Is Premenstrual Syndrome Associated with Inflammation, Oxidative Stress and Antioxidant Status? A Systematic Review of Case–Control and Cross-Sectional Studies
Abstract
:1. Introduction
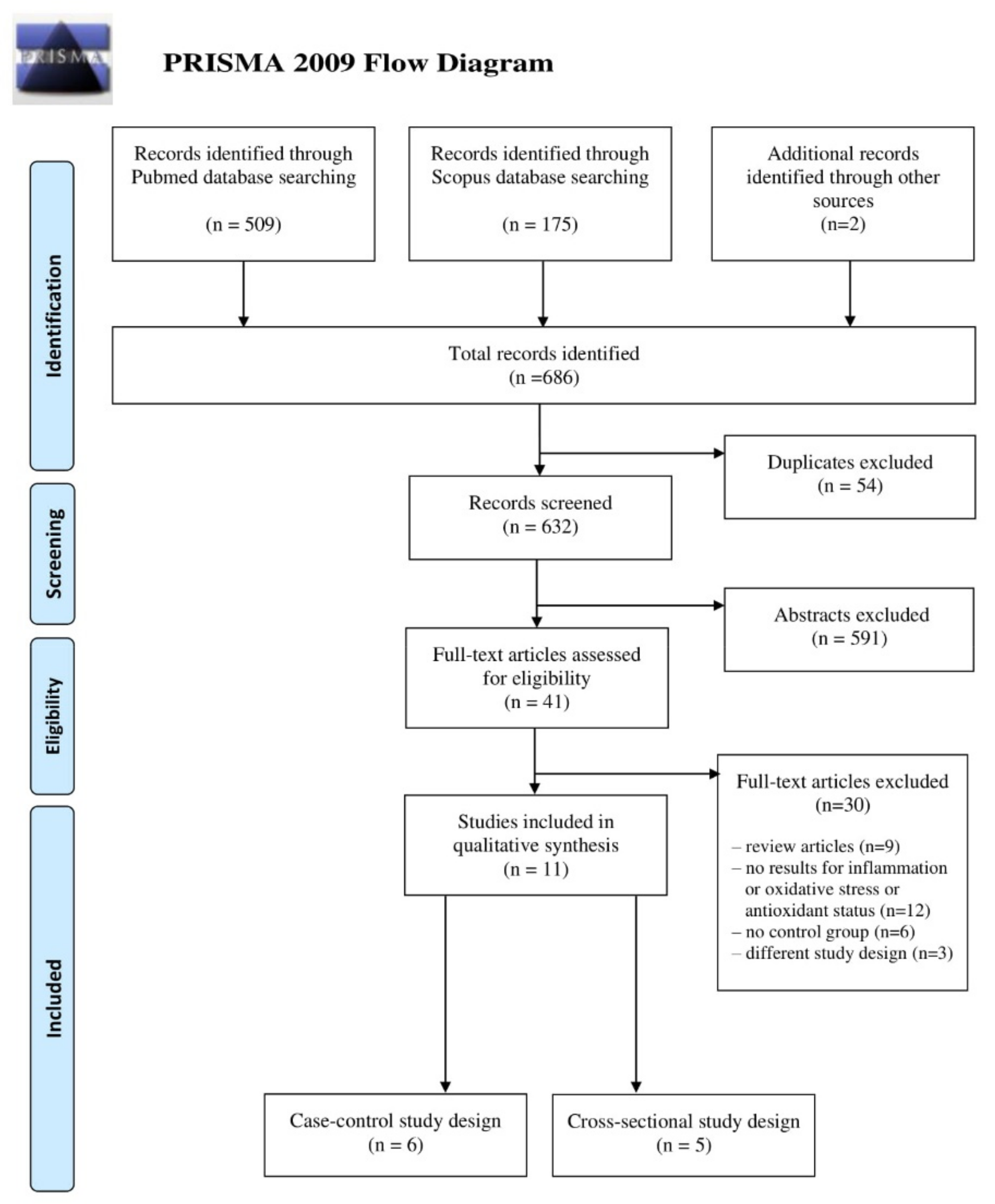
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
3. Results
3.1. Inflammatory Biomarkers
3.2. Oxidative Stress Markers
3.3. Antioxidative Status Parameters
3.3.1. Nonenzymatic Antioxidant Parameters
3.3.2. Enzymatic Antioxidants
4. Discussion
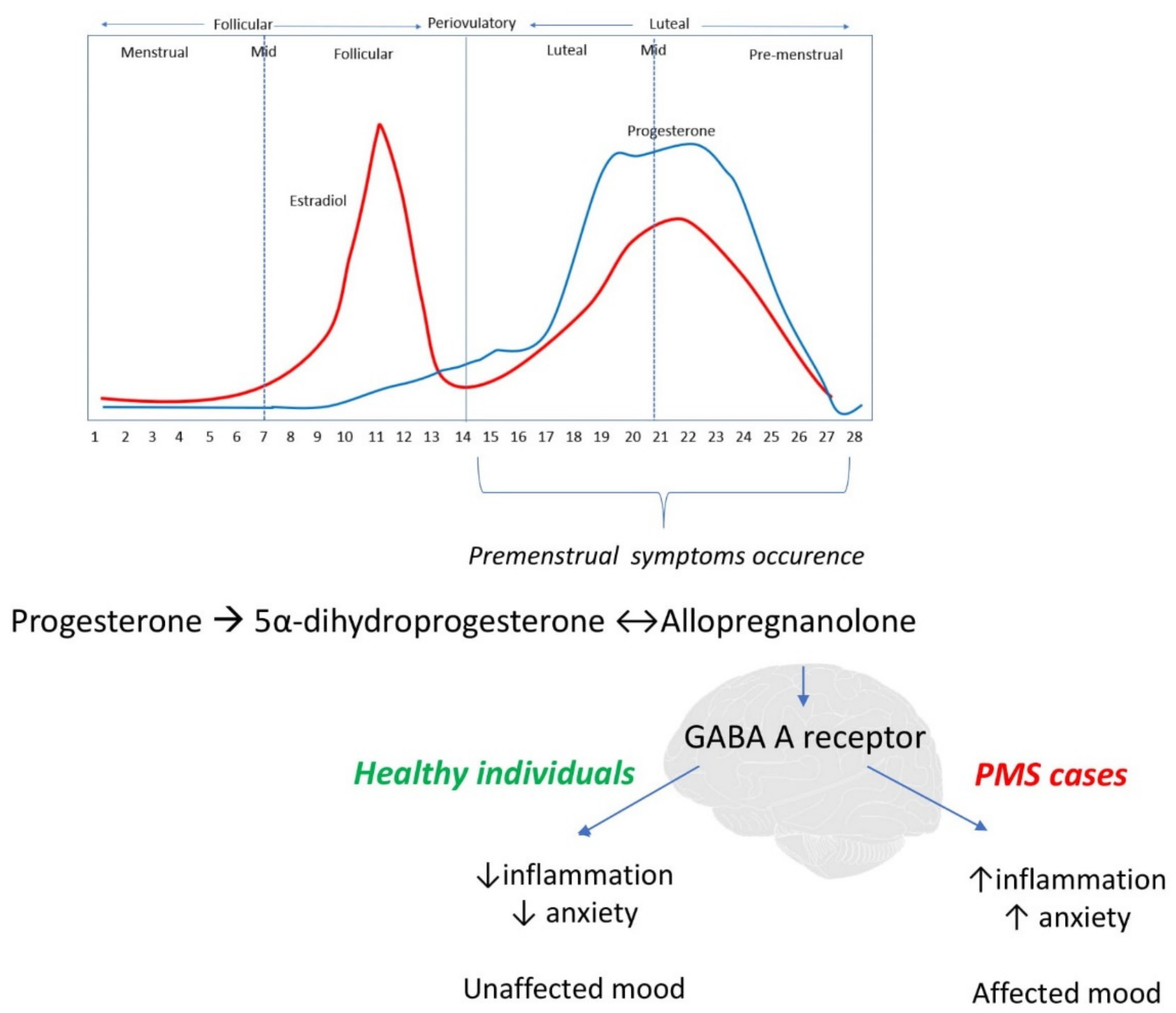
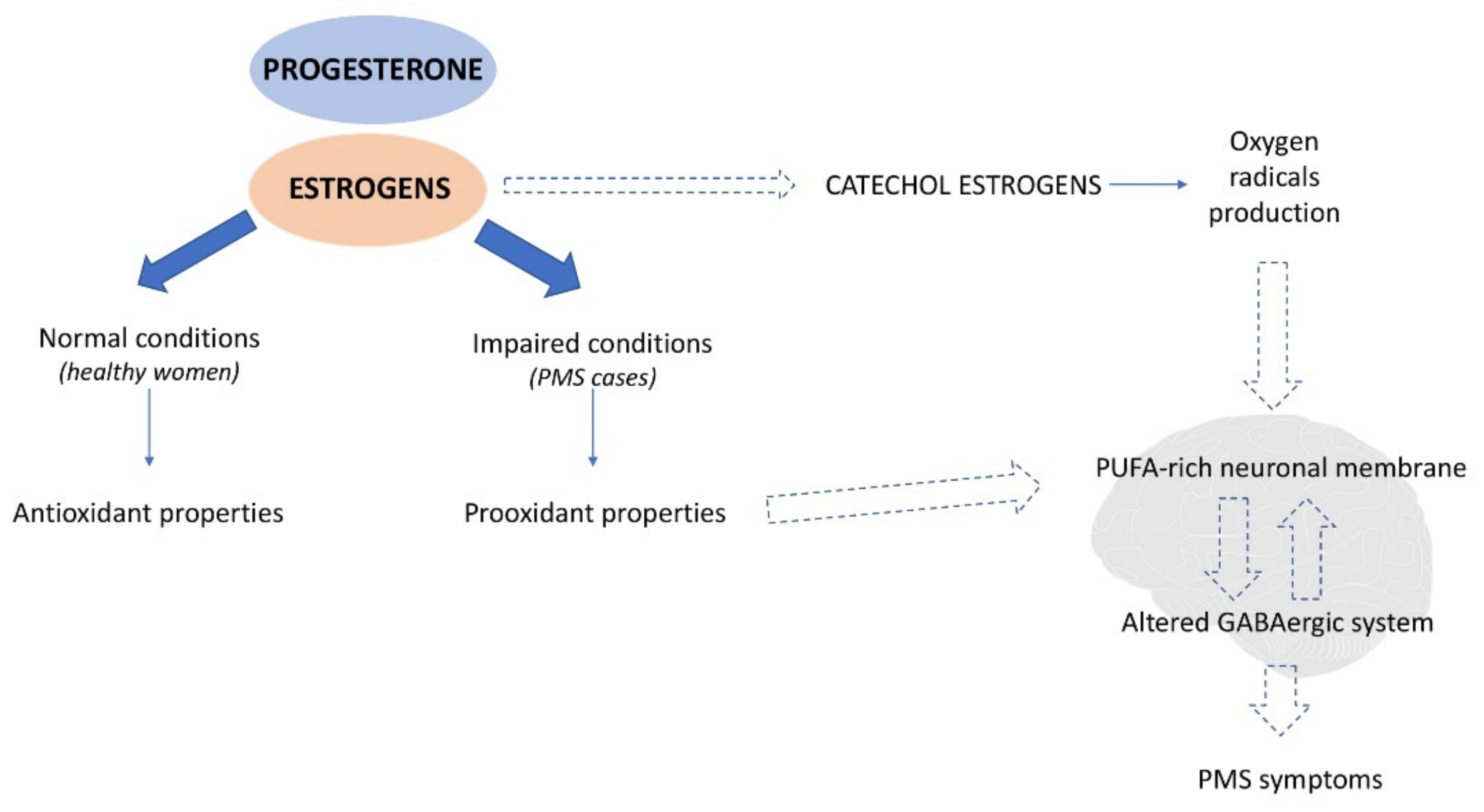
4.1. Potential Mechanism
4.1.1. Inflammation
4.1.2. Oxidative Stress
4.2. Strengths and Limitations
4.3. Implications for Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yonkers, K.A.; Simoni, M.K. Premenstrual Disorders. Am. J. Obstet. Gynecol. 2018, 218, 68–74. [Google Scholar] [CrossRef]
- Hofmeister, S.; Bodden, S. Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Am. Fam. Physician 2016, 94, 236–240. [Google Scholar] [PubMed]
- Direkvand-Moghadam, A.; Sayehmiri, K.; Delpisheh, A.; Kaikhavandi, S. Epidemiology of Premenstrual Syndrome (PMS)—A Systematic Review and Meta-Analysis Study. J. Clin. Diagn. Res. JCDR 2014, 8, 106–109. [Google Scholar] [CrossRef]
- O’Brien, S.; Rapkin, A.; Dennerstein, L.; Nevatte, T. Diagnosis and Management of Premenstrual Disorders. BMJ 2011, 342, d2994. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.J.; Martinez, P.E.; Nieman, L.K.; Koziol, D.E.; Thompson, K.D.; Schenkel, L.; Wakim, P.G.; Rubinow, D.R. Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels but Not Continuous Stable Levels. Am. J. Psychiatry 2017, 174, 980–989. [Google Scholar] [CrossRef]
- Spinelli, M.G. Depression and Hormone Therapy. Clin. Obstet. Gynecol. 2004, 47, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Appleton, S.M. Premenstrual Syndrome: Evidence-Based Evaluation and Treatment. Clin. Obstet. Gynecol. 2018, 61, 52–61. [Google Scholar] [CrossRef]
- Walsh, S.; Ismaili, E.; Naheed, B.; O’Brien, S. Diagnosis, Pathophysiology and Management of Premenstrual Syndrome. Obstet. Gynaecol. 2015, 17, 99–104. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross Talk between ER Stress, Oxidative Stress, and Inflammation in Health and Disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mattina, G.F.; van Lieshout, R.J.; Steiner, M. Inflammation, Depression and Cardiovascular Disease in Women: The Role of the Immune System across Critical Reproductive Events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719851950. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R. Chronic Inflammation and Premenstrual Syndrome: A Missing Link Found? J. Womens Health 2016, 25, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Bannister, E. There Is Increasing Evidence to Suggest That Brain Inflammation Could Play a Key Role in the Aetiology of Psychiatric Illness. Could Inflammation Be a Cause of the Premenstrual Syndromes PMS and PMDD? Post Reprod. Health 2019, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.M.S.; Bäckström, T.; Brown, C.; Dennerstein, L.; Endicott, J.; Epperson, C.N.; Eriksson, E.; Freeman, E.; Halbreich, U.; Ismail, K.M.K.; et al. Towards a Consensus on Diagnostic Criteria, Measurement and Trial Design of the Premenstrual Disorders: The ISPMD Montreal Consensus. Arch. Womens Ment. Health 2011, 14, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 February 2021).