Astaxanthin Ameliorates Diabetic Retinopathy in Swiss Albino Mice via Inhibitory Processes of Neuron-Specific Enolase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Diabetic Retinopathy (DR)
2.3. Experimental Design
2.4. Assessment of DR Associated Changes of Visual Acuity Behaviours
2.4.1. Assessment of Visual Acuity Functions by OMR Test Device
2.4.2. Assessment of Spatial Imagery Transformation Function by PM Test Device
2.5. Biochemical Estimations
2.5.1. Estimation of Catalase as an Indication of Oxidative Stress
2.5.2. Estimation of LDH as an Indication of Retinal Tissue Inflammation
2.5.3. Estimation of NSE as an Indication of Retinal Macular Degeneration
2.5.4. Estimation of Total Protein
2.6. Statistical Analysis
3. Results
3.1. Effect of AST in DR Associated Visual Behavioural Changes
3.1.1. Effect of AST in DR Associated Changes of Visual Acuity Functions
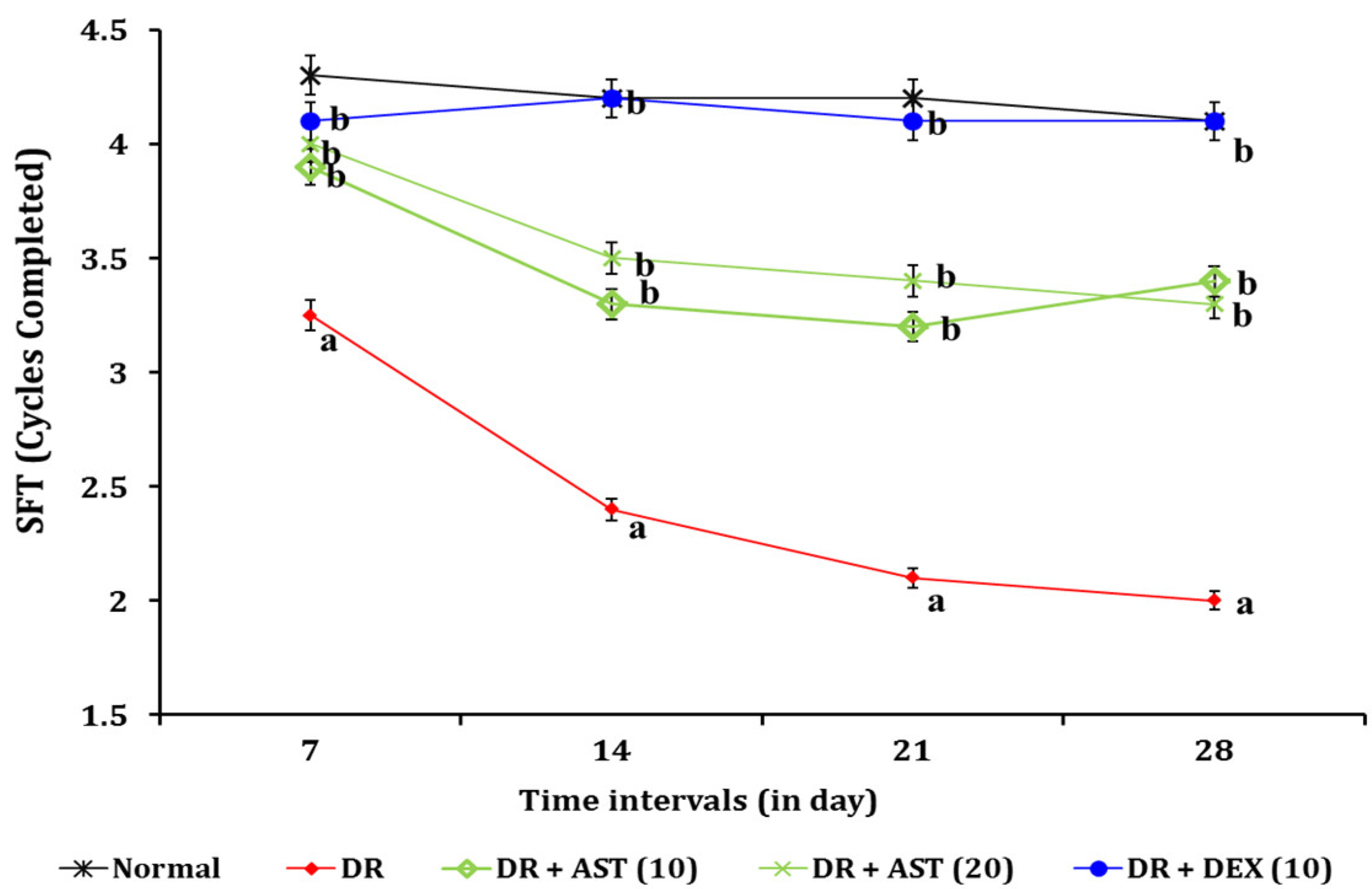
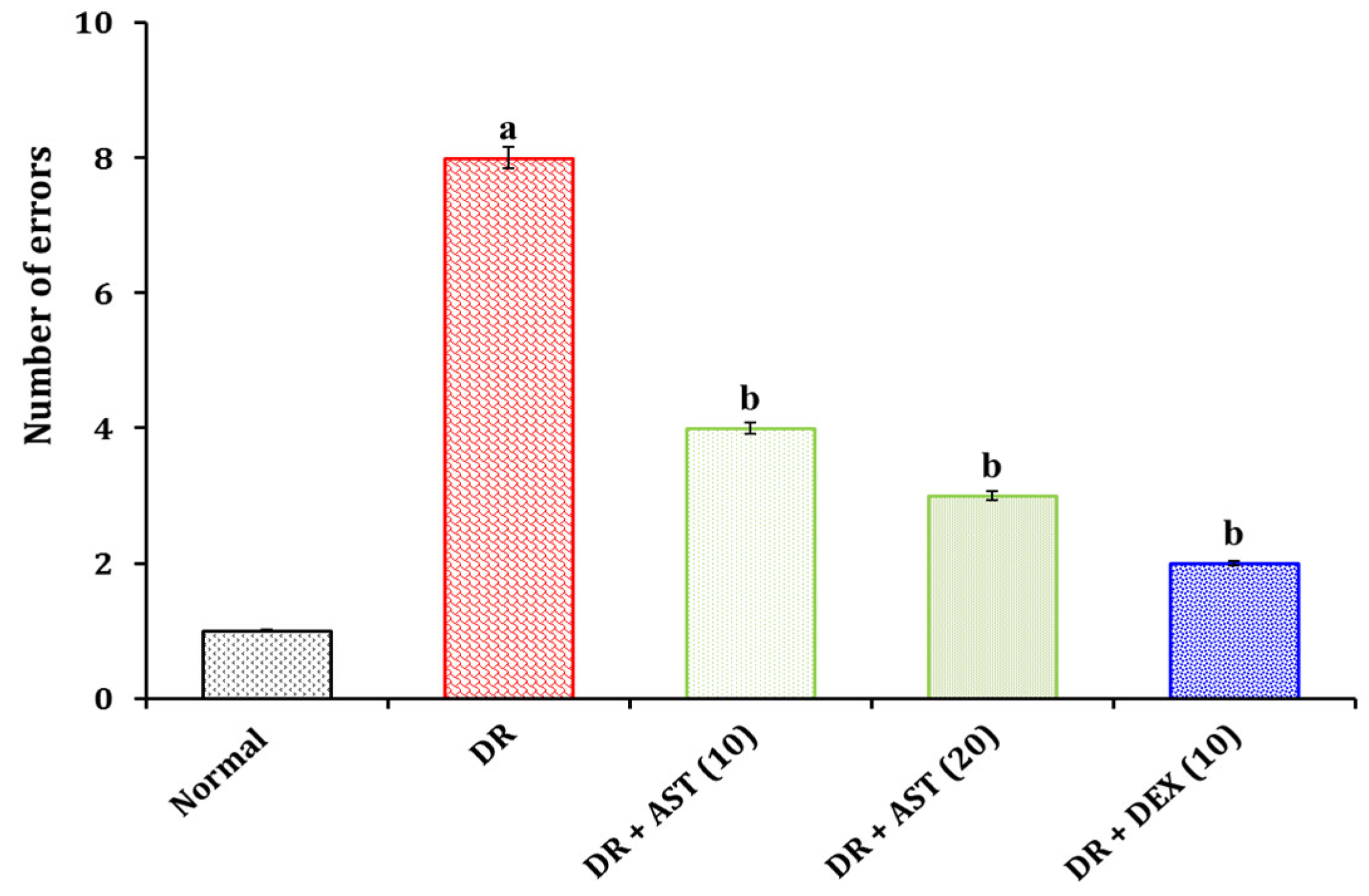
3.1.2. Effect of AST in DR Associated Changes in Spatial Imagery Transformation Function
3.2. Effect of AST in DR Associated Changes in Biochemical Estimations
3.2.1. Effect of AST in DR Associated Changes of Blood Glucose Level
3.2.2. Effect of AST in DR Associated Tissue Biomarker Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.L.; Chan, C.Y.; Abd Shukor, S.R.; Mashitah, M.D. Recovery of Oil and Carotenes from Palm Oil Mill Effluent (POME). Chem. Eng. J. 2008, 141, 383–386. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of Biogas and Performance Evaluation of Existing Treatment Processes in Palm Oil Mill Effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- Wang, J.; Wang, E.; Liu, T.; He, Y.; Wang, K. The Highly Conserved α-Enolase Stimulats Cross-Protective Immunity against Serotype I and II Streptococcus Iniae Infection in Channel Catfish (Ictalurus Punctatus). Aquaculture 2022, 550, 737854. [Google Scholar] [CrossRef]
- Ryans, K.; Omosun, Y.; McKeithen, D.N.; Simoneaux, T.; Mills, C.C.; Bowen, N.; Eko, F.O.; Black, C.M.; Igietseme, J.U.; He, Q. The Immunoregulatory Role of Alpha Enolase in Dendritic Cell Function during Chlamydia Infection. BMC Immunol. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Ghaib, Z.J.; Ghudhaib, K.K.; Mohsen, F.Y. Assessment of Neuron Specific Enolase Level and Some Related Biochemical Factors in Patients with Diabetic Peripheral Nerve Disorders. Indian J. Forensic Med. Toxicol. 2021, 15, 1494–1500. [Google Scholar] [CrossRef]
