A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation
Abstract
:1. Introduction
2. Materials and Methods
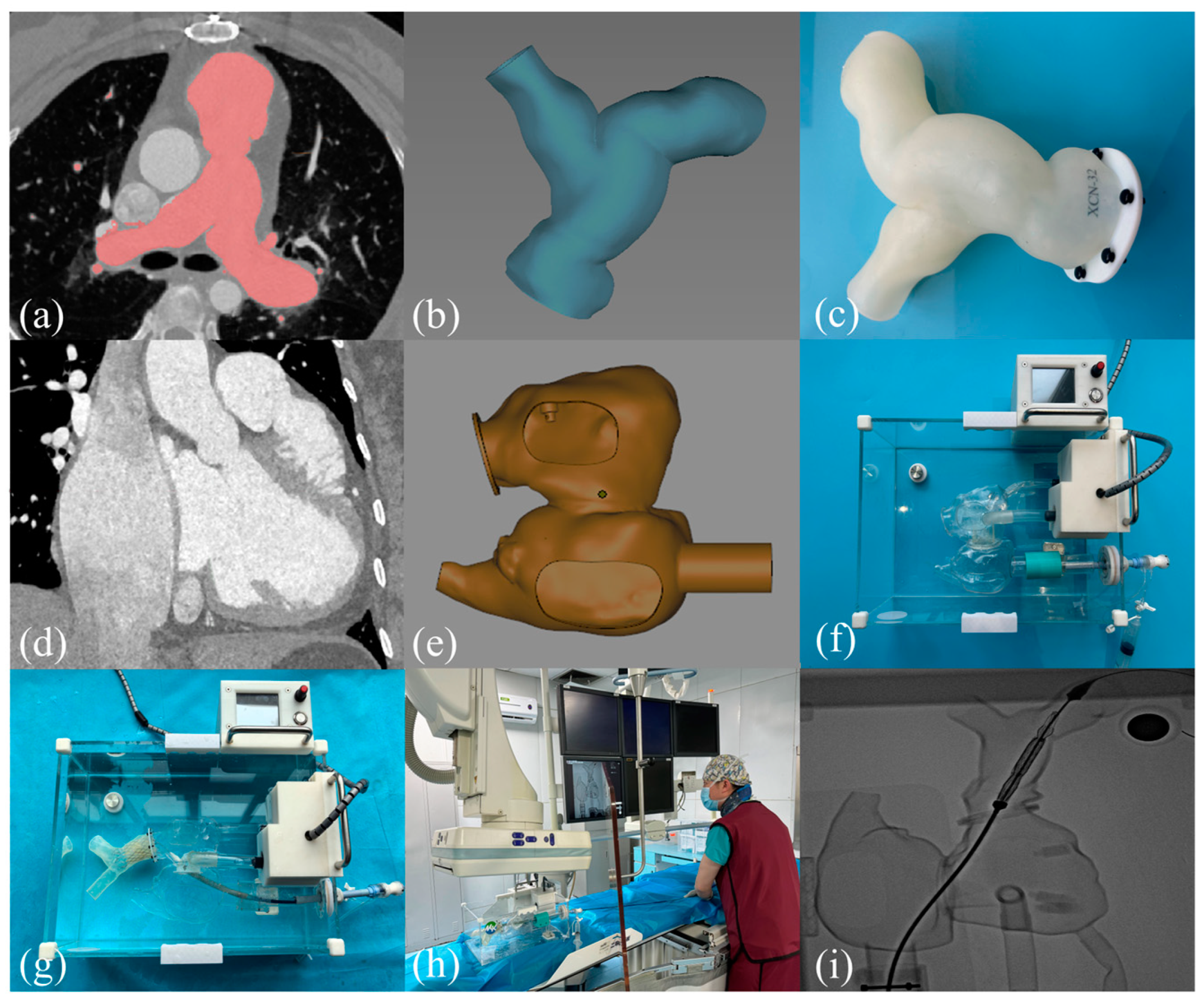
2.1. 3D Printing the RVOT Model Process and Building the TPVR Simulator
2.2. Guidance for 3D Printing
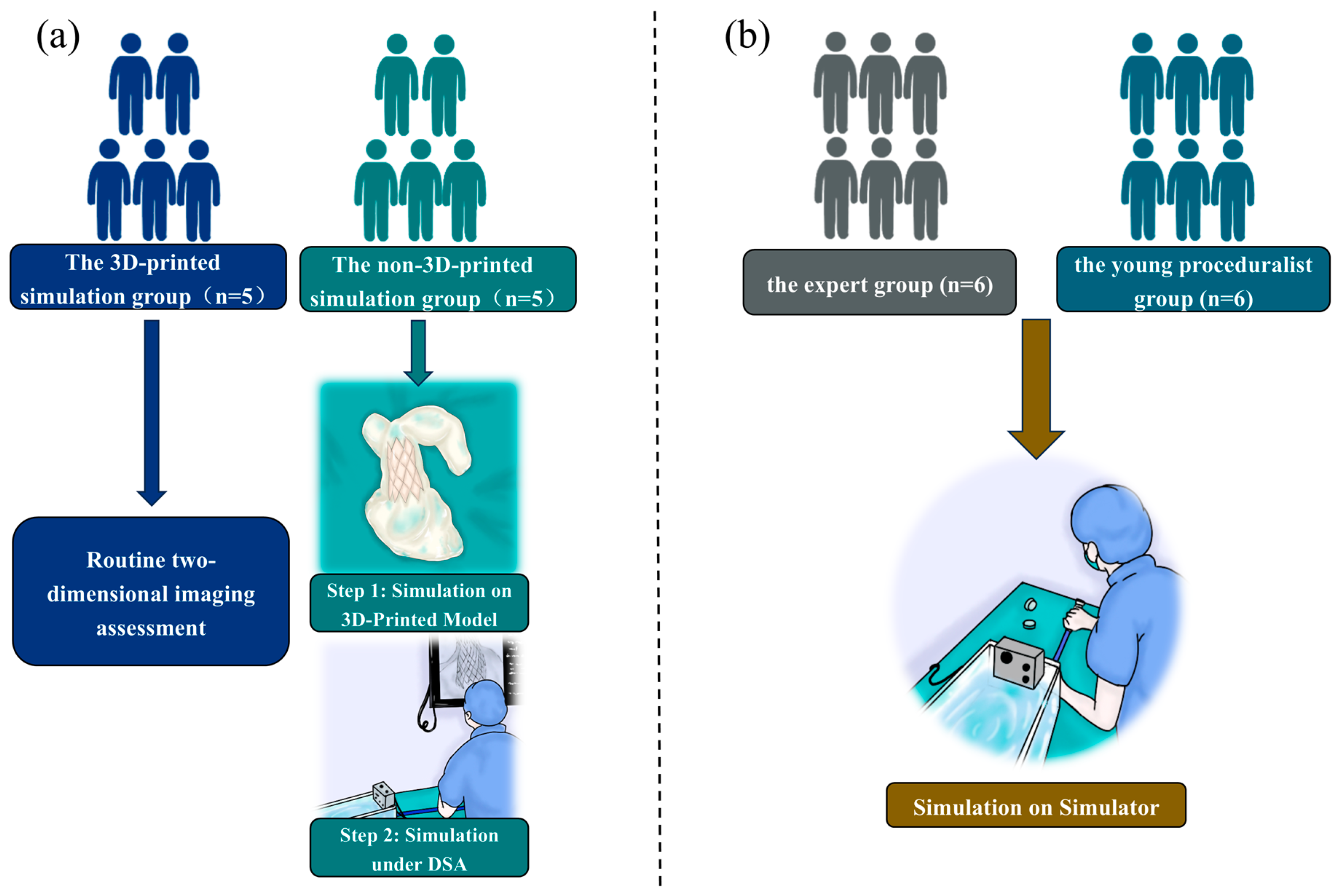
2.3. The 3D-Printed Group Versus the Non-3D-Printed Group
2.4. Experts Versus Young Surgeons in the Simulator
2.5. Statistical Analysis
3. Results
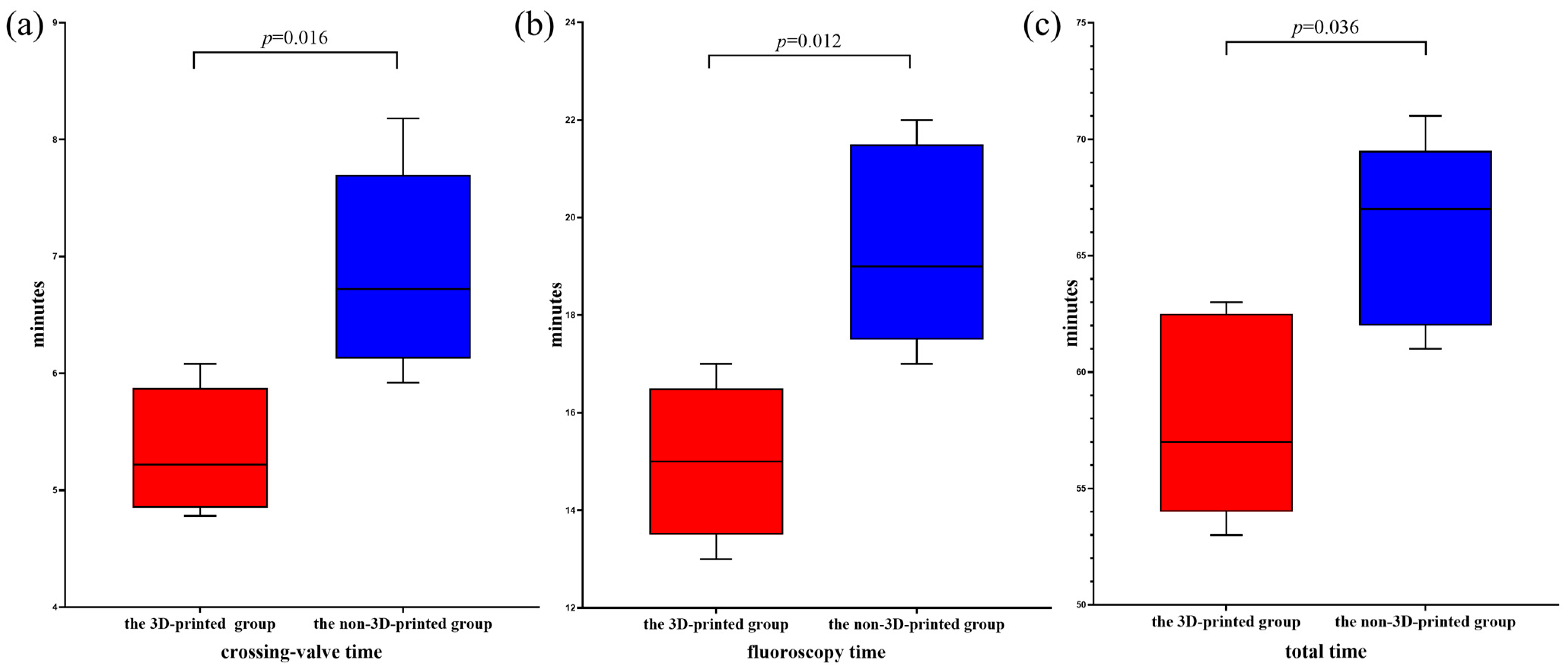
3.1. The 3D-Printed Group Versus the Non-3D-Printed Group
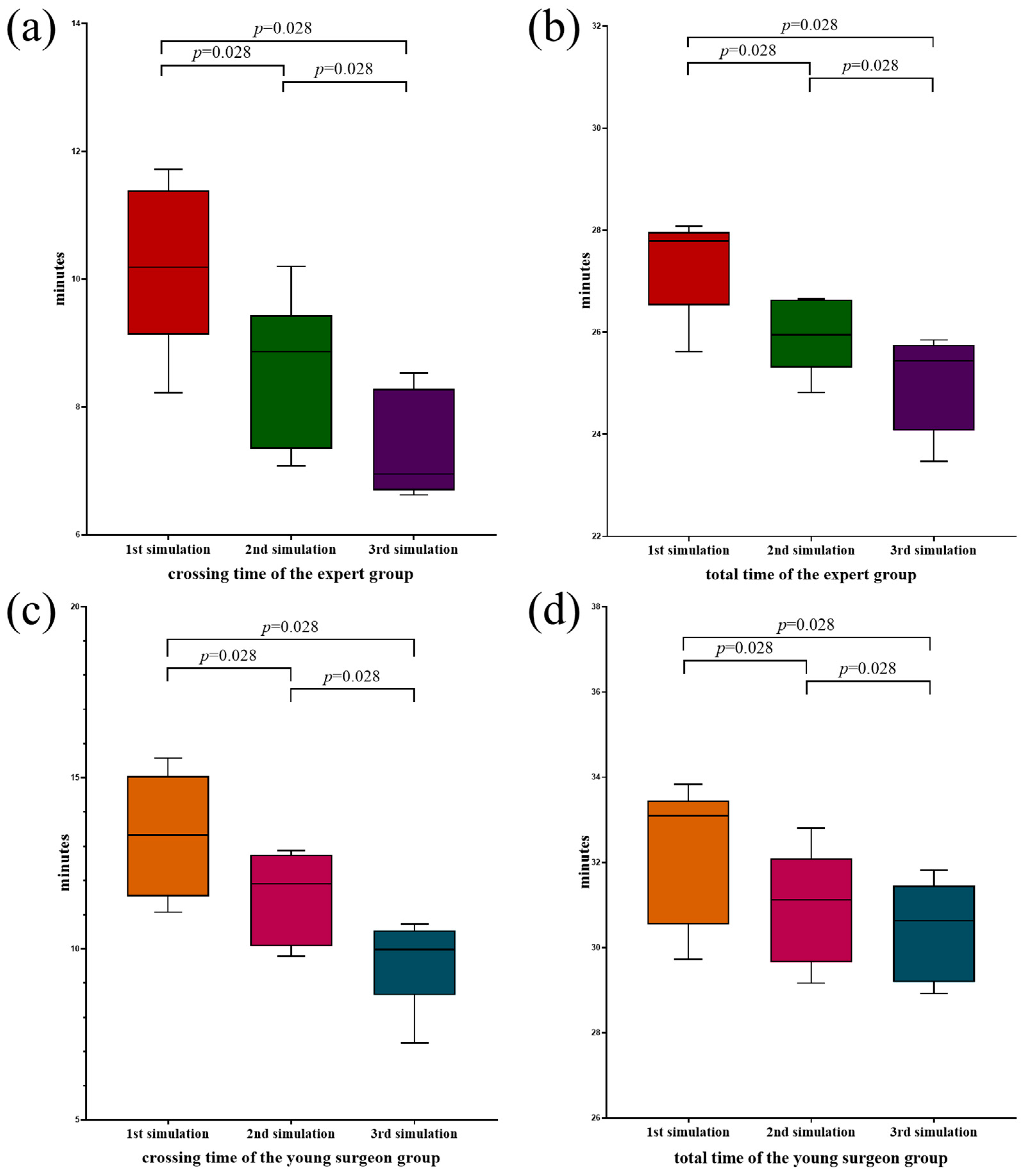
3.2. Experts Versus Young Surgeons in the Simulator
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basquin, A.; Pineau, E.; Galmiche, L.; Bonnet, D.; Sidi, D.; Boudjemline, Y. Transcatheter Valve Insertion in a Model of Enlarged Right Ventricular Outflow Tracts. J. Thorac. Cardiovasc. Surg. 2010, 139, 198–208. [Google Scholar] [CrossRef]
- Latus, H.; Stammermann, J.; Voges, I.; Waschulzik, B.; Gutberlet, M.; Diller, G.P.; Schranz, D.; Ewert, P.; Beerbaum, P.; Kühne, T.; et al. Impact of Right Ventricular Pressure Load after Repair of Tetralogy of Fallot. J. Am. Heart Assoc. 2022, 11, e022694. [Google Scholar]
- Álvarez-Fuente, M.; Toledano, M.; Garrido-Lestache, E.; Sánchez, I.; Molina, I.; Rivero, N.; García-Ormazábal, I.; Del Cerro, M.J. Balloon-Expandable Pulmonary Valves for Patched or Native Right Ventricular Outflow Tracts. Pediatr. Cardiol. 2023, 44, 1285–1292. [Google Scholar]
