Upper Limb Neural Tension Test and Spinal Biomechanics: Insights from a Longitudinal Pilot Study
Abstract
:1. Introduction
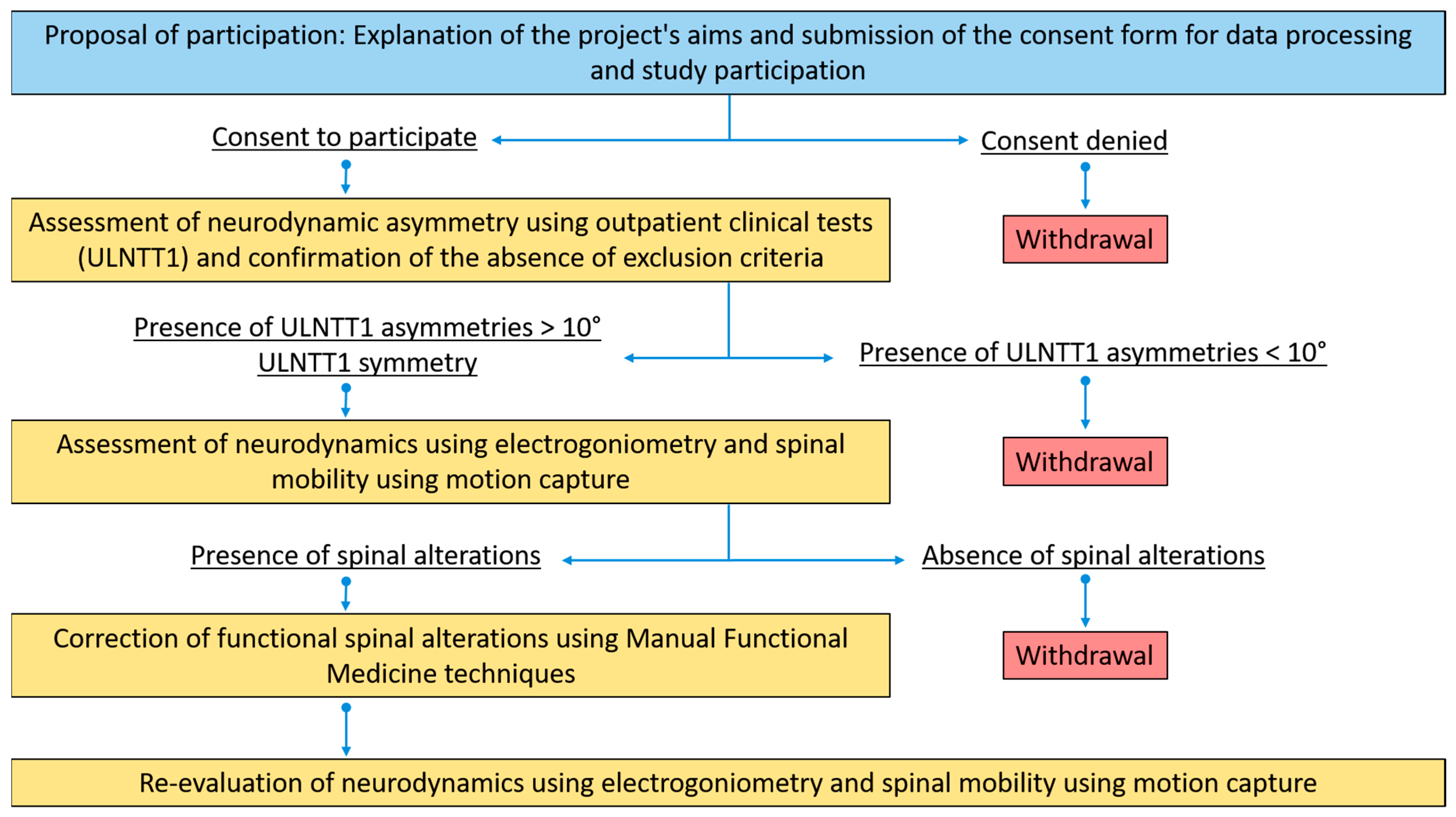
2. Materials and Methods
2.1. Study Design
2.2. Participants
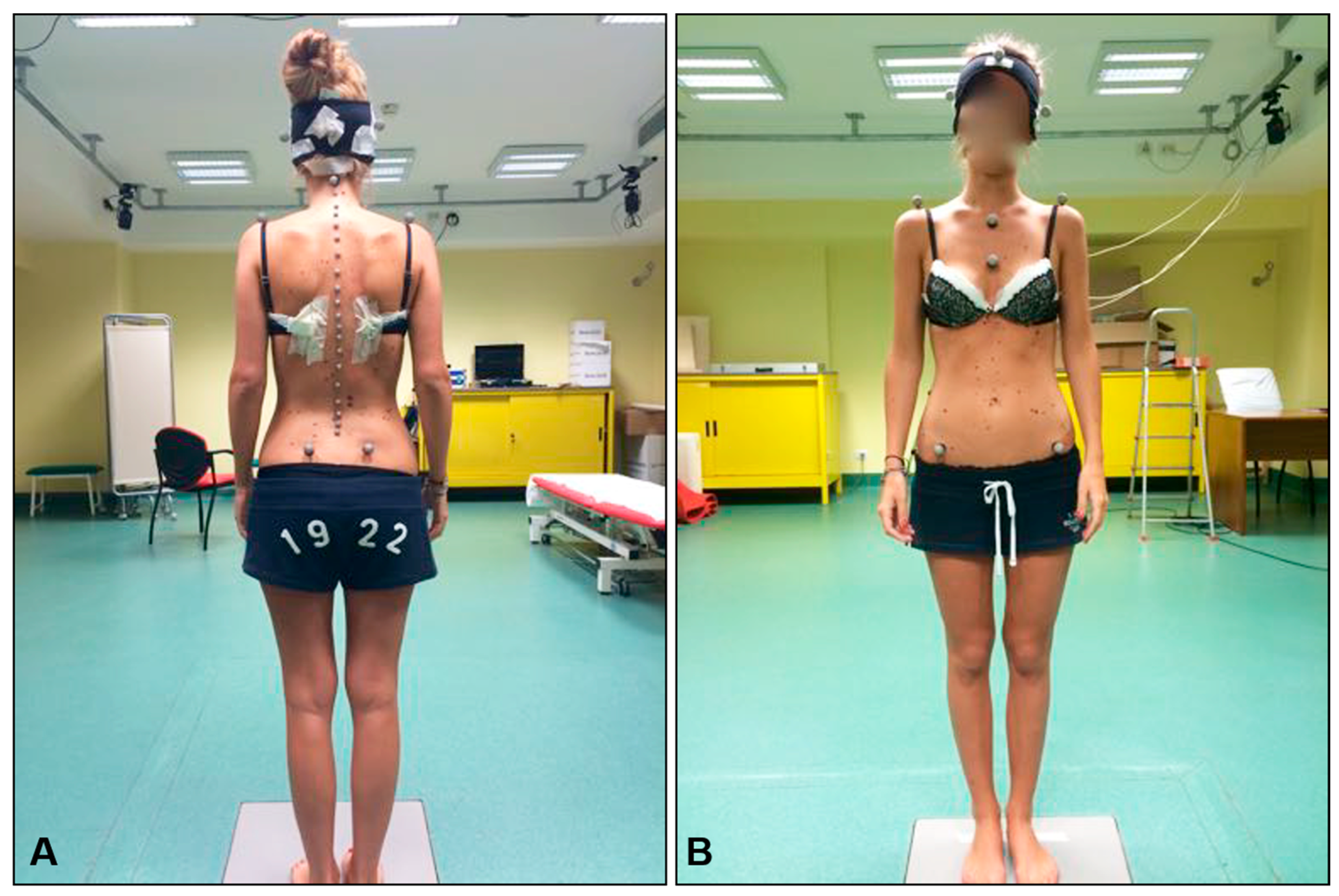
2.3. Neurodynamic Testing
2.4. Spine Mobility Testing
2.5. Spine Intervention
2.6. Spine Biomechanics Data Analysis
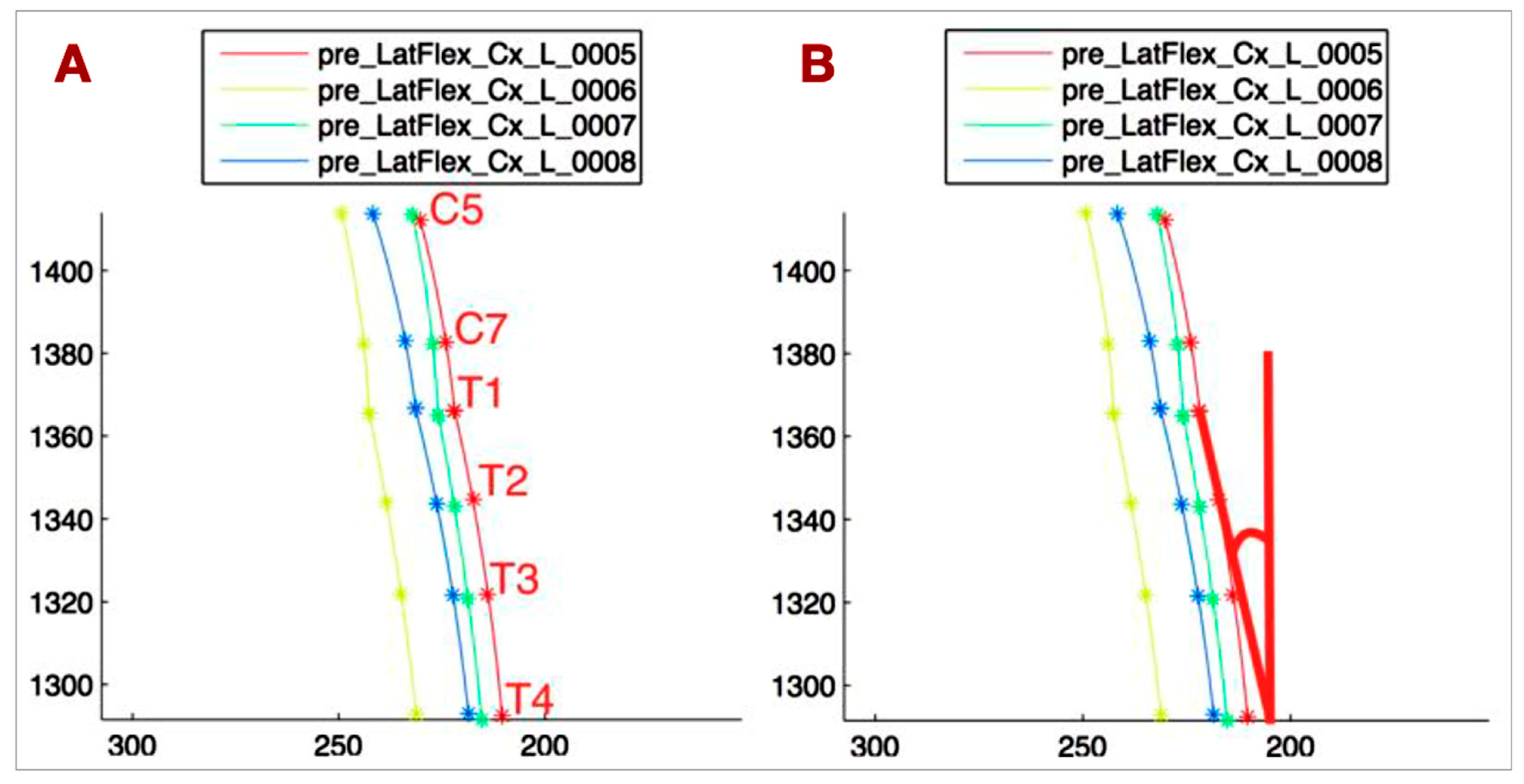
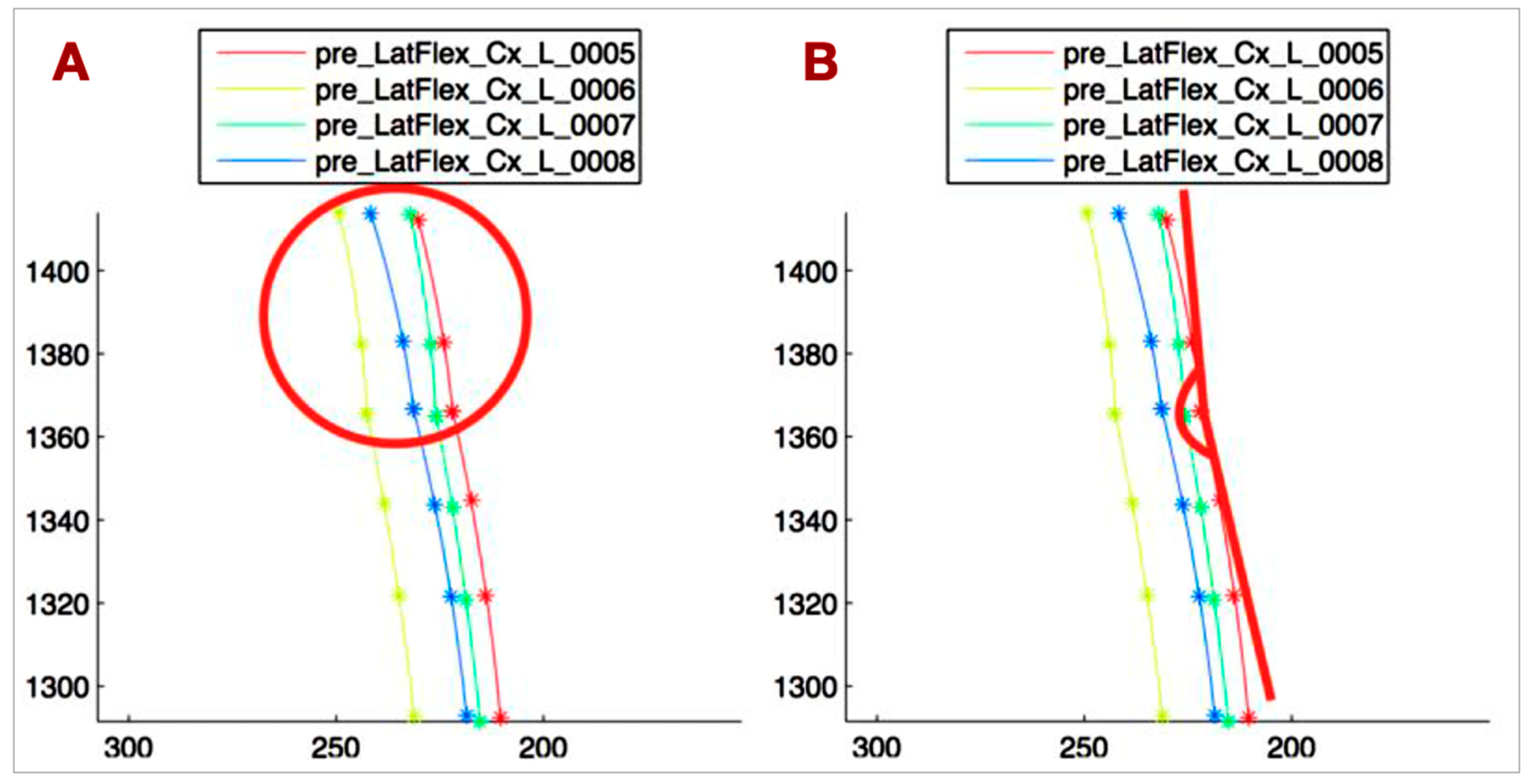
- T1-T4_VERT: The angle between the tangent at T1 to the T1-T4 curve and the vertical line, representing the side bending of the upper thoracic spine (Figure 4B).