- Yu, Z.-W.; Liu, R.; Li, X.; Wang, Y.; Fu, Y.-H.; Li, H.-Y.; Yuan, Y.; Gao, X.-Y. High Serum Neuron-Specific Enolase Level Is Associated with Mild Cognitive Impairment in Patients with Diabetic Retinopathy. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1359–1365. [Google Scholar] [CrossRef]
- Li, J.; Yan, M.; Zhang, Y.; Xie, M.; Yan, L.; Chen, J. Serum Neuron-Specific Enolase Is Elevated as a Novel Indicator of Diabetic Retinopathy Including Macular Oedema. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 102–107. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Cheng, J.L.; Beebe, J.D.; Nepple, K.G.; Zakharia, Y.; Mullins, R.F.; Flamme-Wiese, M.J.; Thurtell, M.J.; Han, I.C. Autoimmune Retinopathy and Optic Neuropathy Associated with Enolase-Positive Renal Oncocytoma. Am. J. Ophthalmol. Case Rep. 2018, 12, 55–60. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yoneda, M.; Hasegawa, T.; Matsunaga, A.; Ikawa, M.; Nakamura, T.; Ezura, M.; Baba, T.; Sugeno, N.; Ishiyama, S.; et al. High Prevalence of Serum Anti-NH2-Terminal of α-Enolase Antibodies in Patients with Multiple System Atrophy and Corticobasal Syndrome. J. Neurol. 2021, 268, 4291–4295. [Google Scholar] [CrossRef]
- Asadova, V.; Gul, Z.; Buyukuysal, R.L.; Yalcinbayir, O. Assessment of Neuron-Specific Enolase, S100B and Malondialdehyde Levels in Serum and Vitreous of Patients with Proliferative Diabetic Retinopathy. Int. Ophthalmol. 2020, 40, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Thomas, S.M.; Lillie, E.; Veroniki, A.A.; Hamid, J.S.; Pham, B.; Lee, T.; Agarwal, A.; Sharpe, J.P.; Scott, A.; et al. Anti-Vascular Endothelial Growth Factor Therapy for Age-Related Macular Degeneration: A Systematic Review and Network Meta-Analysis. Syst. Rev. 2021, 10, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Hupfeld, C.; Chao, D.L. SGLT2 Inhibitor–Induced Low-Grade Ketonemia Ameliorates Retinal Hypoxia in Diabetic Retinopathy—A Novel Hypothesis. J. Clin. Endocrinol. Metab. 2021, 106, 1235–1244. [Google Scholar] [CrossRef]
- Chatziralli, I. Ranibizumab for the Treatment of Diabetic Retinopathy. Exp. Opin. Biol. Ther. 2021, 21, 991–997. [Google Scholar] [CrossRef]
- Iyer, P.G.; Albini, T.A. Drug-Related Adverse Effects of Antivascular Endothelial Growth Factor Agents. Curr. Opin. Ophthalmol. 2021, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I. Herbal Plants: A Boon in the Treatment of Diabetic Retinopathy. Pharmacologia 2015, 6, 1–10. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, R.; Mandal, V. Role of Phytomedicine in Alleviating Oxidative Stress-Mediated Vascular Complications in Diabetes. In Evidence Based Validation of Traditional Medicines; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 141–162. ISBN 9789811581267. [Google Scholar]