- Jalal, Z.; Valdeolmillos, E.; Malekzadeh-Milani, S.; Eicken, A.; Georgiev, S.; Hofbeck, M.; Sieverding, L.; Gewillig, M.; Ovaert, C.; Bouvaist, H.; et al. Mid-Term Outcomes Following Percutaneous Pulmonary Valve Implantation Using the “Folded Melody Valve” Technique. Circ. Cardiovasc. Interv. 2021, 14, e009707. [Google Scholar]
- Martin, M.H.; Meadows, J.; McElhinney, D.B.; Goldstein, B.H.; Bergersen, L.; Qureshi, A.M.; Shahanavaz, S.; Aboulhosn, J.; Berman, D.; Peng, L.; et al. Safety and Feasibility of Melody Transcatheter Pulmonary Valve Replacement in the Native Right Ventricular Outflow Tract: A Multicenter Pediatric Heart Network Scholar Study. JACC Cardiovasc. Interv. 2018, 11, 1642–1650. [Google Scholar]
- Guzeltas, A.; Tanidir, I.C.; Gokalp, S.; Topkarci, M.A.; Sahin, M.; Ergul, Y. Implantation of the Edwards Sapien Xt and Sapien 3 Valves for Pulmonary Position in Enlarged Native Right Ventricular Outflow Tract. Anatol. J. Cardiol. 2021, 25, 96–103. [Google Scholar]
- Tannous, P.; Nugent, A. Transcatheter Pulmonary Valve Replacement in Native and Nonconduit Right Ventricle Outflow Tracts. J. Thorac. Cardiovasc. Surg. 2021, 162, 967–970. [Google Scholar]
- Park, W.Y.; Kim, G.B.; Lee, S.Y.; Kim, A.Y.; Choi, J.Y.; Jang, S.I.; Kim, S.H.; Cha, S.G.; Wang, J.K.; Lin, M.T.; et al. The Adaptability of the Pulsta Valve to the Diverse Main Pulmonary Artery Shape of Native Right Ventricular Outflow Tract Disease. Catheter. Cardiovasc. Interv. 2024, 103, 587–596. [Google Scholar]
- Corrigan, F.E., 3rd; Gleason, P.T.; Condado, J.F.; Lisko, J.C.; Chen, J.H.; Kamioka, N.; Keegan, P.; Howell, S.; Clements, S.D., Jr.; Babaliaros, V.C.; et al. Imaging for Predicting, Detecting, and managing complications After transcatheter aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 904–920. [Google Scholar] [CrossRef]
