How to Implement Clinical 7T MRI—Practical Considerations and Experience with Ultra-High-Field MRI
Abstract
:1. Introduction
1.1. MR Image Formation Background
1.2. Other Applications of MRI
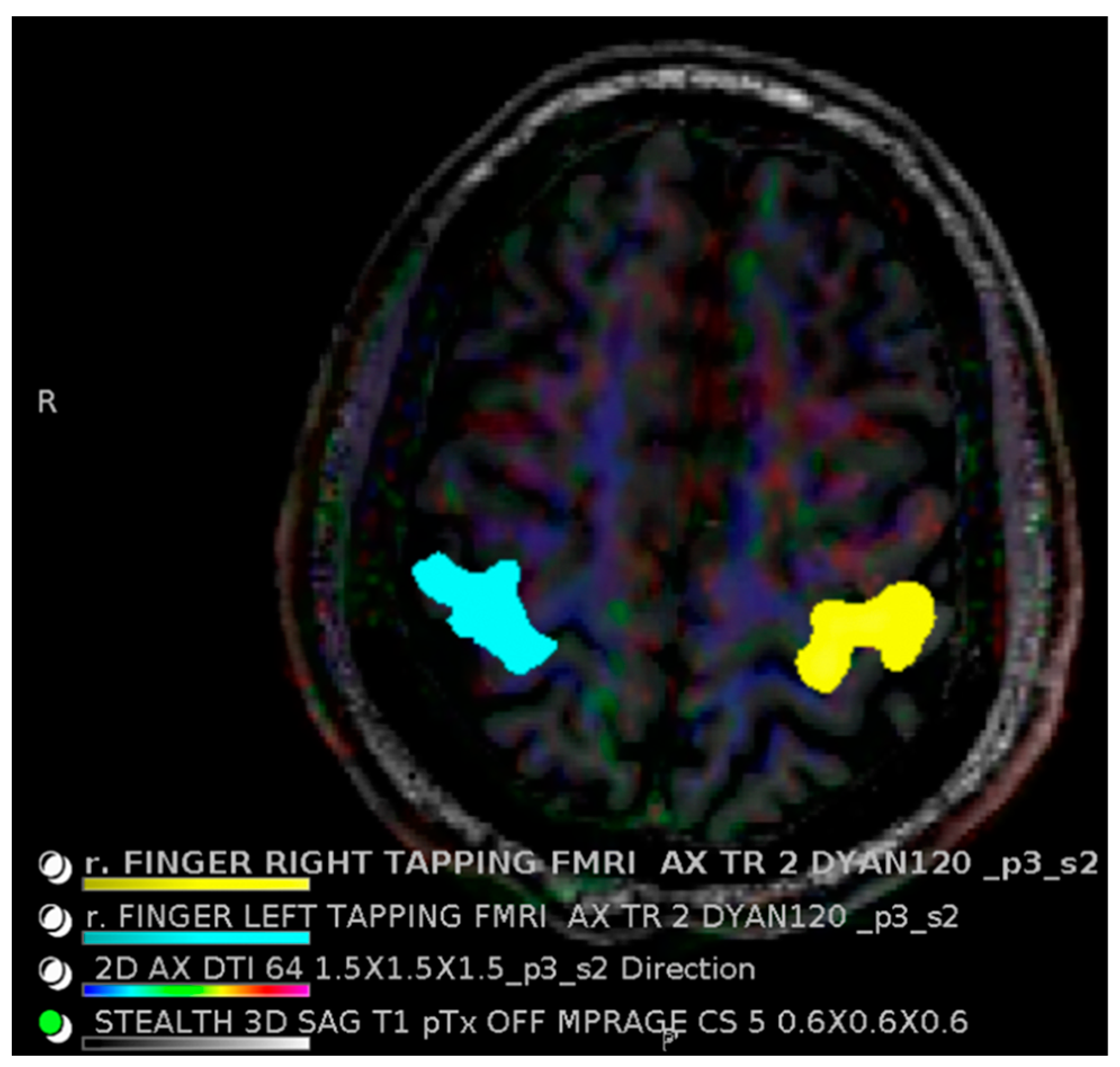
2. 7T Technical Considerations
2.1. 7T Physics Considerations
2.1.1. 7T RF Wavelength Considerations
2.1.2. 7T Relaxation Times and Tissue Contrast
2.1.3. 7T Susceptibility
- i
- Pulse sequence optimization: There are numerous parameters and techniques that can reduce susceptibility for any given sequence. As examples, these include spin-echo imaging, shortening the echo time, decreasing the voxel size, increasing receiver bandwidth, aligning the phase-encoding direction with susceptibility gradients, radial sampling, and parallel imaging [32]. A detailed discussion of these techniques is beyond the scope of this review.
- ii
- Image post-processing techniques: Artificial intelligence (AI) and more conventional image processing algorithms can be applied to reduce artifacts after the scan is acquired [33].
- iii
- Careful patient screening: Patients with certain metallic implants in certain locations may not be suitable candidates for 7T MRI due to excessive susceptibility artifact even if the implant is technically safe.
2.1.4. 7T B0/B1 Inhomogeneity Mitigation
2.2. 7T Hardware Considerations
2.3. 7T Software
2.4. 7T Safety
- i
- Magnetic field-increased magnetic forces: Any ferromagnetic objects, such as metal implants, jewelry, or surgical clips, will be subject to a magnetic force when they are placed in a strong magnetic field. The attractive magnetic force can be divided into two kinetic forces: the translational force and the torque force. The former is responsible for the displacement of an object within a magnetic field (translation and diversion), and the latter is responsible for the object’s rotational movement [36,41]. The rotational force exerted on a ferromagnetic object in a magnetic field is proportional to the square of the magnetic field strength B0, whereas translational forces are proportional to the product of B0 and the spatial field gradient (SFG). The SFG is the change in the static magnetic field with distance from the MRI system, which is greater at 7T than at lower field strengths. Thus, a 7T magnetic field exerts at least 2.3 times the translational force and at least 5.4 times the rotational force on an object compared to a 3T field, and likely much more. Therefore, it is even more critical to ensure that patients and staff are free of any ferromagnetic objects, implants, and devices before entering a 7T MRI room. These metallic objects can be pulled towards the magnet or rotate with great force, potentially causing injury.