- Zhang, H.W.; Zhang, H.; Grant, S.J.; Wan, X.; Li, G. Single Herbal Medicine for Diabetic Retinopathy. Cochrane Database Syst. Rev. 2018, 2018, CD007939. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, Q.; Wu, Q.; Shi, J. Neuroprotective Effects of Dendrobium Alkaloids on Rat Cortical Neurons Injured by Oxygen-Glucose Deprivation and Reperfusion. Phytomedicine 2010, 17, 108–115. [Google Scholar] [CrossRef]
- Saha, L.; Chakrabarti, A.; Kumari, S.; Bhatia, A.; Banerjee, D. Antiapoptotic and Neuroprotective Role of Curcumin in Pentylenetetrazole (PTZ) Induced Kindling Model in Rat. Indian J. Exp. Biol. 2016, 54, 133–141. [Google Scholar]
- Guo, C.; Zhu, Y.; Weng, Y.; Wang, S.; Guan, Y.; Wei, G.; Yin, Y.; Xi, M.; Wen, A. Therapeutic Time Window and Underlying Therapeutic Mechanism of Breviscapine Injection against Cerebral Ischemia/Reperfusion Injury in Rats. J. Ethnopharmacol. 2014, 151, 660–666. [Google Scholar] [CrossRef]
- Asadollahi, M.; Nikdokht, P.; Hatef, B.; Sadr, S.S.; Sahraei, H.; Assarzadegan, F.; Pirzad Jahromi, G. Protective Properties of the Aqueous Extract of Saffron (Crocus Sativus L.) in Ischemic Stroke, Randomized Clinical Trial. J. Ethnopharmacol. 2019, 238, 111833. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin Is Neuroprotective in an Aged Mouse Model of Parkinson’s Disease. Oncotarget 2018, 9, 10388–10401. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.-T.; Huang, H.-W.; Yang, C.-M.; Yang, W.-S.; Yang, C.-H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-T.; Yang, C.-M.; Yang, C.-H. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Y.; Hang, H.; Liu, X.; Chen, X.; Xie, P.; Yuan, S.; Zhang, W.; Lin, X.; Liu, Q. Edaravone Protect against Retinal Damage in Streptozotocin-Induced Diabetic Mice. PLoS ONE 2014, 9, e99219. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohamed, R.; Elsherbiny, N.; DeAngelis, M.; Bartoli, M.; Al-Shabrawey, M. Homocysteine: A Potential Biomarker for Diabetic Retinopathy. J. Clin. Med. 2019, 8, 121. [Google Scholar] [CrossRef]