- Fan, Y.; Wong, R.H.L.; Lee, A.P. Three-Dimensional Printing in Structural Heart Disease and Intervention. Ann. Transl. Med. 2019, 7, 579. [Google Scholar]
- Pluchinotta, F.R.; Sturla, F.; Caimi, A.; Giugno, L.; Chessa, M.; Giamberti, A.; Votta, E.; Redaelli, A.; Carminati, M. 3-Dimensional Personalized Planning for Transcatheter Pulmonary Valve Implantation in a Dysfunctional Right Ventricular Outflow Tract. Int. J. Cardiol. 2020, 309, 33–39. [Google Scholar] [CrossRef]
- Schievano, S.; Migliavacca, F.; Coats, L.; Khambadkone, S.; Carminati, M.; Wilson, N.; Deanfield, J.E.; Bonhoeffer, P.; Taylor, A.M. Percutaneous Pulmonary Valve Implantation Based on Rapid Prototyping of Right Ventricular Outflow Tract and Pulmonary Trunk from Mr Data. Radiology 2007, 242, 490–497. [Google Scholar] [CrossRef]
- Han, Y.; Shao, Z.; Sun, Z.; Han, Y.; Xu, H.; Song, S.; Pan, X.; de Jaegere, P.P.T.; Fan, T.; Zhang, G. In Vitro Bench Testing Using Patient-Specific 3d Models for Percutaneous Pulmonary Valve Implantation with Venus P-Valve. Chin. Med. J. 2024, 137, 990–996. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, P.; Meng, X.; Li, L.; Mao, Y.; Zheng, M.; Liu, L.; Liu, Y.; Yang, J. Treatment of Severe Pulmonary Regurgitation in Enlarged Native Right Ventricular Outflow Tracts: Transcatheter Pulmonary Valve Replacement with Three-Dimensional Printing Guidance. Bioengineering 2023, 10, 1136. [Google Scholar] [CrossRef]
