Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications
Abstract
:1. Obesity and the Ongoing Coronavirus Disease 2019 Crisis
2. Diet and Calories-Independent Patterns
3. Diet and Trefoil Factor Family: From Gastrointestinal Protection to Metabolic Implications
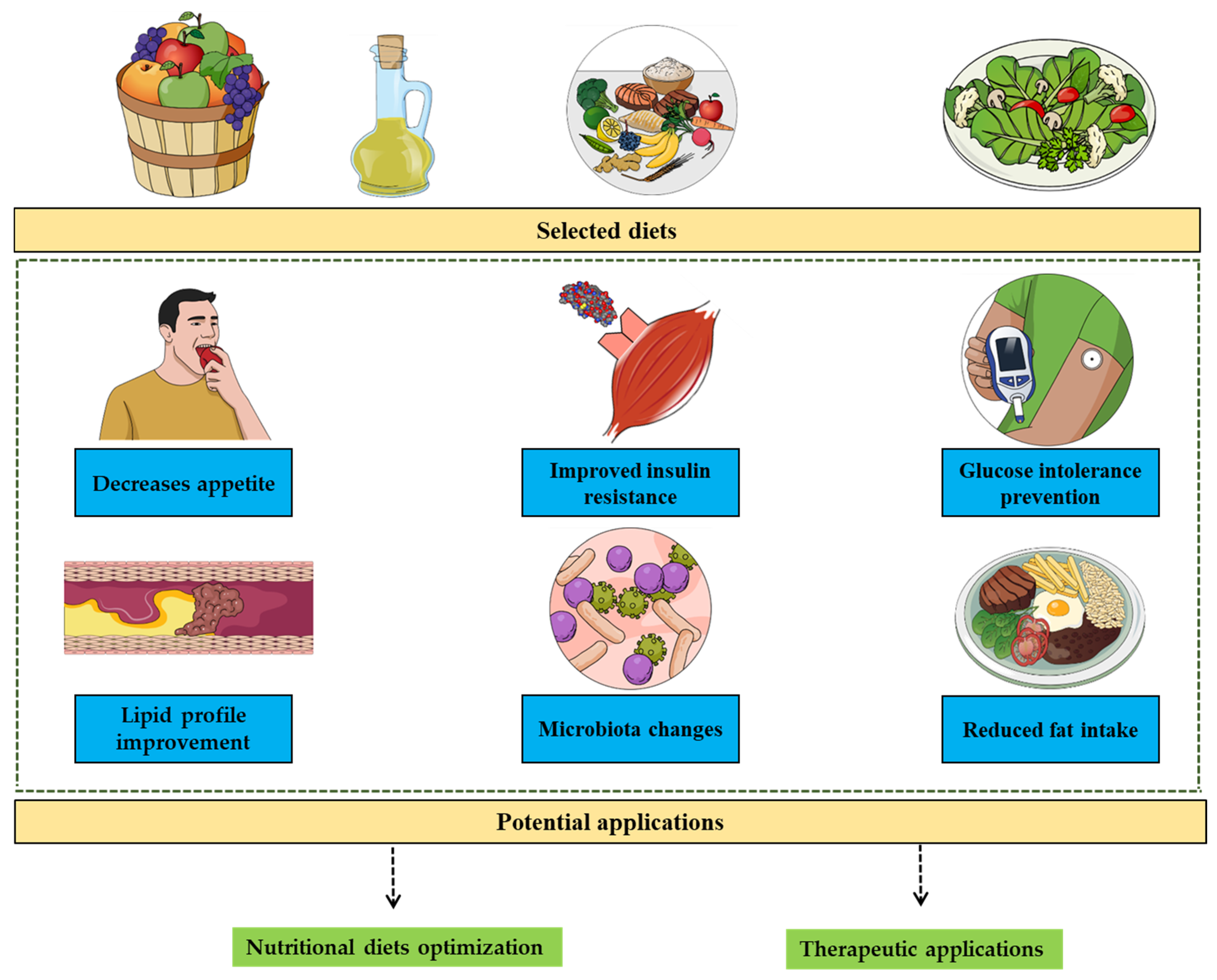
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Hulbert, A.J.; Else, P.L. Basal metabolic rate: History, composition, regulation, and usefulness. Physiol. Biochem. Zool. 2004, 77, 869–876. [Google Scholar] [CrossRef] [Green Version]
- van Baak, M.A. Physical activity and energy balance. Public Health Nutr. 1999, 2, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Roesler, A.; Kazak, L. UCP1-independent thermogenesis. Biochem. J. 2020, 477, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Wang, F.; Deeney, J.T.; Denis, G.V. Brd2 gene disruption causes “metabolically healthy” obesity: Epigenetic and chromatin-based mechanisms that uncouple obesity from type 2 diabetes. Vitam. Horm. 2013, 91, 49–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Liu, H.; Blanton, W.P.; Belkina, A.; Lebrasseur, N.K.; Denis, G.V. Brd2 disruption in mice causes severe obesity without Type 2 diabetes. Biochem. J. 2009, 425, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, H.L.; Raj, N.R.; Marquez de Prado, B.; Kuburas, A.; Luu, A.K.S.; Barr, G.A.; Recober, A. Trigeminal Pain Responses in Obese ob/ob Mice Are Modality-Specific. Neuroscience 2019, 415, 121–134. [Google Scholar] [CrossRef]
- Lindström, P. beta-cell function in obese-hyperglycemic mice [ob/ob Mice]. Adv. Exp. Med. Biol. 2010, 654, 463–477. [Google Scholar] [CrossRef]
- Lopomo, A.; Burgio, E.; Migliore, L. Epigenetics of Obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 151–184. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and epigenetics in obesity. Metab. Clin. Exp. 2019, 92, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.Y.; Lee, Y.S.; Ooi, D.S.Q. Engineering the Gut Microbiome for Treatment of Obesity: A Review of Current Understanding and Progress. Biotechnol. J. 2020, 15, e2000013. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obese Animals as Models for Numerous Diseases: Advantages and Applications. Medicina 2021, 57, 399. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Piché, M.E.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Regeneration during Obesity: An Impaired Homeostasis. Animals 2020, 10, 2344. [Google Scholar] [CrossRef]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharm. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Silva Junior, G.B.; Bentes, A.C.; Daher, E.F.; Matos, S.M. Obesity and kidney disease. J. Bras. Nephrol. 2017, 39, 65–69. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharm. 2016, 29, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Henao-Mejia, J.; Henrickson, S.E. Obesity and immune status in children. Curr. Opin. Pediatr. 2020, 32, 805–815. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Impact of Adiposity and Fat Distribution, Rather Than Obesity, on Antibodies as an Illustration of Weight-Loss-Independent Exercise Benefits. Medicines 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Osornio, S.A.; Cruz-Hernández, T.R.; Drago-Serrano, M.E.; Campos-Rodríguez, R. Immunity to influenza: Impact of obesity. Obes. Res. Clin. Pract. 2019, 13, 419–429. [Google Scholar] [CrossRef]