- Iglicki, M.; Zur, D.; Busch, C.; Okada, M.; Loewenstein, A. Progression of Diabetic Retinopathy Severity after Treatment with Dexamethasone Implant: A 24-Month Cohort Study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018, 55, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Kanar, H.S.; Aki, S.F.; Arsan, A.; Hacisalihoglu, A. Effectiveness and Safety of Coadministration of Intravitreal Dexamethasone Implant and Silicone Oil Endotamponade for Proliferative Diabetic Retinopathy with Tractional Diabetic Macular Edema. J. Ocul. Pharmacol. Ther. 2021, 37, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Taguchi, M.; Harimoto, K.; Karasawa, Y.; Takeuchi, M.; Ito, M. Protective Effects of Dexamethasone on Hypoxia-Induced Retinal Edema in a Mouse Model. Exp. Eye Res. 2019, 178, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rotschild, T.; Nandgaonkar, B.N.; Yu, K.; Higgins, R.D. Dexamethasone Reduces Oxygen Induced Retinopathy in a Mouse Model. Pediatr. Res. 1999, 46, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Prusky, G.T.; Douglas, R.M.; Nelson, L.; Shabanpoor, A.; Sutherland, R.J. Visual Memory Task for Rats Reveals an Essential Role for Hippocampus and Perirhinal Cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 5064–5068. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, F.; Sajgo, S.; Kretschmer, V.; Badea, T.C. A System to Measure the Optokinetic and Optomotor Response in Mice. J. Neurosci. Methods 2015, 256, 91–105. [Google Scholar] [CrossRef]
- Rondi-Reig, L. Impaired Sequential Egocentric and Allocentric Memories in Forebrain-Specific-NMDA Receptor Knock-Out Mice during a New Task Dissociating Strategies of Navigation. J. Neurosci. 2006, 26, 4071–4081. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Assessing Spatial Learning and Memory in Rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef]
- Fouquet, C.; Babayan, B.M.; Watilliaux, A.; Bontempi, B.; Tobin, C.; Rondi-Reig, L. Complementary Roles of the Hippocampus and the Dorsomedial Striatum during Spatial and Sequence-Based Navigation Behavior. PLoS ONE 2013, 8, e67232. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Vanderlinde, R.E. Measurement of Total Lactate Dehydrogenase Activity. Ann. Clin. Lab. Sci. 1985, 15, 13–31. [Google Scholar] [PubMed]