- Müller, J.; Engelhardt, A.; Fratz, S.; Eicken, A.; Ewert, P.; Hager, A. Improved Exercise Performance and Quality of Life after Percutaneous Pulmonary Valve Implantation. Int. J. Cardiol. 2014, 173, 388–392. [Google Scholar] [CrossRef]
- Cheatham, J.P.; Hellenbrand, W.E.; Zahn, E.M.; Jones, T.K.; Berman, D.P.; Vincent, J.A.; McElhinney, D.B. Clinical and Hemodynamic Outcomes up to 7 Years after Transcatheter Pulmonary Valve Replacement in the Us Melody Valve Investigational Device Exemption Trial. Circulation 2015, 131, 1960–1970. [Google Scholar] [CrossRef]
- Schievano, S.; Coats, L.; Migliavacca, F.; Norman, W.; Frigiola, A.; Deanfield, J.; Bonhoeffer, P.; Taylor, A.M. Variations in Right Ventricular Outflow Tract Morphology Following Repair of Congenital Heart Disease: Implications for Percutaneous Pulmonary Valve Implantation. J. Cardiovasc. Magn. Reson. 2007, 9, 687–695. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, W.; Jilaihawi, H.; Zhang, G.; Feng, Y.; Pan, X.; Liu, J.; Yu, S.; Cao, Q.; Ge, J. A Self-Expanding Percutaneous Valve for Patients with Pulmonary Regurgitation and an Enlarged Native Right Ventricular Outflow Tract: One-Year Results. EuroIntervention 2019, 14, 1371–1377. [Google Scholar] [CrossRef]
- Odemis, E.; Aka, I.B.; Ali, M.H.A.; Gumus, T.; Pekkan, K. Optimizing Percutaneous Pulmonary Valve Implantation with Patient-Specific 3d-Printed Pulmonary Artery Models and Hemodynamic Assessment. Front. Cardiovasc. Med. 2023, 10, 1331206. [Google Scholar] [CrossRef]