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. ESH Working Group on CV Risk in Low Resource Settings Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balat, O.; Dikensoy, E.; Ugur, M.G.; Atmaca, R.; Cekmen, M.; Yurekli, M. Malon Dialdehyde, Nitrite and Adrenomedullin Levels in Patients with Premenstrual Syndrome. Arch. Gynecol. Obstet. 2007, 275, 361–365. [Google Scholar] [CrossRef]
- Duvan, C.I.; Cumaoglu, A.; Turhan, N.O.; Karasu, C.; Kafali, H. Oxidant/Antioxidant Status in Premenstrual Syndrome. Arch. Gynecol. Obstet. 2011, 283, 299–304. [Google Scholar] [CrossRef]
- Fathizadeh, S.; Amani, R.; Haghighizadeh, M.H.; Hormozi, R. Comparison of Serum Zinc Concentrations and Body Antioxidant Status between Young Women with Premenstrual Syndrome and Normal Controls: A Case-Control Study. Int. J. Reprod. Biomed. Yazd Iran 2016, 14, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Foster, R.; Vaisberg, M.; Bachi, A.L.L.; dos Santos, J.d.M.B.; de Paula Vieira, R.; Luna, L.A., Jr.; Araújo, M.P.; Parmigiano, T.R.; Borges, F.; di-Bella, Z.I.K.J. Premenstrual Syndrome, Inflammatory Status, and Mood States in Soccer Players. Neuroimmunomodulation 2019, 26, 1–6. [Google Scholar] [CrossRef]
- Incebiyik, A.; Camuzcuoglu, A.; Hilali, N.G.; Ulas, T.; Vural, M.; Camuzcuoglu, H.; Aksoy, N. Serum Oxidative Stress, Visfatin and Apelin in Healthy Women and Those with Premenstrual Syndrome. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2015, 35, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, E.T.; Rao, A. Plasma Protein Oxidation and Total Antioxidant Power in Premenstrual Syndrome. Asian Pac. J. Trop. Med. 2010, 3, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Azizieh, F.Y.; Alyahya, K.O.; Dingle, K. Association of Self-Reported Symptoms with Serum Levels of Vitamin D and Multivariate Cytokine Profile in Healthy Women. J. Inflamm. Res. 2017, 10, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, A.; Bahrami-Taghanaki, H.; Khorasanchi, Z.; Timar, A.; Jaberi, N.; Azaryan, E.; Tayefi, M.; Ferns, G.A.; Sadeghnia, H.R.; Ghayour-Mobarhan, M. Menstrual Problems in Adolescence: Relationship to Serum Vitamins A and E, and Systemic Inflammation. Arch. Gynecol. Obstet. 2020, 301, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Gonoodi, K.; Khayyatzadeh, S.S.; Tayefi, M.; Darroudi, S.; Bahrami-Taghanaki, H.; Eslami, S.; Jaberi, N.; Ferns, G.A.; Farahmand, K.; et al. The Association of Trace Elements with Premenstrual Syndrome, Dysmenorrhea and Irritable Bowel Syndrome in Adolescents. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Ronnenberg, A.G.; Houghton, S.C.; Nobles, C.; Zagarins, S.E.; Takashima-Uebelhoer, B.B.; Faraj, J.L.; Whitcomb, B.W. Association of Inflammation Markers with Menstrual Symptom Severity and Premenstrual Syndrome in Young Women. Hum. Reprod. Oxf. Engl. 2014, 29, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, M.; Allahdadian, M.; Bahadorani, M. Comparison of Serum Level of Some Trace Elements and Vitamin D between Patients with Premenstrual Syndrome and Normal Controls: A Cross-Sectional Study. Int. J. Reprod. Biomed. 2019. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of Vitamin D on Inflammatory and Oxidative Stress Responses of Human Bronchial Epithelial Cells Exposed to Particulate Matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [Green Version]