- Bischof, G.N.; Park, D.C. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Xanthopoulos, M.; Tapia, I.E. Obesity and common respiratory diseases in children. Paediatr. Respir. Rev. 2017, 23, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Jubber, A.S. Respiratory complications of obesity. Int. J. Clin. Pract. 2004, 58, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Bhattacharya, S. Obesity and fertility. Horm. Mol. Biol. Clin. Investig. 2015, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis Measures: Health Protective Properties? Medicines 2021, 8, 49. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Will an obesity pandemic replace the coronavirus disease-2019 (COVID-19) pandemic? Med. Hypotheses 2020, 144, 110042. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis: Losing Our Immunity When We Need It the Most. Biology 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Albashir, A.A.D. The potential impacts of obesity on COVID-19. Clin. Med. 2020, 20, e109–e113. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B.; Coca, A. Physical Activity Change during COVID-19 Confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef]
- Pellegrini, C.A.; Webster, J.; Hahn, K.R.; Leblond, T.L.; Unick, J.L. Relationship between stress and weight management behaviors during the COVID-19 pandemic among those enrolled in an internet program. Obes. Sci. Pract. 2021, 7, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef]
- Caci, G.; Albini, A.; Malerba, M.; Noonan, D.M.; Pochetti, P.; Polosa, R. COVID-19 and Obesity: Dangerous Liaisons. J. Clin. Med. 2020, 9, 2511. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. Int. J. Mol. Sci. 2020, 21, 5793. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. 2017, 11, 73–79. [Google Scholar] [CrossRef]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front. Endocrinol. 2021, 12, 649525. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, A.; Rahimi, L.; Ismail-Beigi, F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J. Diabetes 2020, 12, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Del Turco, S.; Vianello, A.; Ragusa, R.; Caselli, C.; Basta, G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb. Res. 2020, 196, 143–151. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 and diabetes: A bidirectional relationship. Clin. Investig. Arterioscler. 2021, 33, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Post-Coronavirus Disease-2019 (COVID-19): Toward a Severe Multi-Level Health Crisis? Med. Sci. 2021, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 vaccine: Where are we now and where should we go? Expert Rev. Vaccines 2021, 20, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Cole, A.G.; Laxer, R.E.; Leatherdale, S. Social Consequences of Overweight and Obesity among Ontario High School Students. Can. J. Diabetes 2015, 39, S67. [Google Scholar] [CrossRef]
- Bacchini, D.; Licenziati, M.R.; Garrasi, A.; Corciulo, N.; Driul, D.; Tanas, R.; Fiumani, P.M.; Di Pietro, E.; Pesce, S.; Crinò, A.; et al. Bullying and Victimization in Overweight and Obese Outpatient Children and Adolescents: An Italian Multicentric Study. PLoS ONE 2015, 10, e0142715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, V.M.; Breen, D.M.; Fortin, J.P.; Liou, A.; Kuzmiski, J.B.; Loomis, A.K.; Rives, M.L.; Shah, B.; Carpino, P.A. Latest approaches for the treatment of obesity. Expert Opin. Drug Discov. 2015, 10, 825–839. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L. Obesity medications in development. Expert Opin. Investig. Drugs 2020, 29, 63–71. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca-Junior, S.J.; Sá, C.G.; Rodrigues, P.A.; Oliveira, A.J.; Fernandes-Filho, J. Physical exercise and morbid obesity: A systematic review. Arq. Bras. Cir. Dig. 2013, 26 (Suppl. 1), 67–73. [Google Scholar] [CrossRef] [Green Version]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 59–63. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Harvey-Berino, J. Calorie restriction is more effective for obesity treatment than dietary fat restriction. Ann. Behav. Med. 1999, 21, 35–39. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef]