- Etami, H.V.; Rismawanti, R.I.; Hanifah, V.A.N.; Herianto, H.; Yanuar, Y.; Kuswanto, D.; Anggrahini, D.W.; Gharini, P.P.R. Ct-Derived 3d Printing for Coronary Artery Cannulation Simulator Design Manufacturing. Bioengineering 2022, 9, 338. [Google Scholar] [CrossRef]
- Barabas, I.J.; Vegh, D.; Bottlik, O.; Kreuter, P.; Hartyanszky, I.; Merkely, B.; Palkovics, D. The Role of 3d Technology in the Practical Education of Congenital Coarctation and Its Treatment-a Feasibility Pilot Study. BMC Med. Educ. 2024, 24, 357. [Google Scholar]
- Torres, I.O.; De Luccia, N. A Simulator for Training in Endovascular Aneurysm Repair: The Use of Three Dimensional Printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar]
- Chessa, M.; Giugno, L.; Butera, G.; Carminati, M. Multi-Modal Imaging Support in a Staging Percutaneous Pulmonary Valve Implantation. Eur. Heart J. 2016, 37, 66. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, K.; Liu, S.; Zhou, X.; Rajagopal, V.; Meduri, C.; Kauten, J.R.; Chang, Y.H.; Wu, C.; Zhang, C.; et al. Quantitative Prediction of Paravalvular leak in Transcatheter Aortic valve Replacement Based On tissue-Mimicking 3d Printing. JACC Cardiovasc. Imaging 2017, 10, 719–731. [Google Scholar]
- Santoro, G.; Pizzuto, A.; Rizza, A.; Cuman, M.; Federici, D.; Cantinotti, M.; Pak, V.; Clemente, A.; Celi, S. Transcatheter Treatment of “Complex” Aortic Coarctation Guided by Printed 3d Model. JACC Case Rep. 2021, 3, 900–904. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Zhai, M.; Yang, J. Application of and Prospects for 3-Dimensional Printing in Transcatheter Mitral Valve Interventions. Rev. Cardiovasc. Med. 2023, 24, 61. [Google Scholar] [CrossRef]