- ii
- Magnetic field interaction with electronics: The strong magnetic field can interact with certain implanted devices, leading to malfunction or interference. This is especially concerning for patients with pacemakers, defibrillators, or other electronic implants. While some devices are clear for use at 1.5T or 3T, manufacturer instructions should always be reviewed prior to use in a 7T field.
- iii
- Magnetic field-increased Lenz forces: When a conducting material, even if it is not ferromagnetic, moves through a changing magnetic field, an electrical current is induced within it. This induced current, in turn, generates additional magnetic fields that oppose the object’s motion [42]. This phenomenon, known as Lenz’s law, results in Lenz forces that resist the movement of the object. These Lenz forces are significantly greater when the object moves through the stronger 7-tesla SFG compared to its motion through weaker 1.5- or 3-tesla SFGs. This is a consideration even for safe implants such as orthopedic implants. Very slow table movement in and out of the MRI bore is the primary strategy to mitigate Lenz forces [43]. The 7T table always moves slowly to account for this issue as well as bioeffects discussed below.
- iv
- Magnetic field-increased biologic and physiologic effects: Patients undergoing 7T MRI may experience worsened biologic and physiologic effects due to motion through the high magnetic field. These effects can include vertigo, dizziness, false feelings of motion, nausea, nystagmus, magnetophosphenes, and electrogustatory effects [44,45,46,47,48]. In some cases, patients may also experience discomfort or pain, particularly in areas with high concentrations of nerves or blood vessels. The primary way to mitigate these effects is to limit patient motion during scanning. First, the scanner table moves very slowly. Second, patients are coached to limit head motion until they are off the table, as head rotation can also induce physiologic effects. Anecdotally, this has not been a significant limiting factor for 7T imaging at our institution.
- v
- RF transmission heating: The major safety concern with the RF pulse is tissue and implant heating. Specific absorption rate (SAR) is the metric MRI scanners monitor to determine how much energy has been deposited in tissues during the scan, and it is closely monitored for all magnetic field strengths. However, RF heating is significantly greater at 7T. This is due to several factors, including the shorter RF wavelength being more absorbed in tissue and associated reduced tissue penetration, as well as a greater overall RF energy requirement to due higher flip angles required with increasing magnetic field strength. Due to the shorter RF wavelength in tissues at 7T, smaller metallic objects, implants, and foreign bodies are also more susceptible to heating and potential thermal injury during 7T MRI [49,50]. The primary method of mitigating this heating effect is distance from the imaging coil. At our institution, we require most implants and non-removable metallic external devices to be located >30 cm from the head coil to qualify for 7T. Finally, the newer parallel transmit (pTx) head coils have the potential to reduce RF heating as well by creating a more optimized RF field [17].
- vi
- Claustrophobia: Our 7T MRI has a 60 cm bore. This is a very common bore size for even 3T MRI, with 70 cm considered “large bore”. However, the 7T bore is also longer. In theory, the longer bore could contribute to an increased incidence of claustrophobia. While not formally tracked at our institution, anecdotally this has also not been a major limitation.
3. 7T Structural Imaging and Clinical Applications
4. 7T Advanced Imaging Protocols
5. 7T Future Directions
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M.; Boone, J.M. The Essential Physics of Medical Imaging; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 1049. [Google Scholar]
- Bandettini, P.A. What’s new in neuroimaging methods? Ann. N. Y. Acad. Sci. 2009, 1156, 260–293. [Google Scholar] [CrossRef] [PubMed]
- Heiserman, J.E.; Drayer, B.P.; Keller, P.J.; Fram, E.K. Intracranial vascular stenosis and occlusion: Evaluation with three-dimensional time-of-flight MR angiography. Radiology 1992, 185, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.R.; Ross, B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases; CRC Press: New York, NY, USA, 2014; p. 344. [Google Scholar]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Chakeres, D.W.; Abduljalil, A.M.; Novak, P.; Novak, V. Comparison of 1.5 and 8 Tesla High-Resolution Magnetic Resonance Imaging of Lacunar Infarcts. J. Comput. Assist. Tomogr. 2002, 26, 628–632. [Google Scholar] [CrossRef]
- Budinger, T.F.; Bird, M.D. MRI and MRS of the human brain at magnetic fields of 14T to 20T: Technical feasibility, safety, and neuroscience horizons. Neuroimage 2018, 168, 509–531. [Google Scholar] [CrossRef]
- Uğurbil, K. Ultrahigh field and ultrahigh resolution fMRI. Curr. Opin. Biomed. Eng. 2021, 18, 100288. [Google Scholar] [CrossRef]
- Vargas, M.I.; Martelli, P.; Xin, L.; Ipek, O.; Grouiller, F.; Pittau, F.; Trampel, R.; Gruetter, R.; Vulliemoz, S.; Lazeyras, F. Clinical Neuroimaging Using 7 T MRI: Challenges and Prospects. J. Neuroimaging 2017, 28, 5–13. [Google Scholar] [CrossRef]