- Li, A.; Lane, W.; Johnson, L.; Chader, G.; Tombran-Tink, J. Neuron-Specific Enolase: A Neuronal Survival Factor in the Retinal Extracellular Matrix? J. Neurosci. 1995, 15, 385–393. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- McAllister, I.L.; Vijayasekaran, S.; Zhang, D.; McLenachan, S.; Chen, F.K.; Yu, D.-Y. Neuronal Degeneration and Associated Alterations in Cytokine and Protein in an Experimental Branch Retinal Venous Occlusion Model. Exp. Eye Res. 2018, 174, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kovacova, A.; Shotliff, K. Eye Problems in People with Diabetes: More than Just Diabetic Retinopathy. Pract. Diabetes 2022, 39, 34. [Google Scholar] [CrossRef]
- Alam, N.M.; Douglas, R.M.; Prusky, G.T. Treatment of Age-Related Visual Impairment with a Peptide Acting on Mitochondria. Dis. Models Mech. 2022, 15, dmm048256. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-Z.; Yang, N.; Nakajima, N.; Otsu, W.; Fu, C.; Yang, H.H.; Lee, M.P.; Akbar, A.F.; Badea, T.C.; Guo, Z.; et al. Retinal Pigment Epithelium-Specific CLIC4 Mutant Is a Mouse Model of Dry Age-Related Macular Degeneration. Nat. Commun. 2022, 13, 374. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Drel, V.R.; Kumagai, A.K.; Szábo, C.; Pacher, P.; Stevens, M.J. Early Diabetes-Induced Biochemical Changes in the Retina: Comparison of Rat and Mouse Models. Diabetologia 2006, 49, 2525–2533. [Google Scholar] [CrossRef]
- Kermorvant-Duchemin, E.; Pinel, A.C.; Lavalette, S.; Lenne, D.; Raoul, W.; Calippe, B.; Behar-Cohen, F.; Sahel, J.-A.; Guillonneau, X.; Sennlaub, F. Neonatal Hyperglycemia Inhibits Angiogenesis and Induces Inflammation and Neuronal Degeneration in the Retina. PLoS ONE 2013, 8, e79545. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Couper, D.J.; Cooper, L.S.; Shahar, E.; Hubbard, L.D.; Wofford, M.R.; Sharrett, A.R. Retinal Microvascular Abnormalities and Incident Stroke: The Atherosclerosis Risk in Communities Study. Lancet 2001, 358, 1134–1140. [Google Scholar] [CrossRef]
- Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.N.R.S.; Azam, S.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175. [Google Scholar] [CrossRef]
- Bro, T.; Andersson, J. The Effects of Visual-Field Loss from Panretinal Photocoagulation of Proliferative Diabetic Retinopathy on Performance in a Driving Simulator. Eye 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, F.-Y.; Xu, P.; Wu, J.-H. Regulation of Mitophagy by Metformin Improves the Structure and Function of Retinal Ganglion Cells Following Excitotoxicity-Induced Retinal Injury. Exp. Eye Res. 2022, 217, 108979. [Google Scholar] [CrossRef]