- Ghosh, S.; O’Connell, J.F.; Carlson, O.D.; González-Mariscal, I.; Kim, Y.; Moaddel, R.; Ghosh, P.; Egan, J.M. Linoleic acid in diets of mice increases total endocannabinoid levels in bowel and liver: Modification by dietary glucose. Obes. Sci. Pract. 2019, 5, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Lecheminant, J.D.; Gibson, C.A.; Sullivan, D.K.; Hall, S.; Washburn, R.; Vernon, M.C.; Curry, C.; Stewart, E.; Westman, E.C.; Donnelly, J.E. Comparison of a low carbohydrate and low fat diet for weight maintenance in overweight or obese adults enrolled in a clinical weight management program. Nutr. J. 2007, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Brinkworth, G.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight-loss diet and an isocaloric low-fat diet on bone health in obese adults. Nutrition 2016, 32, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- LeCheminant, J.D.; Smith, B.K.; Westman, E.C.; Vernon, M.C.; Donnelly, J.E. Comparison of a reduced carbohydrate and reduced fat diet for LDL, HDL, and VLDL subclasses during 9-months of weight maintenance subsequent to weight loss. Lipids Health Dis. 2010, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Meckling, K.A.; O’Sullivan, C.; Saari, D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J. Clin. Endocrinol. Metab. 2004, 89, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, D.G.; Hill, J.O.; Pope-Cordle, J.; Arnold, D.; Virts, K.L.; Katahn, M. Randomized evaluation of a low fat ad libitum carbohydrate diet for weight reduction. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 623–629. [Google Scholar]
- Astrup, A.; Astrup, A.; Buemann, B.; Flint, A.; Raben, A. Low-fat diets and energy balance: How does the evidence stand in 2002? Proc. Nutr. Soc. 2002, 61, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A. The role of dietary fat in the prevention and treatment of obesity. Efficacy and safety of low-fat diets. Int. J. Obes. Relat. Metab. Disord. 2001, 25 (Suppl. 1), S46–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, P.M.; Hegele, R.A. Functional foods and dietary supplements for the management of dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Aronson, J.K. Defining Nutraceuticals: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharm. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Tramontin, N.D.S.; Luciano, T.F.; Marques, S.O.; de Souza, C.T.; Muller, A.P. Ginger and avocado as nutraceuticals for obesity and its comorbidities. Phytother. Res. 2020, 34, 1282–1290. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Boivin, D.; Blanchette, M.; Barrette, S.; Moghrabi, A.; Béliveau, R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007, 27, 937–948. [Google Scholar]
- Sekizawa, H.; Ikuta, K.; Mizuta, K.; Takechi, S.; Suzutani, T. Relationship between polyphenol content and anti-influenza viral effects of berries. J. Sci. Food Agric. 2013, 93, 2239–2241. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.S.; Varin, T.V.; St-Pierre, P.; Pilon, G.; Tremblay, A.; Marette, A. A polyphenol-rich cranberry extract protects against endogenous exposure to persistent organic pollutants during weight loss in mice. Food Chem. Toxicol. 2020, 146, 111832. [Google Scholar] [CrossRef]
- Pannu, P.K.; Calton, E.K.; Soares, M.J. Calcium and Vitamin D in Obesity and Related Chronic Disease. Adv. Food Nutr. Res. 2016, 77, 57–100. [Google Scholar] [CrossRef]
- Kuroda, M.; Sakaue, H. Role of vitamin D and calcium in obesity and type 2 diabetes. Clin. Calcium. 2016, 26, 349–354. [Google Scholar] [PubMed]
- Sathyamoorthy, N.; Wang, T.T.; Phang, J.M. Stimulation of pS2 expression by diet-derived compounds. Cancer Res. 1994, 54, 957–961. [Google Scholar] [PubMed]