- Balchandani, P.; Naidich, T.P. Ultra-High-Field MR Neuroimaging. AJNR Am. J. Neuroradiol. 2015, 36, 1204–1215. [Google Scholar] [CrossRef]
- Rondinoni, C.; Magnun, C.; da Silva, A.V.; Heinsen, H.M.; Amaro, E. Epilepsy under the scope of ultra-high field MRI. Epilepsy Behav. 2019, 121, 106366. [Google Scholar] [CrossRef]
- Graves, M.J. 3 T: The good, the bad and the ugly. Br. J. Radiol. 2021, 95, 20210708. [Google Scholar] [CrossRef] [PubMed]
- Erturk, M.A.; Li, X.; Moortele, P.F.V.; de Ugurbil, K.; Metzger, G.J. Evolution of UHF Body Imaging in the Human Torso at 7T: Technology, Applications, and Future Directions. Top. Magn. Reson. Imaging 2019, 28, 101. [Google Scholar] [CrossRef] [PubMed]
- Katscher, U.; Börnert, P.; Leussler, C.; Brink, J.S.v.D. Transmit SENSE. Magn. Reson. Med. 2002, 49, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Padormo, F.; Beqiri, A.; Hajnal, J.V.; Malik, S.J. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 2015, 29, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.N.; McElhinney, P.; Gunamony, S. Ultra-high field MRI: Parallel-transmit arrays and RF pulse design. Phys. Med. Biol. 2023, 68, 02TR02. [Google Scholar] [CrossRef]
- Rinck, P.A. Relaxation Times and Basic Pulse Sequences. In Magnetic Resonance in Medicine; Jezzard, P., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Hoult, D.I.; Bhakar, B. NMR signal reception: Virtual photons and coherent spontaneous emission. Concepts Magn. Reson. 1997, 9, 277–297. [Google Scholar] [CrossRef]
- Traficante, D.D. Relaxation. Can T2, be longer than T1? Concepts Magn. Reson. 1991, 3, 171–177. [Google Scholar] [CrossRef]
- Rooney, W.D.; Johnson, G.; Li, X.; Cohen, E.R.; Kim, S.G.; Ugurbil, K.; Springer, C.S., Jr. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 2007, 57, 308–318. [Google Scholar] [CrossRef]
- de Graaf, R.A.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Behar, K.L.; Rothman, D.L. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn. Reason. Med. 2006, 56, 386–394. [Google Scholar] [CrossRef]
- Stanisz, G.J.; Odrobina, E.E.; Pun, J.; Escaravage, M.; Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reason. Med. 2005, 54, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, P.A.; Foster, T.H.; Argersinger, R.E.; Pfeifer, L.M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1–100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 1984, 11, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.H.; Carr, H.Y. Diffusion and Nuclear Spin Relaxation in Water. Phys. Rev. B 1958, 111, 1201–1202. [Google Scholar] [CrossRef]
- Mitchell, D.; Burk, D.; Vinitski, S.; Rifkin, M.D. The biophysical basis of tissue contrast in extracranial MR imaging. Am. J. Roentgenol. 1987, 149, 831–837. [Google Scholar] [CrossRef]
- Spijkerman, J.M.; Petersen, E.T.; Hendrikse, J.; Luijten, P.; Zwanenburg, J.J.M. T 2 mapping of cerebrospinal fluid: 3 T versus 7 T. Magn. Reson. Mater. Physics, Biol. Med. 2017, 31, 415–424. [Google Scholar] [CrossRef]
- Cox, E.F.; Gowland, P.A. Measuring T2 and T2′ in the brain at 1.5T, 3T and 7T using a hybrid gradient echo-spin echo sequence and EPI. Proc. Intl. Soc. Mag. Reason. Med. 2008, 16, 1411. [Google Scholar]
- Visser, F.; Zwanenburg, J.J.M.; Hoogduin, J.M.; Luijten, P.R. High-resolution magnetization-prepared 3D-FLAIR imaging at 7.0 Tesla. Magn. Reason. Med. 2010, 64, 194–202. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Fracasso, A.; Visser, F.; Spliet, W.G.; Luijten, P.R.; Biessels, G.J.; Zwanenburg, J.J. FLAIR images at 7 Tesla MRI highlight the ependyma and the outer layers of the cerebral cortex. NeuroImage 2015, 104, 100–109. [Google Scholar] [CrossRef]
- Keith, G.A.; Woodward, R.A.; Hopkins, T.; Allwood-Spiers, S.; Trinder, J.; Muir, K.W.; Porter, D.A.; Fullerton, N.E. Towards clinical translation of 7 Tesla MRI in the human brain. IPEM-Translation 2024, 9, 100025. [Google Scholar] [CrossRef]
- Huang, S.Y.; Seethamraju, R.T.; Patel, P.; Hahn, P.F.; Kirsch, J.E.; Guimaraes, A.R. Body MR Imaging: Artifacts, k-Space, and Solutions. RadioGraphics 2015, 35, 1439–1460. [Google Scholar] [CrossRef]
- Monsour, R.; Dutta, M.; Mohamed, A.Z.; Borkowski, A.; Viswanadhan, N.A. Neuroimaging in the Era of Artificial Intelligence: Current Applications. Fed. Pract. 2022, 39, S14. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.A.; Schmitt, F.; Landes, H.; de Bever, J.; Wade, T.; Alejski, A.; Rutt, B.K. Gradient and shim technologies for ultra high field MRI. NeuroImage 2016, 168, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Blasche, M.; Fischer, D. Magnet Homogeneity and Shimming. Siemens Healthineers. Erlangen Ger. White Pap. Siemens Healthc. GmbH. 2017, pp. 2–9. Available online: https://www.magnetomworld.siemens-healthineers.com/publications/whitepapers (accessed on 24 September 2024).
- Shokrollahi, P.; Drake, J.M.; Goldenberg, A.A. Quantification of Force and Torque Applied by a High-Field Magnetic Resonance Imaging System on an Ultrasonic Motor for MRI-Guided Robot-Assisted Interventions. Actuators 2017, 6, 29. [Google Scholar] [CrossRef]
- Deniz, C.M. Parallel Transmission for Ultrahigh Field MRI. Top. Magn. Reson. Imaging 2019, 28, 159–171. [Google Scholar] [CrossRef] [PubMed]
- MAGNETOM Terra.X Make the Difference. [Internet]. Available online: https://www.siemens-healthineers.com/en-us/magnetic-resonance-imaging/7t-mri-scanner/magnetom-terra-x (accessed on 11 November 2024).