- C5-T1_CONC: The C5-T1 concavity angle, which defines the side bending of C5-C7, independent of the upper thoracic side bending (Figure 5A).
- ANGLE_TANG: The angle between the tangent at T1 to the C5-T1 curve and the tangent at T1 to the T1-T4 curve, describing the relationship between the lower cervical and upper thoracic spine (Figure 5B).
- Head rotation (HEAD_ROT): The angular variation of the segment connecting the lateral markers on the head projected onto the horizontal plane, used to monitor head rotation. This parameter helps avoid bias in measuring spine lateral bending due to head rotation.
2.7. Statistical Analysis
3. Results
3.1. Intragroup Comparisons at Baseline (Table 2)
- S group:
- AS group:ULNTT confirmed the inclusion criterion of >10° asymmetry (R2 median difference: 13.5°).P2 also showed a significant difference (14.5°), reinforcing R2 findings.T1-T4_VERT showed a significant difference, with 12.13° contralateral to the greater ULNTT ROM side and −1.17° contralateral to the smaller ROM side, indicating opposite T1-T4 movement to the cervical spine.No other spine biomechanics parameters, including HEAD_ROT, showed significant differences.
3.2. Intergroup Comparisons at Baseline (Table 3)
- Significant differences between S and AS groups were observed in R2 and P2 at the smaller ULNTT ROM side, and in T1-T4_VERT contralateral to the smaller ROM side.
- HEAD_ROT also differed significantly, but its clinical relevance is uncertain [28].
- R2 and P2 at the greater ULNTT ROM side, and T1-T4_VERT contralateral to the greater ROM side, were similar between groups.
- These findings suggest a relationship between upper limb neurodynamics and upper thoracic spine biomechanics.
3.3. Pre- to Post-Intervention Comparisons (AS Group—Table 4)
- Significant changes were observed in R2, P2, T1-T4_VERT, and C5-T1_CONC at the smaller ULNTT ROM side.
- These parameters were the same parameters that showed differences between groups at baseline.
- The clinical relevance of C5-T1_CONC changes is uncertain.