- Van Horn, L.; Huffman, M.D. Eat to Treat Heart Failure. Circ. Heart Fail. 2018, 11, e005367. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Kokkinos, A.; Katechaki, E.; Ros, E.; Mantzoros, C.S. Mediterranean diet as a nutritional approach for COVID-19. Metabolism 2021, 114, 154407. [Google Scholar] [CrossRef]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [CrossRef]
- Yoshioka, M.; Imanaga, M.; Ueyama, H.; Yamane, M.; Kubo, Y.; Boivin, A.; St-Amand, J.; Tanaka, H.; Kiyonaga, A. Maximum tolerable dose of red pepper decreases fat intake independently of spicy sensation in the mouth. Br. J. Nutr. 2004, 91, 991–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerterp-Plantenga, M.S.; Smeets, A.; Lejeune, M.P. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int. J. Obes. 2005, 29, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Mathern, J.R.; Raatz, S.K.; Thomas, W.; Slavin, J.L. Effect of fenugreek fiber on satiety, blood glucose and insulin response and energy intake in obese subjects. Phytother. Res. 2009, 23, 1543–1548. [Google Scholar] [CrossRef]
- Choi, B.S.; Daniel, N.; Houde, V.P.; Ouellette, A.; Marcotte, B.; Varin, T.V.; Vors, C.; Feutry, P.; Ilkayeva, O.; Ståhlman, M.; et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat. Commun. 2021, 12, 3377. [Google Scholar] [CrossRef]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR-/-/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samane, S.; Christon, R.; Dombrowski, L.; Turcotte, S.; Charrouf, Z.; Lavigne, C.; Levy, E.; Bachelard, H.; Amarouch, H.; Marette, A.; et al. Fish oil and argan oil intake differently modulate insulin resistance and glucose intolerance in a rat model of dietary-induced obesity. Metabolism 2009, 58, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Steerenberg, P.A.; Beekhof, P.K.; Feskens, E.J.; Lips, C.J.; Höppener, J.W.; Beems, R.B. Long-term effect of fish oil diet on basal and stimulated plasma glucose and insulin levels in ob/ob mice. Diabetes Nutr. Metab. 2002, 15, 205–214. [Google Scholar]
- Gao, X.; Xu, J.; Jiang, C.; Zhang, Y.; Xue, Y.; Li, Z.; Wang, J.; Xue, C.; Wang, Y. Fish oil ameliorates trimethylamine N-oxide-exacerbated glucose intolerance in high-fat diet-fed mice. Food Funct. 2015, 6, 1117–1125. [Google Scholar] [CrossRef]
- Fedor, D.; Kelley, D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Baynes, H.W.; Mideksa, S.; Ambachew, S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 2018, 7, 81–87. [Google Scholar] [CrossRef]
- Richards, P.; Pais, R.; Habib, A.M.; Brighton, C.A.; Yeo, G.S.; Reimann, F.; Gribble, F.M. High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides 2016, 77, 21–27. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Zhou, S.; Li, J.; Gao, J.; Owyang, C. High-fat diet-induced vagal afferent dysfunction via upregulation of 2-pore domain potassium TRESK channel. JCI Insight 2019, 4, e130402. [Google Scholar] [CrossRef] [Green Version]
- Grigorova, N.; Ivanova, Z.; Bjorndal, B.; Vachkova, E.; Penchev, G.; Berge, R.; Ribarski, S.; Georgieva, T.M.; Yonkova, P.; Georgiev, I.P. Effect of fish oil supplementation and restricted feeding on body fat distribution and blood lipid profile in a rabbit model of castration-induced obesity. Res. Vet. Sci. 2019, 124, 99–105. [Google Scholar] [CrossRef]