- Behl, N. Deep Resolve: Unrivaled Speed in MRI. MAGNETOM Flash 2024, 89, 2–11. [Google Scholar]
- Liang, D.; Liu, B.; Wang, J.; Ying, L. Accelerating SENSE using compressed sensing. Magn. Reson. Med. 2009, 62, 1574–1584. [Google Scholar] [CrossRef]
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2004, 87, 185–204. [Google Scholar] [CrossRef]
- Condon, B.; Hadley, D.M. Potential MR Hazard to Patients With Metallic Heart Valves: The Lenz Effect. J. Magn. Reson. Imaging 2000, 12, 171–176. [Google Scholar] [CrossRef]
- Shellock, F.G.; Rosen, M.S.; Webb, A.; Kimberly, W.T.; Rajan, S.; Nacev, A.N.; Crues, J.V. Managing Patients With Unlabeled Passive Implants on MR Systems Operating Below 1.5 T. J. Magn. Reson. Imaging 2023, 59, 1514–1522. [Google Scholar] [CrossRef]
- Heilmaier, C.; Theysohn, J.M.; Maderwald, S.; Kraff, O.; Ladd, M.E.; Ladd, S.C. A large-scale study on subjective perception of discomfort during 7 and 1.5 T MRI examinations. Bioelectromagnetics 2011, 32, 610–619. [Google Scholar] [CrossRef]
- Hoff, M.N.; McKinney, A.; Shellock, F.G.; Rassner, U.; Gilk, T.; Watson, R.E.; Greenberg, T.D.; Froelich, J.; Kanal, E. Safety Considerations of 7-T MRI in Clinical Practice. Radiology 2019, 292, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Theysohn, J.M.; Maderwald, S.; Kraff, O.; Moenninghoff, C.; Ladd, M.E.; Ladd, S.C. Subjective acceptance of 7 Tesla MRI for human imaging. Magn. Reson. Mater. Phys. Biol. Med. 2007, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Versluis, M.J.; Teeuwisse, W.M.; Kan, H.E.; van Buchem, M.A.; Webb, A.G.; van Osch, M.J. Subject tolerance of 7 T MRI examinations. J. Magn. Reson. Imaging 2012, 38, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Rauschenberg, J.; Nagel, A.M.; Ladd, S.C.; Theysohn, J.M.; Ladd, M.E.; Möller, H.E.; Trampel, R.; Turner, R.; Pohmann, R.; Scheffler, K.; et al. Multicenter Study of Subjective Acceptance During Magnetic Resonance Imaging at 7 and 9.4 T. Investig. Radiol. 2014, 49, 249–259. [Google Scholar] [CrossRef]
- Collins, C.M.; Smith, M.B. Signal-to-noise ratio and absorbed power as functions of main magnetic field strength, and definition of “90 degrees” RF pulse for the head in the birdcage coil. Magn. Reason. Med. 2001, 45, 684–691. [Google Scholar] [CrossRef]