- Al-Hajaya, Y.; Karpinska, B.; Foyer, C.H.; Baker, A. Nuclear and Peroxisomal Targeting of Catalase. Plant Cell Environ. 2022, 45, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, C.-H.; Wang, Z.; Fu, Z.; Britton, W.R.; Blomfield, A.K.; Kamenecka, T.M.; Dunaief, J.L.; Solt, L.A.; Chen, J. REV-ERBα Regulates Age-Related and Oxidative Stress-Induced Degeneration in Retinal Pigment Epithelium via NRF2. Redox Biol. 2022, 51, 102261. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, D.-H.; Yang, S.G.; Kim, D.Y. Improved Effect of a Mitochondria-Targeted Antioxidant on Hydrogen Peroxide-Induced Oxidative Stress in Human Retinal Pigment Epithelium Cells. BMC Pharmacol. Toxicol. 2021, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Borțea, C.I.; Stoica, F.; Boia, M.; Iacob, E.R.; Dinu, M.; Iacob, R.; Iacob, D. Risk Factors Associated with Retinopathy of Prematurity in Very and Extremely Preterm Infants. Medicina 2021, 57, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, X.; Yang, C.; Li, Y. Teneligliptin Protects against Hypoxia/Reoxygenation-Induced Endothelial Cell Injury. Biomed. Pharmacother. 2019, 109, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Archibong, V.B.; Ekanem, T.; Igiri, A.; Ofutet, E.O.; Ifie, J.E. The Effect of Codeine Administration on Oxidative Stress Biomarkers and the Expression of the Neuron-Specific Enolase in the Brain of Wistar Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1665–1673. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, T.; Nakamura, A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2301. [Google Scholar] [CrossRef]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Astaxanthin Attenuates Neuroinflammation Contributed to the Neuropathic Pain and Motor Dysfunction Following Compression Spinal Cord Injury. Brain Res. Bull. 2018, 143, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Benlarbi-Ben Khedher, M.; Hajri, K.; Dellaa, A.; Baccouche, B.; Hammoum, I.; Boudhrioua-Mihoubi, N.; Dhifi, W.; Ben Chaouacha-Chekir, R. Astaxanthin Inhibits Aldose Reductase Activity in Psammomys Obesus, a Model of Type 2 Diabetes and Diabetic Retinopathy. Food Sci. Nutr. 2019, 7, 3979–3985. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Cakir, U.; Tayman, C.; Turkmenoglu, T.T.; Gonel, A.; Turan, I.O. Favorable Effects of Astaxanthin on Brain Damage Due to Ischemia- Reperfusion Injury. Comb. Chem. High Throughput Screen. 2020, 23, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Hirose, I.; Noda, K.; Murata, M.; Ishida, S. Glucocorticoid-transactivated TSC22D3 Attenuates Hypoxia- and Diabetes-induced Müller Glial Galectin-1 Expression via HIF-1α Destabilization. J. Cell. Mol. Med. 2020, 24, 4589–4599. [Google Scholar] [CrossRef]