- Schubert, M.M.; Irwin, C.; Seay, R.F.; Clarke, H.E.; Allegro, D.; Desbrow, B. Caffeine, coffee, and appetite control: A review. Int. J. Food Sci. Nutr. 2017, 68, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Gavrieli, A.; Karfopoulou, E.; Kardatou, E.; Spyreli, E.; Fragopoulou, E.; Mantzoros, C.S.; Yannakoulia, M. Effect of different amounts of coffee on dietary intake and appetite of normal-weight and overweight/obese individuals. Obesity 2013, 21, 1127–1132. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Daoust, L.; Pilon, G.; Marette, A.; Tremblay, A. Potential therapeutic applications of the gut microbiome in obesity: From brain function to body detoxification. Int. J. Obes. 2020, 44, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Trefflich, I.; Jabakhanji, A.; Menzel, J.; Blaut, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2990–3004. [Google Scholar] [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- da Silva, T.F.; Casarotti, S.N.; de Oliveira, G.L.V.; Penna, A.L.B. The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit. Rev. Food Sci. Nutr. 2021, 61, 337–355. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of different hypercaloric diets on obesity features in rats: A metagenomics and metabolomics integrative approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Jaiswal, S.; Libby, P. Clonal haematopoiesis: Connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Petcu, E.B.; Capitanescu, B.; Hermann, D.M.; Radu, E.; Gresita, A. Ageing as a risk factor for cerebral ischemia: Underlying mechanisms and therapy in animal models and in the clinic. Mech. Ageing Dev. 2020, 190, 111312. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kozakova, M.; Palombo, C. Vascular Ageing and Aerobic Exercise. Int. J. Environ. Res. Public Health 2021, 18, 666. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.G.; Jackson, E.A.; Richardson, C.R. Exercise Prescriptions in Older Adults. Am. Fam. Physician 2017, 95, 425–432. [Google Scholar]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Flicker, L.; Lautenschlager, N.T.; Almeida, O.P. Healthy mental ageing. J. Br. Menopause Soc. 2006, 12, 92–96. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing. Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.A. Free radicals and redox regulation in ageing. Free Radic. Biol. Med. 2019, 134, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borrás, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Cuadrado, A.; Engedal, N.; Jirsova, Z.; Cahova, M. The Role of Free Radicals in Autophagy Regulation: Implications for Ageing. Oxidative Med. Cell. Longev. 2018, 2018, 2450748. [Google Scholar] [CrossRef] [Green Version]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant Fortification of the Diet for Anti-Ageing Effects: A Review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef]
- Bramorska, A.; Zarzycka, W.; Podolecka, W.; Kuc, K.; Brzezicka, A. Age-Related Cognitive Decline May Be Moderated by Frequency of Specific Food Products Consumption. Nutrients 2021, 13, 2504. [Google Scholar] [CrossRef]
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S5–S8. [Google Scholar] [CrossRef]
- Yin, Y.; Shan, H.Q.; Huang, W.; Wu, Y.M.; Lu, H.; Jin, Y. Bioinformatic analysis of miRNA expression patterns in TFF2 knock-out mice. Genet. Mol. Res. 2014, 13, 8502–8510. [Google Scholar] [CrossRef]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef] [Green Version]
- Thim, L.; May, F.E. Structure of mammalian trefoil factors and functional insights. Cell Mol. Life Sci. 2005, 62, 2956–2973. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Trefoil factors and human gastric cancer (review). Int. J. Mol. Med. 2003, 12, 3–9. [Google Scholar] [CrossRef]
- Dignass, A.; Lynch-Devaney, K.; Kindon, H.; Thim, L.; Podolsky, D.K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J. Clin. Investig. 1994, 94, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef]