- Ding, P.; Li, L.; Liu, Y.; Jin, P.; Tang, J.; Yang, J. Three-Dimensional Printing for Heart Diseases: Clinical Application Review. Biodes Manuf. 2021, 4, 675–687. [Google Scholar]
- Marques, D.M.C.; Silva, J.C.; Serro, A.P.; Cabral, J.M.S.; Sanjuan-Alberte, P.; Ferreira, F.C. 3d Bioprinting of Novel Κ-Carrageenan Bioinks: An Algae-Derived Polysaccharide. Bioengineering 2022, 9, 109. [Google Scholar] [CrossRef]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3d-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef]
| Characteristics | Patient1 | Patient2 | Patient3 | Patient4 | Patient5 | Patient6 | Patient7 | Patient8 | Patient9 | Patient10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years/sex | 19/M | 30/F | 37/F | 52/F | 23/M | 48/F | 12/M | 22/F | 31/F | 42/M |
| Weight (kg) | 68 | 62 | 60 | 55 | 78 | 53 | 51 | 68 | 58 | 81 |
| Height (cm) | 175 | 158 | 162 | 156 | 173 | 156 | 150 | 170 | 166 | 178 |
| NYHA functional class | II | III | III | IV | III | IV | II | III | IV | IV |
| PR severity grade | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ |
| Peak transpulmonary valve gradient | 11 | 17 | 19 | 21 | 17 | 19 | 11 | 18 | 1 7 | 10 |
| TR severity grade | 1+ | 3+ | 3+ | 2+ | 3+ | 3+ | 2+ | 2+ | 3+ | 3+ |
| RV–PA conduit length (mm) | 58 | 52 | 57 | 55 | 59 | 55 | 48 | 53 | 56 | 62 |
| nRVOT diameter (mm) | 44 | 38 | 37 | 36 | 41 | 37 | 32 | 35 | 38 | 43 |
| The narrowest plane/diameter (mm) | Distal MPA/23 | Distal MPA/24 | Mid MPA/28 | Mid MPA/23 | PA/ 33 | Mid MPA/27 | Distal MPA/24 | Distal MPA/22 | Mid MPA/26 | PA/30 |
| Number | Sex | Age (Years) | Years for Proceduralist | Years for Intervention | Patient Number |