- Ladd, M.E. High-field-strength magnetic resonance: Potential and limits. Top. Magn. Reason. Imaging 2007, 18, 139–152. [Google Scholar] [CrossRef]
- Trattnig, S.; Springer, E.; Bogner, W.; Hangel, G.; Strasser, B.; Dymerska, B.; Cardoso, P.L.; Robinson, S.D. Key clinical benefits of neuroimaging at 7 T. NeuroImage 2018, 168, 477–489. [Google Scholar] [CrossRef]
- Veersema, T.J.; Ferrier, C.H.; van Eijsden, P.; Gosselaar, P.H.; Aronica, E.; Visser, F.; Zwanenburg, J.M.; de Kort, G.A.P.; Hendrikse, J.; Luijten, P.R.; et al. Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. Epilepsia Open 2017, 2, 162–171. [Google Scholar] [CrossRef]
- Okromelidze, L.; Patel, V.; Singh, R.B.; Chiriboga, A.S.L.; Tao, S.; Zhou, X.; Straub, S.; Westerhold, E.M.; Gupta, V.; Agarwal, A.K.; et al. Central Vein Sign in Multiple Sclerosis: A Comparison Study of the Diagnostic Performance of 3T versus 7T MRI. Am. J. Neuroradiol. 2023, 45, 76–81. [Google Scholar] [CrossRef]
- Clarke, M.A.; Cheek, R.; Kazimuddin, H.F.; Hernandez, B.; Clarke, R.; McKnight, C.D.; Derwenskus, J.; Eaton, J.; Irlmeier, R.; Ye, F.; et al. Paramagnetic rim lesions and the central vein sign: Characterizing multiple sclerosis imaging markers. J. Neuroimaging 2023, 34, 86–94. [Google Scholar] [CrossRef]
- Wermer, M.J.; van Walderveen, M.A.; Garpebring, A.; van Osch, M.J.; Versluis, M.J. 7 Tesla MRA for the differentiation between intracranial aneurysms and infundibula. Magn. Reson. Imaging 2017, 37, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Vysotski, S.; Madura, C.; Swan, B.; Holdsworth, R.; Lin, Y.; Del Rio, A.M.; Wood, J.; Kundu, B.; Penwarden, A.; Voss, J.; et al. Preoperative FMRI associated with decreased mortality and morbidity in brain tumor patients. Interdiscip. Neurosurg. 2018, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, E.; Shmuel, A.; Pfeuffer, J.; Van De Moortele, P.; Adriany, G.; Andersen, P.; Vaughan, J.T.; Merkle, H.; Ugurbil, K.; Hu, X. Imaging brain function in humans at 7 Tesla. Magn. Reson. Med. 2001, 45, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, S.; Mennecke, A.; Konerth, L.; Rösch, J.; Nagel, A.M.; Laun, F.B.; Uder, M.; Dörfler, A.; Hess, A. 3T vs. 7T fMRI: Capturing early human memory consolidation after motor task utilizing the observed higher functional specificity of 7T. Front. Neurosci. 