3.4. Post-Intervention Intragroup Comparisons (AS Group—Table 5)
3.5. Data Interpretation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lohman, C.; Gilbert, K.; Sobczak, S.; Brismée, J.-M.; James, R.C.; Day, M.; Smith, M.P.; Taylor, L.; Dugailly, P.-M.; Pendergrass, T.; et al. Cervical Nerve Root Displacement and Strain During Upper Limb Neural Tension Testing. Spine 2015, 40, 793–800. [Google Scholar] [CrossRef]
- Kleinrensink, G.; Stoeckart, R.; Mulder, P.G.H.; Hoek, G.V.D.; Broek, T.H.; Vleeming, A.; Snijders, C.J. Upper limb tension tests as tools in the diagnosis of nerve and plexus lesions. Anatomical and biomechanical aspects. Clin. Biomech. 2000, 15, 9–14. [Google Scholar] [CrossRef]
- Selvaratnam, P.J. Brachial Plexus Tension Test in Patients And cadavers. Ph.D. Thesis, Department of Anatomy, Faculty of Medicine, Monash University, Clayton VIC, Australia, 1991. [Google Scholar]
- Vanti, C.; Conteddu, L.; Guccione, A.; Filomena, M.; Sergio, P.; Carlotta, V.; Paolo, P. The Upper Limb Neurodynamic Test 1: Intra- and intertester reliability and the effect of several repetitions on pain and resistance. J. Manip. Physiol. Ther. 2010, 33, 292–299. [Google Scholar]
- Van der Heide, B.; Allison, G.T.; Zusman, M. Pain and muscular responses to a neural tissue provocation test in the upper limb. Man. Ther. 2001, 6, 154–162. [Google Scholar] [CrossRef]
- Coppieters, M.; Stappaerts, K.; Janssens, K.; Jull, G. Reliability of detecting ‘onset of pain’ and ‘submaximal pain’ during neural provocation testing for the upper quadrant. Physiother. Res. Int. 2002, 7, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, P.J. Noninvasive Discrimination of Brachial Plexus Involvement in Upper Limb Pain. Spine 1994, 19, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Elvey, R.L. Brachial plexus tension test and the pathoanatomical origin of arm pain. In Aspects of Manipulative Therapy, 2nd ed.; Glasgow, E.F., Twomey Scull, E.R., Kleynhans, A.M., Eds.; Churchill Livingstone: Melbourne, VIC, Australia, 1985; pp. 116–122. [Google Scholar]
- Elvey, R.L. The investigation of arm pain: Signs of adverse responses to the physical examination of the brachial plexus and related tissues. In Grieve’s Modern Manual Therapy, 2nd ed.; Boyling, J.D., Palastanga, N., Eds.; Churchill Livingstone: New York, NY, USA, 1994; pp. 577–585. [Google Scholar]
- Ferrario, V.; Sforza, C. Active range of motion of the head and cervical spine: A three dimensional investigation in healthy young adults. J. Orthop. Res. 2002, 20, 122–129. [Google Scholar] [CrossRef]
- Sidorkewicz, N.; McGill, S. Male Spine Motion During Coitus. Implications for the Low Back Pain Patient. Spine 2014, 39, 1633–1639. [Google Scholar] [CrossRef]
- Grip, H.; Gerdle, G.; Karlsson, S. Cervical helical axis characteristics and its center of rotation during active head and upper arm movements—Comparisons of whiplash-associated disorders, non-specific neck pain and asymptomatic individuals. J. Biomech. 2008, 41, 2799–2805. [Google Scholar] [CrossRef]
- Duc, C.; Salvia, P.; Lubansu, A.; Feipel, V.; Aminian, K. A wearable inertial system to assess the cervical spine mobility: Comparison with an optoelectronic-based motion capture evaluation. Med. Eng. Phys. 2014, 36, 49–56. [Google Scholar] [CrossRef]
- Cobian, D.; Daehn, N.; Anderson, P.; Heiderscheit, B.C. Active Cervical and Lumbar Range of Motion During Performance of Activities of Daily Living in Healthy Young Adults. Spine 2013, 38, 1754–1763. [Google Scholar] [CrossRef]
