.; Witten, L.; et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol. Dis. 2010, 40, 503–517. [Google Scholar] [CrossRef]
- Winner, B.; Melrose, H.L.; Zhao, C.; Hinkle, K.M.; Yue, M.; Kent, C.; Braithwaite, A.T.; Ogholikhan, S.; Aigner, R.; Winkler, J.; et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol. Dis. 2011, 41, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Matikainen-Ankney, B.A.; Kezunovic, N.; Mesias, R.E.; Tian, Y.; Williams, F.M.; Huntley, G.W.; Benson, D.L. Altered Development of Synapse Structure and Function in Striatum Caused by Parkinson's Disease-Linked LRRK2-G2019S Mutation. J. Neurosci 2016, 36, 7128–7141. [Google Scholar] [CrossRef] [PubMed]
- Skiteva, O.; Yao, N.; Sitzia, G.; Chergui, K. LRRK2-G2019S mice display alterations in glutamatergic synaptic transmission in midbrain dopamine neurons. J. Neurochem. 2022, 161, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Skelton, P.D.; Tokars, V.; Parisiadou, L. LRRK2 at Striatal Synapses: Cell-Type Specificity and Mechanistic Insights. Cells 2022, 11, 169. [Google Scholar] [CrossRef]
- Ibañez-Sandoval, O.; Hernández, A.; Florán, B.; Galarraga, E.; Tapia, D.; Valdiosera, R.; Erlij, D.; Aceves, J.; Bargas, J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J. Neurophysiol. 2006, 95, 1800–1811. [Google Scholar] [CrossRef][Green Version]
- Qin, Q.; Zhi, L.T.; Li, X.T.; Yue, Z.Y.; Li, G.Z.; Zhang, H. Effects of LRRK2 Inhibitors on Nigrostriatal Dopaminergic Neurotransmission. CNS Neurosci. Ther. 2017, 23, 162–173. [Google Scholar] [CrossRef]
- Sitzia, G.; Mantas, I.; Zhang, X.; Svenningsson, P.; Chergui, K. NMDA receptors are altered in the substantia nigra pars reticulata and their blockade ameliorates motor deficits in experimental parkinsonism. Neuropharmacology 2020, 174, 108136. [Google Scholar] [CrossRef]
- Yao, N.; Skiteva, O.; Zhang, X.; Svenningsson, P.; Chergui, K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol. Psychiatry 2018, 23, 2066–2077. [Google Scholar] [CrossRef]
- Ferreira-Pinto, M.J.; Kanodia, H.; Falasconi, A.; Sigrist, M.; Esposito, M.S.; Arber, S. Functional diversity for body actions in the mesencephalic locomotor region. Cell 2021, 184, 4564–4578.e18. [Google Scholar] [CrossRef]
- Zhou, F.M.; Lee, C.R. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience 2011, 198, 69–94. [Google Scholar] [CrossRef]
- Dupuis, J.P.; Feyder, M.; Miguelez, C.; Garcia, L.; Morin, S.; Choquet, D.; Hosy, E.; Bezard, E.; Fisone, G.; Bioulac, B.H.; et al. Dopamine-Dependent Long-Term Depression at Subthalamo-Nigral Synapses Is Lost in Experimental Parkinsonism. J. Neurosci. 2013, 33, 14331–14341. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wei, W.; Zhou, F.-M. Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J. Neurophysiol. 2011, 106, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Tepper, J.M. Morphological and physiological properties of parvalbumin- and calretinin-containing gamma-aminobutyric acidergic neurons in the substantia nigra. J. Comp. Neurol. 2007, 500, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Tan, K.R. Synergistic Nigral Output Pathways Shape Movement. Cell Rep. 2019, 27, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Ramonet, D.; Daher, J.P.; Lin, B.M.; Stafa, K.; Kim, J.; Banerjee, R.; Westerlund, M.; Pletnikova, O.; Glauser, L.; Yang, L.; et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS ONE 2011, 6, e18568. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Bang, Y.; Choi, J.H.; Han, A.; Kwon, M.S.; Liu, K.H.; Choi, H.J. LRRK2 G2019S Induces Anxiety/Depression-like Behavior before the Onset of Motor Dysfunction with 5-HT(1A) Receptor Upregulation in Mice. J. Neurosci. 2018, 38, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Matikainen-Ankney, B.A.; Kezunovic, N.; Menard, C.; Flanigan, M.E.; Zhong, Y.; Russo, S.J.; Benson, D.L.; Huntley, G.W. Parkinson’s Disease-Linked LRRK2-G2019S Mutation Alters Synaptic Plasticity and Promotes Resilience to Chronic Social Stress in Young Adulthood. J. Neurosci. 2018, 38, 9700–9711. [Google Scholar] [CrossRef] [PubMed]
- Guevara, C.A.; Matikainen-Ankney, B.A.; Kezunovic, N.; LeClair, K.; Conway, A.P.; Menard, C.; Flanigan, M.E.; Pfau, M.; Russo, S.J.; Benson, D.L.; et al. LRRK2 mutation alters behavioral, synaptic, and nonsynaptic adaptations to acute social stress. J. Neurophysiol. 2020, 123, 2382–2389. [Google Scholar] [CrossRef]
- Foster, N.N.; Barry, J.; Korobkova, L.; Garcia, L.; Gao, L.; Becerra, M.; Sherafat, Y.; Peng, B.; Li, X.; Choi, J.H.; et al. The mouse cortico-basal ganglia-thalamic network. Nature 2021, 598, 188–194. [Google Scholar] [CrossRef]


| Measurement | WT Mice n = 11 Neurons | G2019S Mice n = 15 Neurons | Test | p Value |
|---|---|---|---|---|
| Interspike membrane potential (mV) | −51.92 ± 1.39 | −50.56 ± 0.79 | Unpaired t-test | 0.3711 |
| AP amplitude (mV) | 62.71 ± 2.33 | 57.80 ± 1.48 | Mann-Whitney | 0.2171 |
| AP half width (ms) | 0.53 ± 0.04 | 0.50 ± 0.03 | Unpaired t-test | 0.592 |
| AP threshold (mV) | −38.80 ± 0.95 | −38.19 ± 1.18 | Unpaired t-test | 0.7088 |
| AHP * amplitude (mV) | −47.89 ± 1.95 | −46.92 ± 1.13 | Unpaired t-test | 0.6521 |
| AHP * duration (ms) | 1.90 ± 0.26 | 2.20 ± 0.26 | Mann-Whitney | 0.4648 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitzia, G.; Skiteva, O.; Chergui, K. Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice. Biomolecules 2022, 12, 1635. https://doi.org/10.3390/biom12111635
Sitzia G, Skiteva O, Chergui K. Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice. Biomolecules. 2022; 12(11):1635. https://doi.org/10.3390/biom12111635
Chicago/Turabian StyleSitzia, Giacomo, Olga Skiteva, and Karima Chergui. 2022. "Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice" Biomolecules 12, no. 11: 1635. https://doi.org/10.3390/biom12111635
APA StyleSitzia, G., Skiteva, O., & Chergui, K. (2022). Neuronal Firing and Glutamatergic Synapses in the Substantia Nigra Pars Reticulata of LRRK2-G2019S Mice. Biomolecules, 12(11), 1635. https://doi.org/10.3390/biom12111635