- Gold, E.B.; Wells, C.; Rasor, M.O. The Association of Inflammation with Premenstrual Symptoms. J. Womens Health 2016, 25, 865–874. [Google Scholar] [CrossRef]
- Puder, J.J.; Blum, C.A.; Mueller, B.; de Geyter, C.; Dye, L.; Keller, U. Menstrual Cycle Symptoms Are Associated with Changes in Low-Grade Inflammation. Eur. J. Clin. Investig. 2006, 36, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Matsumoto, A.K.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.G.; Sirivichayakul, S.; Carvalho, A.; Barbosa, D.S.; Maes, M. The Role of Immune and Oxidative Pathways in Menstrual Cycle Associated Depressive, Physio-Somatic, Breast and Anxiety Symptoms: Modulation by Sex Hormones. J. Psychosom. Res. 2020, 135, 110158. [Google Scholar] [CrossRef]
- Purnawati, J.; Sinrang, A.W.; Jusuf, E.C.; Limoa, E.; Ahmad, M.; Usman, A.N. Nutrition, Mental Status and Level of 8-Hydroxy-2-Deoxyguanosine (OHDG) Urine as Predictors of Premenstrual Syndrome (PMS) in Adolescent Girls. Int. J. Curr. Res. Rev. 2020, 12, 7–13. [Google Scholar] [CrossRef]
- Szmidt, M.K.; Granda, D.; Sicinska, E.; Kaluza, J. Primary Dysmenorrhea in Relation to Oxidative Stress and Antioxidant Status: A Systematic Review of Case-Control Studies. Antioxidants 2020, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Amani, R.; Feizi, A.; Askari, G.; Kohan, S.; Tavasoli, P. Vitamin D Supplementation for Premenstrual Syndrome-Related Inflammation and Antioxidant Markers in Students with Vitamin D Deficient: A Randomized Clinical Trial. Sci. Rep. 2019, 9, 14939. [Google Scholar] [CrossRef]
- Tartagni, M.; Cicinelli, M.V.; Tartagni, M.V.; Alrasheed, H.; Matteo, M.; Baldini, D.; de Salvia, M.; Loverro, G.; Montagnani, M. Vitamin D Supplementation for Premenstrual Syndrome-Related Mood Disorders in Adolescents with Severe Hypovitaminosis D. J. Pediatr. Adolesc. Gynecol. 2016, 29, 357–361. [Google Scholar] [CrossRef]
- Abdollahi, R.; Abiri, B.; Sarbakhsh, P.; Kashanian, M.; Vafa, M. The Effect of Vitamin D Supplement Consumption on Premenstrual Syndrome in Vitamin D-Deficient Young Girls: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Complement. Med. Res. 2019, 26, 336–342. [Google Scholar] [CrossRef]
- Bahrami, A.; Avan, A.; Sadeghnia, H.R.; Esmaeili, H.; Tayefi, M.; Ghasemi, F.; Nejati Salehkhani, F.; Arabpour-Dahoue, M.; Rastgar-Moghadam, A.; Ferns, G.A.; et al. High Dose Vitamin D Supplementation Can Improve Menstrual Problems, Dysmenorrhea, and Premenstrual Syndrome in Adolescents. Gynecol. Endocrinol. 2018, 34, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, H.; Ebrahimi, E.; Fathizadeh, N. Evaluating the Effects of Vitamin D and Vitamin E Supplement on Premenstrual Syndrome: A Randomized, Double-Blind, Controlled Trial. Iran. J. Nurs. Midwifery Res. 2016, 21, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, A.C.; El-Sohemy, A. Association between Vitamin D Status and Premenstrual Symptoms. J. Acad. Nutr. Diet 2019, 119, 115–123. [Google Scholar] [CrossRef]