2023, 17, 1215400. [Google Scholar] [CrossRef]
- Sayin, E.S.; Duffin, J.; Poublanc, J.; Venkatraghavan, L.; Mikulis, D.J.; Fisher, J.A.; Sobczyk, O. Determining the effects of elevated partial pressure of oxygen on hypercapnia-induced cerebrovascular reactivity. J. Cereb. Blood Flow Metab. 2023, 43, 2085–2095. [Google Scholar] [CrossRef]
- Khanna, N.; Altmeyer, W.; Zhuo, J.; Steven, A. Functional Neuroimaging: Fundamental Principles and Clinical Applications. Neuroradiol. J. 2015, 28, 87–96. [Google Scholar] [CrossRef]
- Agarwal, S.; Welker, K.M.; Black, D.F.; Little, J.T.; DeLone, D.R.; Messina, S.A.; Passe, T.J.; Bettegowda, C.; Pillai, J.J. Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas. Cancers 2023, 15, 4473. [Google Scholar] [CrossRef]
- Piepgras, A.; Schmiedek, P.; Leinsinger, G.; Haberl, R.L.; Kirsch, C.M.; Einhaeupl, K.M. A simple test to assess cerebrovascular reserve capacity using transcranial Dopper sonography and acetazolamide. Stroke 1990, 21, 1306–1311. [Google Scholar] [CrossRef]
- Liu, P.; De Vis, J.B.; Lu, H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. NeuroImage 2019, 187, 104–115. [Google Scholar] [CrossRef]
- Agarwal, S.; Sair, H.I.; Pillai, J.J. The Problem of Neurovascular Uncoupling. Neuroimaging Clin. N. Am. 2020, 31, 53–67. [Google Scholar] [CrossRef]
- Fisher, J.A.; Venkatraghavan, L.; Mikulis, D.J. Magnetic Resonance Imaging–Based Cerebrovascular Reactivity and Hemodynamic Reserve. Stroke 2018, 49, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.K.; An, Z.; Tiwari, V.; McNeil, S.; Pinho, M.C.; Pan, E.; Mickey, B.E.; Maher, E.A.; Choi, C. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magn. Reson. Med. 2016, 77, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Völzke, Y.; Pracht, E.D.; Hattingen, E.; Tse, D.Y.H.; Stöcker, T. On the reproducibility of hippocampal MEGA-sLASER GABA MRS at 7T using an optimized analysis pipeline. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 427–436. [Google Scholar] [CrossRef]