|---|---|---|---|---|---|
| A1 | Male | 46 | 16 | 11 | 1 |
| A2 | Male | 42 | 13 | 7 | 2 |
| A3 | Male | 43 | 15 | 10 | 3 |
| A4 | Male | 39 | 12 | 8 | 4 |
| A5 | Male | 38 | 11 | 9 | 5 |
| B1 | Male | 42 | 13 | 8 | 6 |
| B2 | Male | 40 | 10 | 9 | 7 |
| B3 | Male | 45 | 16 | 12 | 8 |
| B4 | Male | 37 | 10 | 8 | 9 |
| B5 | Male | 43 | 11 | 7 | 10 |
| Time | Crossing Valve | Fluoroscopy | Total | Residual PR | Complications |
|---|---|---|---|---|---|
| A1 | 5′13″ | 15′00″ | 53′00″ | None | None |
| A2 | 4′55″ | 14′00″ | 57′00″ | None | None |
| A3 | 5′40″ | 16′00″ | 62′00″ | None | None |
| A4 | 4′47″ | 13′00″ | 55′00″ | None | None |
| A5 | 6′05″ | 17′00″ | 63′00″ | None | None |
| B1 | 5′55″ | 17′00″ | 61′00″ | None | None |
| B2 | 7′13″ | 21′00″ | 71′00″ | Trace | Hemoptysis |
| B3 | 6′43″ | 19′00″ | 68′00″ | None | None |
| B4 | 6′20″ | 18′00″ | 67′00″ | None | None |
| B5 | 8′11″ | 22′00″ | 63′00″ | None | Hemoptysis |
| Number | Sex | Age (Years) | Years for Proceduralist | Years for Intervention |
|---|---|---|---|---|
| C1 | Male | 43 | 12 | 10 |
| C2 | Male | 47 | 17 | 11 |
| C3 | Male | 41 | 12 | 12 |
| C4 | Male | 45 | 19 | 10 |
| C5 | Male | 39 | 13 | 9 |
| C6 | Male | 41 | 11 | 7 |
| D1 | Male | 31 | 2 | 2 |
| D2 | Male | 30 | 4 | 2 |
| D3 | Male | 33 | 3 | 1 |
| D4 | Male | 29 | 3 | 2 |
| D5 | Male | 31 | 2 | 1 |
| D6 | Male | 30 | 4 | 1 |
| Rank | 1st | 2nd | 3rd | |||
|---|---|---|---|---|---|---|
| Device | Total | Device | Total | Device | Total | |
| C1 | 10′35″ | 27′49″ | 8′56″ | 26′07″ | 6′37″ | 25′43″ |