- Abdi, F.; Ozgoli, G.; Rahnemaie, F.S. A Systematic Review of the Role of Vitamin D and Calcium in Premenstrual Syndrome. Obstet. Gynecol. Sci. 2019, 62, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Golpour-Hamedani, S.; Rafie, N. The Association Between Vitamin D and Premenstrual Syndrome: A Systematic Review and Meta-Analysis of Current Literature. J. Am. Coll. Nutr. 2019, 38, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Posaci, C.; Erten, O.; Uren, A.; Acar, B. Plasma Copper, Zinc and Magnesium Levels in Patients with Premenstrual Tension Syndrome. Acta Obstet. Gynecol. Scand. 1994, 73, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.J.; Dawson, E.B. Zinc and Copper Levels in Premenstrual Syndrome. Fertil. Steril. 1994, 62, 313–320. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H. Insight into Zinc Signaling from Dietary Zinc Deficiency. Brain Res. Rev. 2009, 62, 33–44. [Google Scholar] [CrossRef]
- Jafari, F.; Amani, R.; Tarrahi, M.J. Effect of Zinc Supplementation on Physical and Psychological Symptoms, Biomarkers of Inflammation, Oxidative Stress, and Brain-Derived Neurotrophic Factor in Young Women with Premenstrual Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2020, 194, 89–95. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic Diseases, Inflammation, and Spices: How Are They Linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come From? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First?: Inflammation in Psychiatric Disorders. Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Lorenz, T.K.; Demas, G.E.; Heiman, J.R. Partnered Sexual Activity Moderates Menstrual Cycle–Related Changes in Inflammation Markers in Healthy Women: An Exploratory Observational Study. Fertil. Steril. 2017, 107, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual Cycle Rhythmicity: Metabolic Patterns in Healthy Women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Bixo, M.; Johansson, M.; Nyberg, S.; Ossewaarde, L.; Ragagnin, G.; Savic, I.; Strömberg, J.; Timby, E.; van Broekhoven, F.; et al. Allopregnanolone and Mood Disorders. Prog. Neurobiol. 2014, 113, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; de Nicola, A.F.; Guennoun, R. Revisiting the Roles of Progesterone and Allopregnanolone in the Nervous System: Resurgence of the Progesterone Receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Sotler, R. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58. [Google Scholar] [CrossRef]
- Reed, S.C.; Levin, F.R.; Evans, S.M. Changes in Mood, Cognitive Performance and Appetite in the Late Luteal and Follicular Phases of the Menstrual Cycle in Women with and without PMDD (Premenstrual Dysphoric Disorder). Horm. Behav. 2008, 54, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Case–Control Studies | |||||
|---|---|---|---|---|---|
| Authors | Selection (Max. 4 Stars) | Comparability (Max. 2 Stars) | Exposure (Max. 3 Stars) | Total Points (Max. 9) | Quality |
| Balat et al. [18] | 3 | 2 | 2 | 7 | High |
| Duvan et al. [19] | 3 | 2 | 2 | 7 | High |
| Fathizadeh et al. [20] | 3 | 2 | 2 | 7 | High |
| Foster et al. [21] | 3 | 2 | 2 | 7 | High |