- Shah, A.A.; Leidinger, P.; Keller, A.; Wendschlag, A.; Meese, E.; Blin, N. Altered miRNA expression patterns in Tff2 knock-out mice correlate with cellular pathways of neoplastic development and caloric metabolism. Int. J. Mol. Med. 2012, 29, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga Emidio, N.; Brierley, S.M.; Schroeder, C.I.; Muttenthaler, M. Structure, Function, and Therapeutic Potential of the Trefoil Factor Family in the Gastrointestinal Tract. ACS Pharm. Transl. Sci. 2020, 3, 583–597. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, L.; Cui, Y.; Zhang, H.; Chen, Q.; Cui, A.; Fang, F.; Chang, Y. Tff3, as a novel peptide, regulates hepatic glucose metabolism. PLoS ONE 2013, 8, e75240. [Google Scholar] [CrossRef] [Green Version]
- Arnold, P.; Rickert, U.; Helmers, A.K.; Spreu, J.; Schneppenheim, J.; Lucius, R. Trefoil factor 3 shows anti-inflammatory effects on activated microglia. Cell Tissue Res. 2016, 365, 3–11. [Google Scholar] [CrossRef]
- Braga Emidio, N.; Meli, R.; Tran, H.N.T.; Baik, H.; Morisset-Lopez, S.; Elliott, A.G.; Blaskovich, M.A.T.; Spiller, S.; Beck-Sickinger, A.G.; Schroeder, C.I.; et al. Chemical Synthesis of TFF3 Reveals Novel Mechanistic Insights and a Gut-Stable Metabolite. J. Med. Chem. 2021, 64, 9484–9495. [Google Scholar] [CrossRef]
- Belle, N.M.; Ji, Y.; Herbine, K.; Wei, Y.; Park, J.; Zullo, K.; Hung, L.Y.; Srivatsa, S.; Young, T.; Oniskey, T.; et al. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat. Commun. 2019, 10, 4408. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Gardner, J.; Wu, X.; Rulifson, I.; Wang, J.; Xiong, Y.; Ye, J.; Belouski, E.; Cao, P.; Tang, J.; et al. Trefoil Factor 3 (TFF3) Is Regulated by Food Intake, Improves Glucose Tolerance and Induces Mucinous Metaplasia. PLoS ONE 2015, 10, e0126924. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Bujak, I.T.; Sobočanec, S.; Mihalj, M.; Novak, S.; Ćosić, A.; Levak, M.T.; Kopačin, V.; Mihaljević, B.; Balog, T.; et al. Trefoil Factor 3 Deficiency Affects Liver Lipid Metabolism. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, H.; Yang, R.; Luan, X.; Zhang, L.; Jin, Q.; Jin, Y.; Xue, J. Mouse trefoil factor 3 ameliorated high-fat-diet-induced hepatic steatosis via increasing peroxisome proliferator-activated receptor-α-mediated fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E436–E445. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.M.; Chalinor, H.V.; Walduck, A.; Pavlic, D.I.; Däbritz, J.; Dubeykovskaya, Z.; Wang, T.C.; Menheniott, T.R.; Giraud, A.S. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G12–G24. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007, 75, 471–480. [Google Scholar] [CrossRef] [Green Version]
- De Giorgio, M.R.; Yoshioka, M.; Riedl, I.; Moreault, O.; Cherizol, R.G.; Shah, A.A.; Blin, N.; Richard, D.; St-Amand, J. Trefoil factor family member 2 (Tff2) KO mice are protected from high-fat diet-induced obesity. Obesity 2013, 21, 1389–1395. [Google Scholar] [CrossRef]
- Lebherz-Eichinger, D.; Tudor, B.; Ankersmit, H.J.; Reiter, T.; Haas, M.; Einwallner, E.; Roth-Walter, F.; Krenn, C.G.; Roth, G.A. Increased trefoil factor 2 levels in patients with chronic kidney disease. PLoS ONE 2017, 12, e0174551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2 (TFF2) as an Inflammatory-Induced and Anti-Inflammatory Tissue Repair Factor. Animals 2020, 10, 1646. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. High-Fat Diet-Induced Trefoil Factor Family Member 2 (TFF2) to Counteract the Immune-Mediated Damage in Mice. Animals 2021, 11, 258. [Google Scholar] [CrossRef]
- Baus-Loncar, M.; Kayademir, T.; Takaishi, S.; Wang, T. Trefoil factor family 2 deficiency and immune response. Cell. Mol. Life Sci. 2005, 62, 2947–2955. [Google Scholar] [CrossRef]
- Yoshioka, M.; Bolduc, C.; Raymond, V.; St-Amand, J. High-fat meal-induced changes in the duodenum mucosa transcriptome. Obesity 2008, 16, 2302–2307. [Google Scholar] [CrossRef]