| C2 | 9′48″ | 26′55″ | 9′11″ | 25′28″ | 7′06″ | 25′19″ |
| C3 | 8′13″ | 25′37″ | 7′26″ | 24′49″ | 6′43″ | 24′17″ |
| C4 | 11′17″ | 27′46″ | 8′48″ | 26′39″ | 8′12″ | 25′51″ |
| C5 | 9′26″ | 26′50″ | 7′05″ | 25′47″ | 6′48″ | 23′28″ |
| C6 | 11′43″ | 28′05″ | 10′12″ | 26′38″ | 8′32″ | 25′33″ |
| D1 | 12′49″ | 33′19″ | 12′03″ | 31′26″ | 10′29″ | 30′51″ |
| D2 | 11′41″ | 30′49″ | 9′47″ | 29′49″ | 7′15″ | 29′17″ |
| D3 | 13′50″ | 32′53″ | 12′43″ | 30′49″ | 10′22″ | 30′25″ |
| D4 | 11′05″ | 29′44″ | 10′11″ | 29′10″ | 9′36″ | 28′55″ |
| D5 | 15′34″ | 33′50″ | 12′52″ | 32′48″ | 10′43″ | 31′49″ |
| D6 | 14′52″ | 33′18″ | 11′46″ | 31′52″ | 9′07″ | 31′20″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Mao, Y.; Wang, Y.; Jin, P.; Zhai, M.; Liu, Y.; Yang, J. A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation. Bioengineering 2025, 12, 344. https://doi.org/10.3390/bioengineering12040344
Liu Y, Mao Y, Wang Y, Jin P, Zhai M, Liu Y, Yang J. A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation. Bioengineering. 2025; 12(4):344. https://doi.org/10.3390/bioengineering12040344
Chicago/Turabian StyleLiu, Yuanzhang, Yu Mao, Yiwei Wang, Ping Jin, Mengen Zhai, Yang Liu, and Jian Yang. 2025. "A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation" Bioengineering 12, no. 4: 344. https://doi.org/10.3390/bioengineering12040344
APA StyleLiu, Y., Mao, Y., Wang, Y., Jin, P., Zhai, M., Liu, Y., & Yang, J. (2025). A 3D Printing-Based Transcatheter Pulmonary Valve Replacement Simulator: Development and Validation. Bioengineering, 12(4), 344. https://doi.org/10.3390/bioengineering12040344