| Incebiyik et al. [22] | 1 | 2 | 1 | 4 | Medium |
| Tuladhar and Rao [23] | 2 | 2 | 2 | 6 | Medium |
| Cross-Sectional Studies | |||||
| Authors | Selection (Max. 5 Stars) | Comparability (Max. 2 Stars) | Outcome (Max. 3 Stars) | Sum (Max. 10) | Quality |
| Azizieh et al. [24] | 1 | 2 | 2 | 5 | Medium |
| Bahrami et al. [25] | 3 | 2 | 3 | 8 | High |
| Bahrami et al. [26] | 2 | 2 | 3 | 7 | High |
| Bertone-Johnson et al. [27] | 2 | 2 | 3 | 7 | High |
| Fatemi et al. [28] | 2 | 0 | 3 | 5 | Medium |
| Authors | Location | Cases n | Case Definition | Controls n | Participants Characteristics | Age Cases Mean ± SD (Range), Years | Age Controls Mean ± SD (Range), Years | Biomarkers of Interest | Adjustment |
|---|---|---|---|---|---|---|---|---|---|
| Case–control studies | |||||||||
| Balat et al. [18] | Turkey | 11 | ACOG, DSRS | 10 | non-pregnant, not under treatment for PMS, no history of psychiatric disorders, not taking oral contraceptives | 34.2 ± 3.5 (28–37) | 32.9 ± 3.1 (28–37) | MDA, NO, AM | no |
| Duvan et al. [19] | Turkey | 20 | DSRS | 21 | regular menses for at least six previous cycles, non-pregnant, non-breastfeeding, not under PMS treatment, no history of psychiatric disorders, thyroid and other endocrine disorders, not taking oral contraceptive pills | 29.23 ± 4.79 (22–39) | 28.05 ± 4.66 (22–39) | LHP, MDA, PC, T-SH, TAC | no |
| Fathizadeh et al. [20] | Iran | 23 | DSRS | 25 | medical students from dormitories, no history of chronic disease, no nutritional supplements or medication intake * | 24.17 ± 0.55 (21–31) | 23.64 ± 0.60 (21–31) | TAC, zinc | no |
| Foster et al. [21] | Brazil | 31 | DSRS | 21 | non-pregnant, regularly attending training sessions, with regular menstrual cycles, no fractures, no severe ligament injuries, no presented genetic or acquired kidney disease; no hormonal contraceptive or antidepressants, anxiolytics, diuretics, steroids or illegal drugs | 18.7 ± 3.99 (N/A) | 20.68 ± 3.70 (N/A) | IL-1β, IL-6, IL-8, IL-10, TNF-α | no |
| Incebiyik et al. [22] | Turkey | 40 | ACOG, DSRS | 40 | non-pregnant, non-breastfeeding, with regular menstrual cycles, no chronic illnesses, such as thyroid or metabolic diseases; no psychiatric disorders, such as psychosis or bipolar disorder; no hormonal contraceptives, no serotonin reuptake inhibitors or antidepressants; no antioxidant medication, no previous hysterectomy or ovarian surgery, no smokers | 28.93 ± 7.16 (18–45) | 29.45 ± 5.10 (18–45) | TAC (TAS), TOS, OSI, LHP (LOOH), -SH | no |
| Tuladhar and Rao [23] | India | 74 | COPE | 80 | regular menstrual cycles with no other illness and were not on any medications, no history of polycystic ovarian disease, smoking, alcohol consumption, drug abuse, insulin resistance and use of contraceptive pills | 23 ** (20–24) | 24 ** (20–24) | TAC (FRAP), PPT, PC | no |
| Cross-sectional studies | |||||||||
| Azizieh et al. [24] | Kuwait | 94 | self-assessment | 23 | Kuwaiti citizens only; non-pregnant, non-breastfeeding, free from infections/chronic diseases, and not taking any medications or vitamin/mineral supplements/injections for the previous 6 months | 28.5 (19–47) | 25 (19–47) | IL-1β, IL-8, IL-17, IL-4, TNF-α, IFN-γ, vitamin D | no |