- Mucunguzi, O.; Melouane, A.; Ghanemi, A.; Yoshioka, M.; Boivin, A.; Calvo, E.L.; St-Amand, J. Identification of the principal transcriptional regulators for low-fat and high-fat meal responsive genes in small intestine. Nutr. Metab. 2017, 14, 66. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2 Expression as an Indicator of the Severity of the High-Fat Diet-Induced Obesity. Genes 2021, 12, 1505. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Trefoil Factor Family Member 2: From a High-Fat-Induced Gene to a Potential Obesity Therapy Target. Metabolites 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chien, E.Y.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A. Targeting G protein coupled receptor-related pathways as emerging molecular therapies. Saudi Pharm. J. 2015, 23, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubeykovskaya, Z.; Dubeykovskiy, A.; Solal-Cohen, J.; Wang, T.C. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J. Biol. Chem. 2009, 284, 3650–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, K.; Kodama, S.; Nakano, Y.; Minaki-Nakagawa, Y.; Aoyama, Y.; Sakikubo, M.; Goto, T.; Yoshida, M.; Masui, T.; Yamamoto, T.; et al. Exocrine tissue-driven TFF2 prevents apoptotic cell death of endocrine lineage during pancreas organogenesis. Sci Rep. 2019, 9, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orime, K.; Shirakawa, J.; Togashi, Y.; Tajima, K.; Inoue, H.; Ito, Y.; Sato, K.; Nakamura, A.; Aoki, K.; Goshima, Y.; et al. Trefoil factor 2 promotes cell proliferation in pancreatic β-cells through CXCR-4-mediated ERK1/2 phosphorylation. Endocrinology 2013, 154, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Chen, W.; Dai, G.; Huang, Y. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4. Int. J. Mol. Med. 2020, 45, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Heuser-Baker, J.; Herlea-Pana, O.; Zhang, N.; Szweda, L.I.; Griffin, T.M.; Barlic-Dicen, J. Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J. 2014, 28, 4534–4550. [Google Scholar] [CrossRef] [Green Version]
- Mouhieddine, T.H.; Ahmad, Y.; Barlogie, B.; Jagannath, S.; Teruya-Feldstein, J.; Richter, J. Increased Muscle CXCR4 Expression in the Setting of Rare Muscle-invasive Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, e341–e344. [Google Scholar] [CrossRef]
- Banisadr, G.; Fontanges, P.; Haour, F.; Kitabgi, P.; Rostène, W.; Mélik Parsadaniantz, S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002, 16, 1661–1671. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, Y.; Liu, X. CXCR4 as a prognostic biomarker in gastrointestinal cancer: A meta-analysis. Biomarkers 2019, 24, 510–516. [Google Scholar] [CrossRef]
- Urosevic, J.; Blasco, M.T.; Llorente, A.; Bellmunt, A.; Berenguer-Llergo, A.; Guiu, M.; Cañellas, A.; Fernandez, E.; Burkov, I.; Clapés, M.; et al. ERK1/2 Signaling Induces Upregulation of ANGPT2 and CXCR4 to Mediate Liver Metastasis in Colon Cancer. Cancer Res. 2020, 80, 4668–4680. [Google Scholar] [CrossRef]
- Shibata, M.; Banno, R.; Sugiyama, M.; Tominaga, T.; Onoue, T.; Tsunekawa, T.; Azuma, Y.; Hagiwara, D.; Lu, W.; Ito, Y.; et al. AgRP Neuron-Specific Deletion of Glucocorticoid Receptor Leads to Increased Energy Expenditure and Decreased Body Weight in Female Mice on a High-Fat Diet. Endocrinology 2016, 157, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y. Role of central melanocortin signaling in eating disorders. Psychopharmacol. Bull. 2001, 35, 45–65. [Google Scholar] [PubMed]
- Benoit, S.; Schwartz, M.; Baskin, D.; Woods, S.C.; Seeley, R.J. CNS melanocortin system involvement in the regulation of food intake. Horm. Behav. 2000, 37, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Biebermann, H.; Kühnen, P.; Kleinau, G.; Krude, H. The neuroendocrine circuitry controlled by POMC, MSH, and AGRP. Handb. Exp. Pharm. 2012, 209, 47–75. [Google Scholar] [CrossRef]