- Harsh, S.; Supreeth, S.K.; Ayushi, K.A.; Vemuri, K. The role of individual physical body measurements and activity on spine kinematics during flexion, lateral bending and twist tasks in healthy young adults–Comparing marker(less) data. Biomed. Signal Process. Control. 2023, 82, 104517. [Google Scholar] [CrossRef]
- Digo, E.; Pierro, G.; Pastorelli, S.; Gastaldi, L. Tilt-Twist Method Using Inertial Sensors to Assess Spinal Posture During Gait. In Advances in Service and Industrial Robotics. RAAD 2019; Advances in Intelligent Systems and Computing; Berns, K., Görges, D., Eds.; Springer: Cham, Switzerland, 2020; Volume 980. [Google Scholar] [CrossRef]
- Lohkamp, M.; Small, K. Normal response to Upper Limb Neurodynamic Test 1 and 2A. Man. Ther. 2011, 16, 125–130. [Google Scholar] [CrossRef]
- Van Hoof, T.; Vangestel, C.; Shacklock, M.; Kerckaert, I.; D’Herde, K. Asymmetry of the ULNT1 elbow extension range-of-motion in a healthy population: Conse-quences for clinical practice and research. Phys. Ther. Sport 2012, 13, 141–149. [Google Scholar] [CrossRef]
- Stalioraitis, V.; Robinson, K.; Hall, T. Side-to-side range of movement variability in variants of the median and radial neurodynamic test sequences in asymptomatic people. Man. Ther. 2014, 19, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.J.; Brew, J. A single Blind Investigation into the potential differences in Passive range of movement at the elbow, between dominant and non-dominant arm when using the upper limb tension test 1. Physiotherapy 2000, 86, 40. [Google Scholar] [CrossRef]
- Oliver, S.G.; Rushton, A. A study to explore the reliability and precision of intra and inter-rater measures of ULNT1 on an asymptomatic population. Man. Ther. 2011, 16, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.S. The Sensitive Nervous System; Uley, Noigroup Publication: Adelaide, SA, Australia, 2000. [Google Scholar]
- Quarteroni, A.; Sacco, R.; Saleri, F. Approximation of a Function in the Least Square sense. In Numerical Mathematics; Quarteroni, A., Sacco, R., Saleri, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 431–433. [Google Scholar]
- Covill, L.; Petersen, S. Upper extremity neurodynamic tests: Range of motion asymmetry may not indicate impairment. Physiother. Theory Pract. 2012, 28, 535–541. [Google Scholar] [CrossRef]
- Block, C.A.; Cantrall, C.E.; Threlkeld, A.J. Responses of asymptomatic subjects to the upper limb tension test. Phys. Ther. 1998, 78. [Google Scholar]
- Hines, T.; Noakes, R.; Manners, B. The upper limb tension test: Inter-tester reliability for assessing the onset of passive resistance R1. J. Man. Manip. Ther. 1993, 1, 95–98. [Google Scholar] [CrossRef]
- Pullos, J. The upper limb tension test. Aust. J. Physiother. 1986, 32, 258e9. [Google Scholar]
- White, A.; Panjabi, M. The occipital-atlanto-axial complex. In Clinical Biomechanics of the Spine, 2nd ed.; White, A., Panjabi, M., Eds.; Lippincot Williams and Wilkins: Philadelphia, PA, USA, 1990; pp. 97–102. [Google Scholar]

| PZ1 | hvla L3 N S left R left | PZ8 | hvla L3 N S left R left |
| mit T9-L1 ERS right | mit T9-L1 ERS right | ||
| mit T9-L1 ERS right | |||
| PZ3 | hvla L4 N S left R left | PZ9 | hvla L3 N S left R left |
| mit T8-T12 ERS right | mit T8-T12 ERS right | ||
| hvla C5 N S left R left | |||
| PZ4 | hvla L3 N S left R left | PZ10 | hvla L4 N S left R left |
| mit T9-L1 ERS right | mit T9-L1 ERS right | ||
| hvla C5 N S left R left | hvla C6 N S left R left | ||
| mit T7-T9 ERS right | |||