- Gast, L.V.; Platt, T.; Nagel, A.M.; Gerhalter, T. Recent technical developments and clinical research applications of sodium (23Na) MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 138–139, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bai, J.; Zong, R.; Zhou, J.; Zuo, Z.; Chai, X.; Wang, Z.; An, J.; Zhuo, Y.; Boada, F.; et al. Sodium MRI at 7T for Early Response Evaluation of Intracranial Tumors following Stereotactic Radiotherapy Using the CyberKnife. Am. J. Neuroradiol. 2022, 43, 181–187. [Google Scholar] [CrossRef]
- Paech, D.; Weckesser, N.; Franke, V.L.; Breitling, J.; Görke, S.; Deike-Hofmann, K.; Wick, A.; Scherer, M.; Unterberg, A.; Wick, W.; et al. Whole-Brain Intracellular pH Mapping of Gliomas Using High-Resolution 31P MR Spectroscopic Imaging at 7.0 T. Radiol. Imaging Cancer 2024, 6, e220127. [Google Scholar] [CrossRef]
- Özütemiz, C.; White, M.; Elvendahl, W.; Eryaman, Y.; Marjańska, M.; Metzger, G.J.; Patriat, R.; Kulesa, J.; Harel, N.; Watanabe, Y.; et al. Use of a Commercial 7-T MRI Scanner for Clinical Brain Imaging: Indications, Protocols, Challenges, and Solutions—A Single-Center Experience. Am. J. Roentgenol. 2023, 221, 788–804. [Google Scholar] [CrossRef]
- Feinberg, D.A.; Beckett, A.J.S.; Vu, A.T.; Stockmann, J.; Huber, L.; Ma, S.; Ahn, S.; Setsompop, K.; Cao, X.; Park, S.; et al. Next-generation MRI scanner designed for ultra-high-resolution human brain imaging at 7 Tesla. Nat. Methods 2023, 20, 2048–2057. [Google Scholar] [CrossRef]
- Mir, M.; Miller, N.P.; White, M.; Elvendahl, W.; Danyeli, A.E.; Özütemiz, C. Prevalence of Rathke’s Cleft and Other Incidental Pituitary Gland Findings on Contrast-Enhanced 3D Fat-Saturated T1-MPRAGE at 7 Tesla MRI. Am. J. Neuroradiol. 2024, 45, 1811–1818. Available online: https://www.ajnr.org/content/early/2024/06/24/ajnr.A8393 (accessed on 28 August 2024). [CrossRef]
- Middlebrooks, E.H.; Tipton, P.W.; Greco, E.; Okromelidze, L.; Patel, V.; Wszolek, Z.K.; Zhou, X.; Tao, S.; Westerhold, E.M.; Straub, S.; et al. Enhancing outcomes in deep brain stimulation: A comparative study of direct targeting using 7T versus 3T MRI. J. Neurosurg. 2024, 1, 1–8. [Google Scholar] [CrossRef]