| Bahrami et al. [25] | Iran | 134 | COPE | 148 | without any autoimmune abnormalities, carcinoma, metabolic or cardiovascular disorders (CVD), liver or renal failure, periodontal disease, and endocrinopathy diagnosed by physicians or self-reported; no anti-inflammatory, antidepressant, vitamins supplement consumption and hormone therapy over the past year | 14.6 ± 1.4 (12–18) | 14.6 ± 1.7 (12–18) | Anti-Hsp27, hsCRP, vitamin A, vitamin E | no |
| Bahrami et al. [26] | Iran | 67 | COPE | 74 | no acute or chronic physical/psychological disease even without drug consumption; no mineral supplementation during the six months before recruitment | (12–18) | (12–18) | zinc, copper, zinc/copper ratio, SOD | no |
| Bertone-Johnson et al. [27] | USA | 37 | modified COPE | 67 | currently menstruating women; not reporting a history of high blood pressure or elevated cholesterol, kidney or liver disease, bone disease such as osteomalacia, digestive disorders, rheumatologic disease, multiple sclerosis, thyroid disease, hyperparathyroidism, cancer, type 1 or type 2 diabetes or polycystic ovaries; not taking corticosteroids, anabolic steroids, anticonvulsants, cimetidine or propranolol. | 21.6 ± 3.1 (18–30) | 21.1 ± 2.7 (18–30) | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, TNF-α, GMCSF, IFN-γ, hsCRP | age, BMI, smoking status |
| Fatemi et al. [28] | Iran | 115 | PSST | 163 | no endocrine disorders, no vitamin and mineral supplements, no corticosteroid antidepressants, no oral contraceptive pill | N/A (19–21) | N/A (19–21) | zinc, vitamin D | no |
| Inflammatory Parameters | Oxidative Stress Parameters | Antioxidative Status Parameters |
|---|---|---|
| Interleukins | Total | Total |
| IL-1β [21,24,27] | TOS [22] | TAC (TAS/FRAP) [19,20,22,23] |
| IL-2 [27] | OSI [22] | Specific vitamins/minerals |
| IL-4 [24,27] | Lipid peroxidation and protein oxidation | vitamin A [25] |
| IL-5 [27] | MDA [18,19] | vitamin D [24,28] |
| IL-6 [27] | LHP (LOOH) [19,22] | vitamin E [25] |
| IL-7 [21,27] | PC [19,23] | cooper [26] |
| IL-8 [21,24,27] | PPT [23] | zinc [20,26,28] |
| IL-10 [21,27] | Others | zinc/cooper ratio [26] |
| IL-12 [27] | -SH [22] | Enzymes |
| IL-13 [27] | T-SH [19] | SOD [26] |
| IL-17 [24] | NO [18] | |
| Others | AM [18] | |
| TNF-α [21,24,27] | ||
| IFN-γ [24,27] | ||
| GMCSF [27] | ||
| hsCRP [25,27] | ||
| Anti-Hsp27 [25] |
| Authors | Biological Sample | Results Presentation Method (Unit) | No. of Cases | No. of Controls | Results PMS Cases | Results Controls | p-Value |
|---|---|---|---|---|---|---|---|
| INTERLEUKINS | |||||||
| IL-1β | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 0.3 | 0.2 | 0.32 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 0.72 | 0.28 | 0.27 |
| Foster et al. [21] | Urine | Square mean root ± SD (pg/mL) | 31 | 21 | 1.12 ±0.23 | 0.90 ± 0.25 | 0.01 |
| IL-2 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 1.94 | 1.09 | 0.09 |
| IL-4 | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 163.9 | 35.2 | 0.18 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 8.01 | 4.16 | 0.01 |
| IL-5 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 0.33 | 0.20 | 0.05 |
| IL-6 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 2.68 | 1.88 | 0.27 |