| PZ5 | hvla L3 N S left R left | PZ11 | hvla L3 N S left R left |
| mit T10-L1 ESR left | mit T9-L1 ERS left | ||
| hvla C6 N S right R right | hvla C6 N S left R left | ||
| PZ6 | hvla L3 N S left R left | PZ12 | hvla L3 N S right R right |
| mit T9-T12 ERS right | mit T10-L1 ERS left | ||
| hvla C5 N S left R left | hvla C5 N S left R left | ||
| PZ7 | hvla L4 N S left R left | PZ13 | hvla L4 N S right R right |
| mit T9-L1 ERS right | mit T9-L1 ERS right | ||
| hvla C6 N S left R left | hvla C6 N S left R right |
| Null Hypothesis | Group | p Value | Median (Min/Max) Greater ROM Side | Median (Min/Max) Smaller ROM Side |
|---|---|---|---|---|
| Median of following parameter differences equals zero | ||||
| R2 greater ROM side/R2 smaller ROM side | S | 0.003 * | −2.25 (−7.25/3.75) | 0.25 (−7.00/5.75) |
| AS | 0.002 * | 0.12 (−7.25/6.25) | 13.87 (7.25/23.25) | |
| P2 greater ROM side/P2 smaller ROM side | S | 0.123 | 0.00 (−7.25/12.00) | 0.5 (−7.00/12.00) |
| AS | 0.002 * | 3.12 (−5/7.75) | 17.62 (9.26/28.00) | |
| T1-T4_VERT controlat ULNTT greater ROM side/T1-T4_VERT controlat ULNTT smaller ROM side | S | 0.306 | 11.72 (2.68/24.37) | 12.73 (5.38/22.22) |
| AS | 0.003 * | 12.13 (4.70/19.83) | −1.17 (−3.90/7.63) | |
| C5-T1_CONC controlat ULNTT greater ROM side/C5-T1_CONC controlat ULNTT smaller ROM side | S | 0.824 | 0.0029 (−0.0025/0.0086) | 0.0024 (0.0006/0.0056) |
| AS | 0.195 | 0.0022 (0.0003/0.112) | 0.0026 (−0.001/0.0083) | |
| ANGLE_TANG controlat ULNTT greater ROM side /ANGLE_TANG controlat ULNTT smaller ROM side | S | 0.722 | 4.34 (0.05/9.78) | 4.6 (1.55/6.83) |
| AS | 0.077 | 5.73 (1.50/15.51) | 3.26 (0.13/18.02) | |
| ROT_CERV controlat ULNTT greater ROM side/ROT_CERV controlat ULNTT smaller ROM side | S | 1 | 12.45 (2.81/46.42) | 17.05 (7.44/24.64) |
| AS | 0.099 | 12.74 (3.97/31.48) | 6.51 (2.18/18.42) |
| Null Hypothesis | p Value | Median (Min/Max) S | Median (Min/Max) AS |
|---|---|---|---|
| Parameter distribution is the same in S and AS groups | |||
| R2 greater ROM side | 0.104 | −2.25 (−7.25/3.75) | 0.12 (−7.25/6.25) |
| R2 smaller ROM side | <0.001 * | 0.25 (−7.00/5.75) | 13.87 (7.25/23.25) |
| P2 greater ROM side | 0.535 | 0.00 (−7.25/12.00) | 3.12 (−5/7.75) |
| P2 smaller ROM side | <0.001 * | 0.5 (−7.00/12.00) | 17.62 (9.26/28.00) |
| T1-T4_VERT controlat ULNTT greater ROM side | 1 | 11.72 (2.68/24.37) | 12.13 (4.70/19.83) |
| T1-T4_VERT controlat ULNTT smaller ROM side | <0.001 * | 12.73 (5.38/22.22) | −1.17 (−3.90/7.63) |
| C5-T1_CONC controlat ULNTT greater ROM side | 0.695 | 0.0029 (−0.0025/0.0086) | 0.0022 (0.0003/0.112) |
| C5-T1_CONC controlat ULNTT smaller ROM side | 0.695 | 0.0024 (0.0006/0.0056) | 0.0026 (−0.001/0.0083) |
| ANGLE_TANG controlat ULNTT greater ROM side | 0.487 | 4.34 (0.05/9.78) | 5.73 (1.50/15.51) |
| ANGLE_TANG controlat ULNTT smaller ROM side | 0.651 | 4.6 (1.55/6.83) | 3.26 (0.13/18.02) |
| ROT_CERV controlat ULNTT greater ROM side | 0.976 | 12.45 (2.81/46.42) | 17.05 (7.44/24.64) |
| ROT_CERV controlat ULNTT smaller ROM side | 0.007 * | 12.74 (3.97/31.48) | 6.51 (2.18/18.42) |
| Null Hypothesis | p Value | Median (Min/Max) Pre | Median (Min/Max) Post |
|---|---|---|---|
| Median of following parameter differences equals zero | |||
| R2 greater ROM side pre/R2 greater ROM side post | 0.182 | 0.12 (−7.25/6.25) | −1.62 (−8.00/4.00) |
| R2 smaller ROM side pre/R2 smaller ROM side post | 0.002 * | 13.87 (7.25/23.25) | −0.12 (−8.25/5.75) |
| P2 greater ROM side pre/P2 greater ROM side post | 0.109 | 3.12 (−5/7.75) | 0.62 (−8.00/6.75) |