- Aringhieri, G.; Zampa, V.; Tosetti, M. Musculoskeletal MRI at 7 T: Do we need more or is it more than enough? Eur. Radiol. Exp. 2020, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Rietsch, S.H.G.; Orzada, S.; Maderwald, S.; Brunheim, S.; Philips, B.W.J.; Scheenen, T.W.J.; Ladd, M.E.; Quick, H.H. 7T ultra-high field body MR imaging with an 8-channel transmit/32-channel receive radiofrequency coil array. Med. Phys. 2018, 45, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
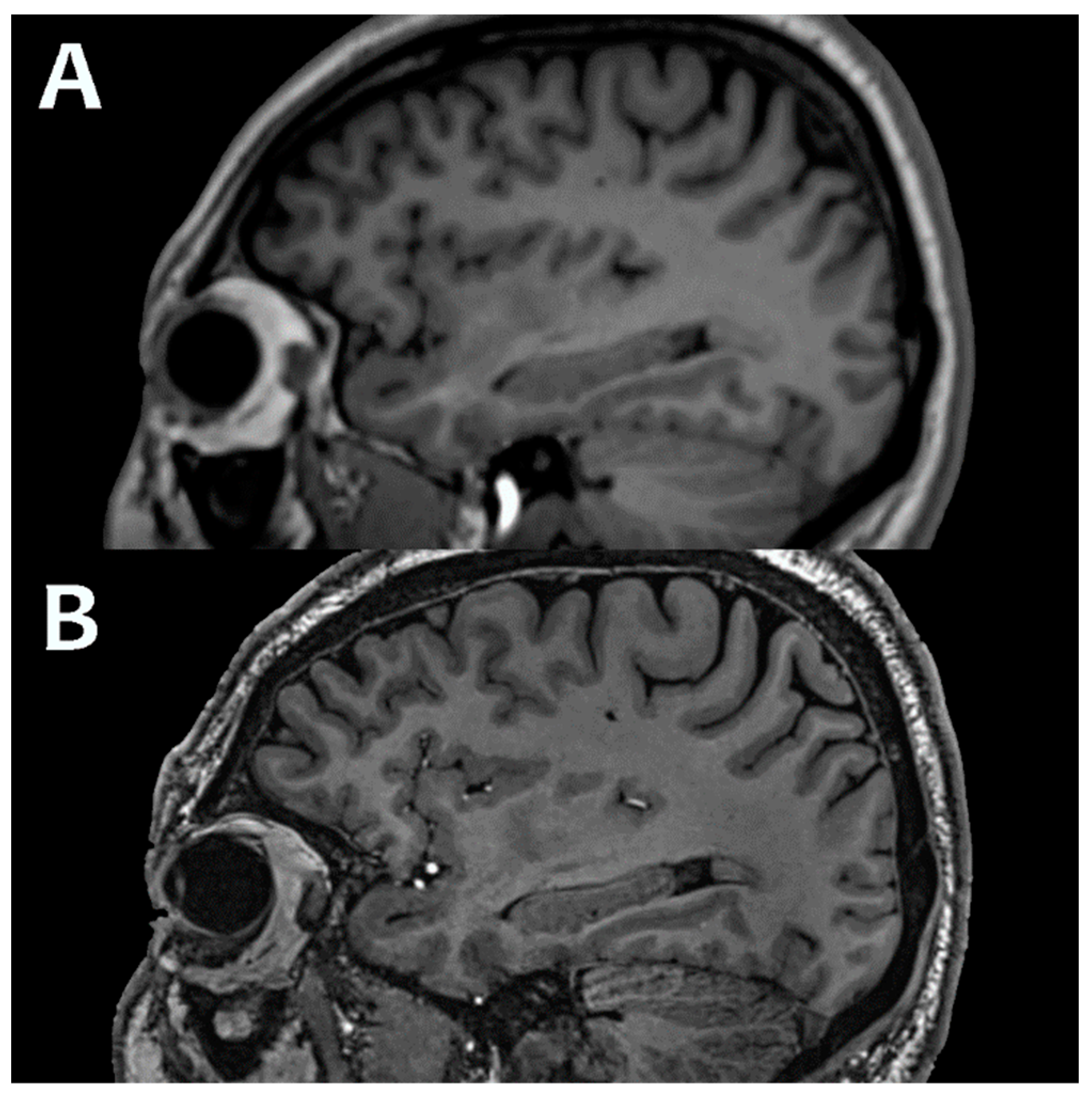
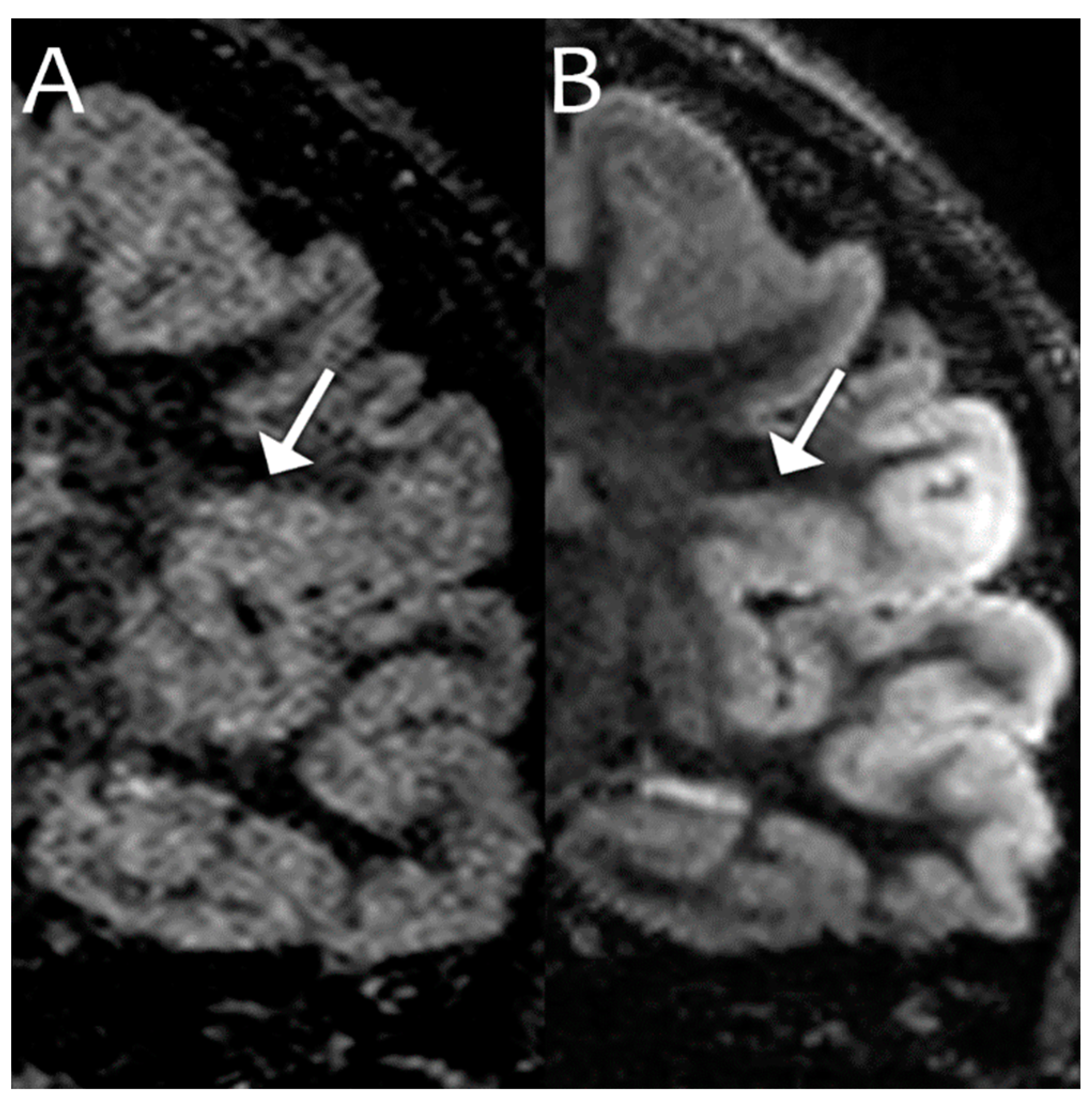
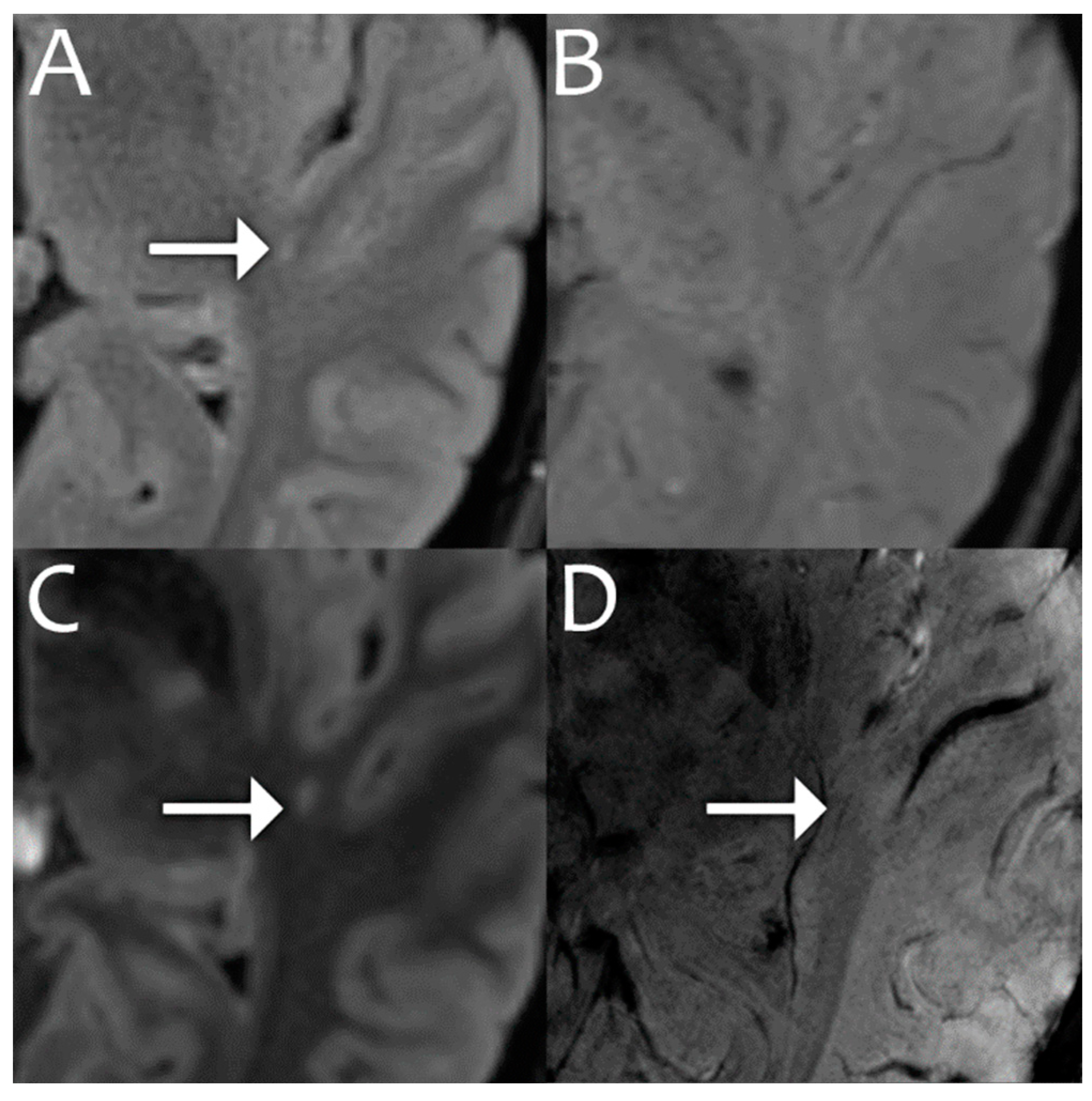
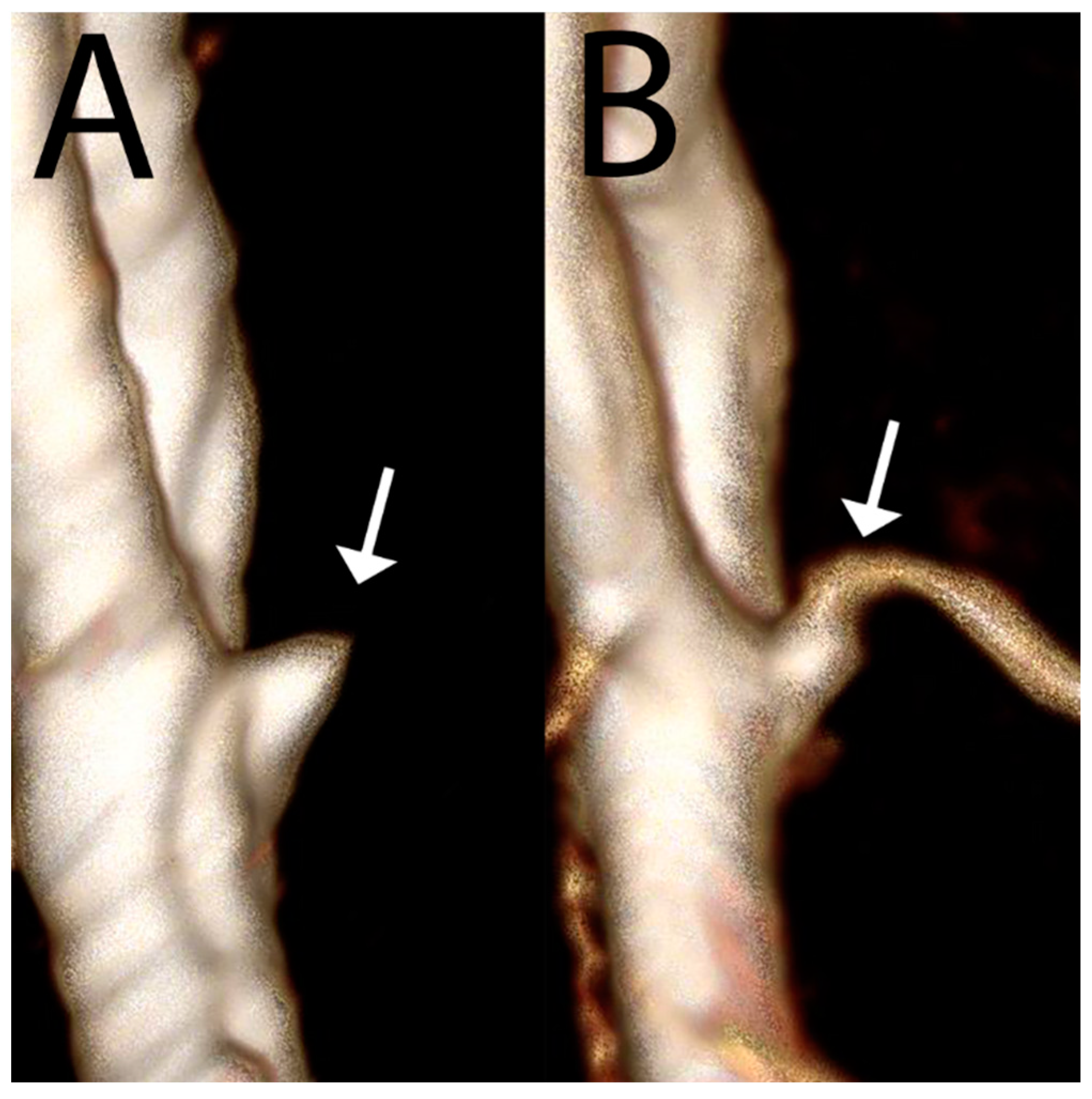
| 1.5T | 3.0T | 7.0T | ||||
|---|---|---|---|---|---|---|
| T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | |
| White Matter | 600–700 | 80 | 850–950 | 75 | 1250–1350 | 70 |
| Gray Matter | 950–1100 | 100 | 1615 | 95 | 2065 | 90 |
| CSF | 4500 | 220 | 5500 | 200 | >6000 | 1000 |
| Muscle | 900 | 50 | 1300 | 40 | 1750 | 40 |
| Fat | 250 | 60 | 310 | 55 | 400 | 50 |
| Blood | 1200 | 100~200 | 1900 | 275 | 2500 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cramer, J.; Ikuta, I.; Zhou, Y. How to Implement Clinical 7T MRI—Practical Considerations and Experience with Ultra-High-Field MRI. Bioengineering 2024, 11, 1228. https://doi.org/10.3390/bioengineering11121228
Cramer J, Ikuta I, Zhou Y. How to Implement Clinical 7T MRI—Practical Considerations and Experience with Ultra-High-Field MRI. Bioengineering. 2024; 11(12):1228. https://doi.org/10.3390/bioengineering11121228
Chicago/Turabian StyleCramer, Justin, Ichiro Ikuta, and Yuxiang Zhou. 2024. "How to Implement Clinical 7T MRI—Practical Considerations and Experience with Ultra-High-Field MRI" Bioengineering 11, no. 12: 1228. https://doi.org/10.3390/bioengineering11121228
APA StyleCramer, J., Ikuta, I., & Zhou, Y. (2024). How to Implement Clinical 7T MRI—Practical Considerations and Experience with Ultra-High-Field MRI. Bioengineering, 11(12), 1228. https://doi.org/10.3390/bioengineering11121228