| IL-7 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 2.11 | 1.20 | 0.09 |
| Foster et al. [21] | Urine | Square mean ± SD (pg/mL) | 31 | 21 | 9.48 ± 1.68 | 6.45 ± 1.01 | 0.001 |
| IL-8 | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 14.5 | 10.7 | 0.009 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 2.26 | 1.72 | 0.20 |
| Foster et al. [21] | Urine | Square mean ± SD (pg/mL) | 31 | 21 | 1.26 ± 0.48 | 0.81 ± 0.10 | 0.04 |
| IL-10 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 17.15 | 9.19 | 0.03 |
| Foster et al. [21] | Urine | Square mean ± SD (pg/mL) | 31 | 21 | 0.98 ± 0.12 | 0.97 ± 0.09 | 0.74 |
| IL-12 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 5.57 | 2.06 | 0.04 |
| IL-13 | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 2.35 | 1.23 | 0.08 |
| IL-17 | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 4.0 | 4.3 | 0.47 |
| OTHERS | |||||||
| TNF-α | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 10.6 | 7.6 | 0.002 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 4.78 | 4.35 | 0.59 |
| Foster et al. [21] | Urine | Square mean ± SD (pg/mL) | 31 | 21 | 1.23 ± 0.36 | 1.15 ± 0.23 | 0.58 |
| IFN-γ | |||||||
| Azizieh et al. [24] | Serum | Median (pg/mL) | 94 | 23 | 11.0 | 10.7 | 0.97 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 3.59 | 1.39 | 0.01 |
| hsCRP | |||||||
| Bahrami et al. [25] | Serum | Median (IR) (mg/L) | 134 | 148 | 0.98 (0.64–1.72) | 0.82 (0.41–1.61) | >0.05 |
| Bertone-Johnson et al. [27] | Serum | Geometric mean (mg/L) | 28 | 41 | 1.36 | 0.91 | 0.30 |
| GMCSF | |||||||
| Bertone-Johnson et al. [27] | Serum | Geometric mean (pg/mL) | 37 | 67 | 8.42 | 5.41 | 0.23 |
| Anti-Hsp27 | |||||||
| Bahrami et al. [25] | Serum | Mean ± SD * (N/A) | 134 | 148 | 0.26 ± 0.17 | 0.29 ± 0.24 | >0.05 |
| Authors | Biological Sample | Results Presentation Method (Unit) | No. of Cases | No. of Controls | Results PMS Cases | Results Controls | p-Value |
|---|---|---|---|---|---|---|---|
| TOTAL OXIDATIVE STATUS | |||||||
| TOS | |||||||
| Incebiyik et al. [22] | Serum | Mean ± SD * (μmol H2O2 equivalent/L) | 40 | 40 | 46.78 ± 23.32 | 41.89 ± 18.64 | 0.304 |
| OSI | |||||||
| Incebiyik et al. [22] | Serum | Mean ± SD (mmol trolox/L) | 40 | 40 | 5.10 ± 2.87 | 4.44 ± 2.12 | 0.243 |
| LIPID PEROXIDATION AND PROTEIN OXIDATION | |||||||
| MDA | |||||||
| Balat et al. [18] | Plasma | Mean ± SD (N/A) | 11 | 10 | 1.4 ± 0.1 | 1.6 ± 0.1 | >0.05 |
| Duvan et al. [19] | Plasma | Mean ± SD (nmol/mL) | 20 | 21 | 4.46 ± 0.54 | 4.50 ± 0.46 | 0.78 |
| LHP (LOOH) | |||||||
| Duvan et al. [19] | Plasma | Mean ± SD (nmol/mL) | 20 | 21 | 0.28 ± 0.08 | 0.20 ± 0.04 | 0.01 |
| Incebiyik et al. [22] | Serum | Mean ± SD (mmol H2O2 equivalent/l) | 40 | 40 | 23.21 ± 13.80 | 20.43 ± 10.90 | 0.321 |
| PC | |||||||
| Duvan et al. [19] | Serum | Mean ± SD (nmol/mL) | 20 | 21 | 13.9 ± 1.38 | 13.4 ± 1.45 | 0.18 |
| Tuladhar and Rao [23] | Plasma | Median (IR) (nmol/mg protein) | 74 | 80 | 0.90 (0.68–1.20) | 0.84 (0.65–1.12) | >0.05 |
| PPT | |||||||
| Tuladhar and Rao [23] | Plasma | Median (IR) (µmol) | 74 | 80 | 410 (371–449) | 410 (371–468) | >0.05 |
| OTHERS | |||||||
| -SH | |||||||