| P2 smaller ROM side pre/P2 smaller ROM side post | 0.002 * | 17.62 (9.26/28.00) | 1.87 (−8.25/5.75) |
| T1-T4_VERT controlat ULNTT greater ROM side pre/ T1-T4_VERT controlat ULNTT greater ROM side post | 0.638 | 12.13 (4.70/19.83) | 9.84 (6.52/20.68) |
| T1-T4_VERT controlat ULNTT smaller ROM side pre/ T1-T4_VERT controlat ULNTT smaller ROM side post | 0.003 * | −1.17 (−3.90/7.63) | 7.33 (3.11/14.58) |
| C1-C5_CONC controlat ULNTT greater ROM side pre/ C1-C5_CONC controlat ULNTT greater ROM side post | 0.41 | 0.0022 (0.0003/0.112) | 0.0023 (0.0003/0.0010) |
| C1-C5_CONC controlat ULNTT smaller ROM side pre/ C1-C5_CONC controlat ULNTT smaller ROM side post | 0.034 * | 0.0026 (−0.001/0.0083) | 0.0043 (0.0005/0.0010) |
| ANGLE_TANG controlat ULNTT greater ROM side pre/ANGLE_TANG controlat ULNTT greater ROM side post | 0.754 | 5.73 (1.50/15.51) | 4.71 (2.51/14.80) |
| ANGLE_TANG controlat ULNTT smaller ROM side pre/ANGLE_TANG controlat ULNTT smaller ROM side post | 0.754 | 3.26 (0.13/18.02) | 4.01 (1.96/10.87) |
| CONC_CERV controlat ULNTT greater ROM side pre/CONC_CERV controlat ULNTT greater ROM side post | 0.117 | 17.05 (7.44/24.64) | 15.82 (1.73/38.73) |
| CONC_CERV controlat ULNTT smaller ROM side pre/CONC_CERV controlat ULNTT smaller ROM side post | 0.308 | 6.51 (2.18/18.42) | 6.69 (2.66/25.50) |
| Null Hypothesis | p Value | Median (Min/Max) Greater ROM Side | Median (Min/Max) Smaller ROM Side |
|---|---|---|---|
| Median of following parameter differences equals zero | |||
| R2 greater ROM side/R2 smaller ROM side | 0.789 | −1.62 (−8.00/4.00) | −0.12 (−8.25/5.75) |
| P2 greater ROM side/P2 smaller ROM side | 0.755 | 0.62 (−8.00/6.75) | 1.87 (−8.25/5.75) |
| T1-T4_VERT controlat ULNTT greater ROM side/T1-T4_VERT controlat ULNTT smaller ROM side | 0.158 | 9.84 (6.52/20.68) | 7.33 (3.11/14.58) |
| C5-T1_CONC controlat ULNTT greater ROM side/C5-T1_CONC controlat ULNTT smaller ROM side | 0.13 | 0.0023 (0.0003/0.0010) | 0.0043 (0.0005/0.0010) |
| ANGLE_TANG controlat ULNTT greater ROM side /ANGLE_TANG controlat ULNTT smaller ROM side | 0.99 | 4.71 (2.51/14.80) | 4.01 (1.96/10.87) |
| ROT_CERV controlat ULNTT greater ROM side/ROT_CERV controlat ULNTT smaller ROM side | 0.209 | 15.82 (1.73/38.73) | 6.69 (2.66/25.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.; Signorini, M.; Baram, A.; De Robertis, M.; Capo, G.; Riva, M.; Fornari, M.; Pessina, F.; Brembilla, C. Upper Limb Neural Tension Test and Spinal Biomechanics: Insights from a Longitudinal Pilot Study. Bioengineering 2025, 12, 487. https://doi.org/10.3390/bioengineering12050487
Rossi M, Signorini M, Baram A, De Robertis M, Capo G, Riva M, Fornari M, Pessina F, Brembilla C. Upper Limb Neural Tension Test and Spinal Biomechanics: Insights from a Longitudinal Pilot Study. Bioengineering. 2025; 12(5):487. https://doi.org/10.3390/bioengineering12050487
Chicago/Turabian StyleRossi, Massimo, Marianna Signorini, Ali Baram, Mario De Robertis, Gabriele Capo, Marco Riva, Maurizio Fornari, Federico Pessina, and Carlo Brembilla. 2025. "Upper Limb Neural Tension Test and Spinal Biomechanics: Insights from a Longitudinal Pilot Study" Bioengineering 12, no. 5: 487. https://doi.org/10.3390/bioengineering12050487
APA StyleRossi, M., Signorini, M., Baram, A., De Robertis, M., Capo, G., Riva, M., Fornari, M., Pessina, F., & Brembilla, C. (2025). Upper Limb Neural Tension Test and Spinal Biomechanics: Insights from a Longitudinal Pilot Study. Bioengineering, 12(5), 487. https://doi.org/10.3390/bioengineering12050487