- Seeley, R.J.; Drazen, D.L.; Clegg, D.J. The critical role of the melanocortin system in the control of energy balance. Annu. Rev. Nutr. 2004, 24, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Scholven, J.; Taras, D.; Sharbati, S.; Schön, J.; Gabler, C.; Huber, O.; Meyer zum Büschenfelde, D.; Blin, N.; Einspanier, R. Intestinal expression of TFF and related genes during postnatal development in a piglet probiotic trial. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 23, 143–156. [Google Scholar] [CrossRef]
- Dossinger, V.; Kayademir, T.; Blin, N.; Gött, P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell. Physiol. Biochem. 2002, 12, 197–206. [Google Scholar] [CrossRef]
- Capuano, E.; Janssen, A.E.M. Food Matrix and Macronutrient Digestion. Annu. Rev. Food Sci. Technol. 2021, 12, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E559–E572. [Google Scholar] [CrossRef]
- Vettor, R.; Di Vincenzo, A.; Maffei, P.; Rossato, M. Regulation of energy intake and mechanisms of metabolic adaptation or maladaptation after caloric restriction. Rev. Endocr. Metab. Disord. 2020, 21, 399–409. [Google Scholar] [CrossRef]
- Jequier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Kirwan, J.P.; Greenway, F.L. Obesity, the most common comorbidity in SARS-CoV-2: Is leptin the link? Int. J. Obes. 2020, 44, 1810–1817. [Google Scholar] [CrossRef]
- Ishioka, K.; Hatai, H.; Komabayashi, K.; Soliman, M.M.; Shibata, H.; Honjoh, T.; Kimura, K.; Saito, M. Diurnal variations of serum leptin in dogs: Effects of fasting and re-feeding. Vet. J. 2005, 169, 85–90. [Google Scholar] [CrossRef]
- Kiani, A. Temporal Changes in Plasma Concentration of Leptin, IGF-1, Insulin and Metabolites Under Extended Fasting and Re-Feeding Conditions in Growing Lambs. Int. J. Endocrinol. Metab. 2013, 11, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Ko, Y.H.; Hsu, Y.P.; Ho, L.T. Plasma leptin response to oral glucose tolerance and fasting/re-feeding tests in rats with fructose-induced metabolic derangements. Life Sci. 2006, 78, 1155–1162. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharm. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Ghanemi, A. Is mapping borders between pharmacology and toxicology a necessity? Saudi Pharm. J. 2014, 22, 489–490. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A. How to define a pharmacological or a toxic food? Alex. J. Med. 2015, 51, 359–360. [Google Scholar] [CrossRef]
- Ghanemi, A. Cell cultures in drug development: Applications, challenges and limitations. Saudi Pharm. J. 2015, 23, 453–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thérien, A.; Cieślak, A.; Verreault, M.; Perreault, M.; Trottier, J.; Gobeil, S.; Vohl, M.-C.; Barbier, O. Omega-3 Polyunsaturated Fatty Acid: A Pharmaco-Nutraceutical Approach to Improve the Responsiveness to Ursodeoxycholic Acid. Nutrients 2021, 13, 2617. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical--definition and introduction. AAPS Pharm. Sci. 2003, 5, E25. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Yoshioka, M.; St-Amand, J. Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications. Biomolecules 2021, 11, 1830. https://doi.org/10.3390/biom11121830
Ghanemi A, Yoshioka M, St-Amand J. Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications. Biomolecules. 2021; 11(12):1830. https://doi.org/10.3390/biom11121830
Chicago/Turabian StyleGhanemi, Abdelaziz, Mayumi Yoshioka, and Jonny St-Amand. 2021. "Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications" Biomolecules 11, no. 12: 1830. https://doi.org/10.3390/biom11121830
APA StyleGhanemi, A., Yoshioka, M., & St-Amand, J. (2021). Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications. Biomolecules, 11(12), 1830. https://doi.org/10.3390/biom11121830