- Bhandari, S.; Gabrielle, P.-H.; Nguyen, V.; Daien, V.; Viola, F.; Bougamha, W.; Young, S.; Romero-Nuñez, B.; Figueras-Roca, M.; Zarranz-Ventura, J.; et al. Dexamethasone Implant for Diabetic Macular Oedema: 1-Year Treatment Outcomes from the Fight Retinal Blindness! Registry. Ophthalmol. Ther. 2022, 11, 797–810. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, T.; Deng, T.; Wang, Z. Protective Effects of Astaxanthin against Diabetic Retinal Vessels and Pro-Inflammatory Cytokine Synthesis. Int. J. Clin. Exp. Med. 2019, 12, 4725–4734. [Google Scholar]
| Groups | Day 0 (mmol/L) | Day 3 (mmol/L) | Day 28 (mmol/L) |
|---|---|---|---|
| Normal | 6.1 ± 0.4 | 6.4 ± 0.8 | 6.3 ± 0.4 |
| DR | 6.3 ± 0.8 | 24.3 ± 1.4 a | 25.1 ± 1.5 a |
| DR + AST (10) | 6.2 ± 0.6 | 25.2 ± 0.9 a | 13.4 ± 1.4 b |
| DR + AST (20) | 6.0 ± 0.7 | 25.1 ± 1.3 a | 14.6 ± 0.5 b |
| STZ + DEX (10) | 6.1 ± 0.5 | 23.3 ± 1.6 a | 23.1 ± 0.6 a |
| Groups | Catalase (U/mg of Protein) | LDH (U/mg of Protein) | NSE (pg/mL) |
|---|---|---|---|
| Normal | 14.4 ± 1.3 | 6.7 ± 1.0 | 17.3 ± 1.5 |
| DR (35) | 3.6 ± 1.1 a | 12.9 ± 1.2 a | 84.7 ± 2.9 a |
| DR + AST (10) | 9.3 ± 0.9 b | 8.1 ± 0.9 b | 32.2 ± 1.2 b |
| DR + AST (20) | 11.6 ± 0.6 b | 7.6 ± 0.4 b | 23.4 ± 1.6 b |
| DR + DEX (10) | 13.9 ± 1.2 b | 7.2 ± 0.8 b | 19.2 ± 2.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, A.; Thirunavukkarasu, J.; Muthuraman, A. Astaxanthin Ameliorates Diabetic Retinopathy in Swiss Albino Mice via Inhibitory Processes of Neuron-Specific Enolase Activity. Processes 2022, 10, 1318. https://doi.org/10.3390/pr10071318
Subramanian A, Thirunavukkarasu J, Muthuraman A. Astaxanthin Ameliorates Diabetic Retinopathy in Swiss Albino Mice via Inhibitory Processes of Neuron-Specific Enolase Activity. Processes. 2022; 10(7):1318. https://doi.org/10.3390/pr10071318
Chicago/Turabian StyleSubramanian, Aswinprakash, Jayaraman Thirunavukkarasu, and Arunachalam Muthuraman. 2022. "Astaxanthin Ameliorates Diabetic Retinopathy in Swiss Albino Mice via Inhibitory Processes of Neuron-Specific Enolase Activity" Processes 10, no. 7: 1318. https://doi.org/10.3390/pr10071318
APA StyleSubramanian, A., Thirunavukkarasu, J., & Muthuraman, A. (2022). Astaxanthin Ameliorates Diabetic Retinopathy in Swiss Albino Mice via Inhibitory Processes of Neuron-Specific Enolase Activity. Processes, 10(7), 1318. https://doi.org/10.3390/pr10071318