| Incebiyik et al. [22] | Serum | Mean ± SD (mmol/L) | 40 | 40 | 0.46 ± 0.09 | 0.43 ± 0.06 | 0.064 |
| T-SH | |||||||
| Duvan et al. [19] | Serum | Mean ± SD (nmol/L) | 20 | 21 | 0.39 ± 0.07 | 0.37 ± 0.05 | 0.59 |
| NO | |||||||
| Balat et al. [18] | Plasma | Mean ± SD (N/A) | 11 | 10 | 42 ± 2.4 | 43 ± 2.4 | >0.05 |
| AM | |||||||
| Balat et al. [18] | Plasma | Mean ± SD (N/A) | 11 | 10 | 37 ± 2.2 | 26 ± 1.4 | <0.05 |
| Authors | Biological Sample | Results Presentation Method (Unit) | No. of Cases | No. of Controls | Results PMS Cases | Results Controls | p-Value |
|---|---|---|---|---|---|---|---|
| Nonenzymatic Antioxidant Parameters | |||||||
| Total Antioxidant Status Parameters | |||||||
| TAC (TAS/FRAP) | |||||||
| Duvan et al. [19] | Plasma | Mean ± SD * (mmol/L) | 20 | 21 | 0.55 ± 0.22 | 0.73 ± 0.09 | 0.01 |
| Fathizadeh et al. [20] | Serum | Mean ± SD (mmol/L) | 23 | 25 | 0.81 ± 0.041 | 1.075 ± 0.06 | <0.01 |
| Incebiyik et al. [22] | Serum | Mean ± SD (mmol Trolox equivalent/L) | 40 | 40 | 0.96 ± 0.19 | 0.96 ± 0.15 | 0.982 |
| Tuladhar and Rao [23] | Plasma | Median (IR) (µmol/L) | 74 | 80 | 955 (775–1110) | 1020 (860–1175) | >0.05 |
| Specific Vitamins And Minerals | |||||||
| Vitamin A | |||||||
| Bahrami et al. [25] | Serum | Median (IR) (μmol/L) | 134 | 148 | 1.12 (0.26–9.30) | 7.2 (2.66–19.27) | 0.007 |
| Vitamin D | |||||||
| Azizieh et al. [24] | Serum | Median (nmol/L) | 94 | 23 | 17.0 | 14.0 | 0.30 |
| Fatemi et al. [28] | Serum | Mean ± SD (ng/mL) | 115 | 163 | 22.44 ± 13.91 | 26.58 ± 6.74 | 0.043 |
| Vitamin E | |||||||
| Bahrami et al. [25] | Serum | Median (IR) (μmol/L) | 134 | 148 | 3.75 (2.87–5.80) | 4.0 (2.69–5.80) | >0.05 |
| Cooper | |||||||
| Bahrami et al. [26] | Serum | Mean ± SD (μmol/L) | 67 | 74 | 9.2 ± 7.6 | 18.4 ± 9.0 | NCD ** |
| Zinc | |||||||
| Bahrami et al. [26] | Serum | Mean ± SD (μmol/L) | 67 | 74 | 14.3 ± 3.1 | 14.3 ± 14.3 | NCD ** |
| Fatemi et al. [28] | Serum | Mean ± SD (μg/dl) | 115 | 163 | 83.49 ± 11.58 | 84.41 ± 11.80 | 0.521 |
| Fathizadeh et al. [20] | Serum | Mean ± SD (μg/dl) | 23 | 25 | 108.20 ± 3.73 | 153.8 ± 18.77 | 0.026 |
| Zinc to cooper ratio | |||||||
| Bahrami et al. [26] | Serum | Mean ± SD | 67 | 74 | 0.8 ± 0.4 | 1.0 ± 7.0 | NCD ** |
| Enzymes | |||||||
| SOD | |||||||
| Bahrami et al. [26] | Serum | Mean ± SD (U/mL) | 67 | 74 | 0.05 ± 0.02 | 0.05 ± 0.02 | NCD ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granda, D.; Szmidt, M.K.; Kaluza, J. Is Premenstrual Syndrome Associated with Inflammation, Oxidative Stress and Antioxidant Status? A Systematic Review of Case–Control and Cross-Sectional Studies. Antioxidants 2021, 10, 604. https://doi.org/10.3390/antiox10040604
Granda D, Szmidt MK, Kaluza J. Is Premenstrual Syndrome Associated with Inflammation, Oxidative Stress and Antioxidant Status? A Systematic Review of Case–Control and Cross-Sectional Studies. Antioxidants. 2021; 10(4):604. https://doi.org/10.3390/antiox10040604
Chicago/Turabian StyleGranda, Dominika, Maria Karolina Szmidt, and Joanna Kaluza. 2021. "Is Premenstrual Syndrome Associated with Inflammation, Oxidative Stress and Antioxidant Status? A Systematic Review of Case–Control and Cross-Sectional Studies" Antioxidants 10, no. 4: 604. https://doi.org/10.3390/antiox10040604






