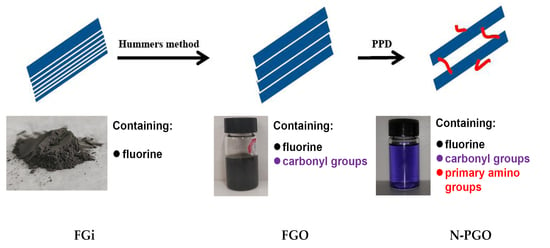
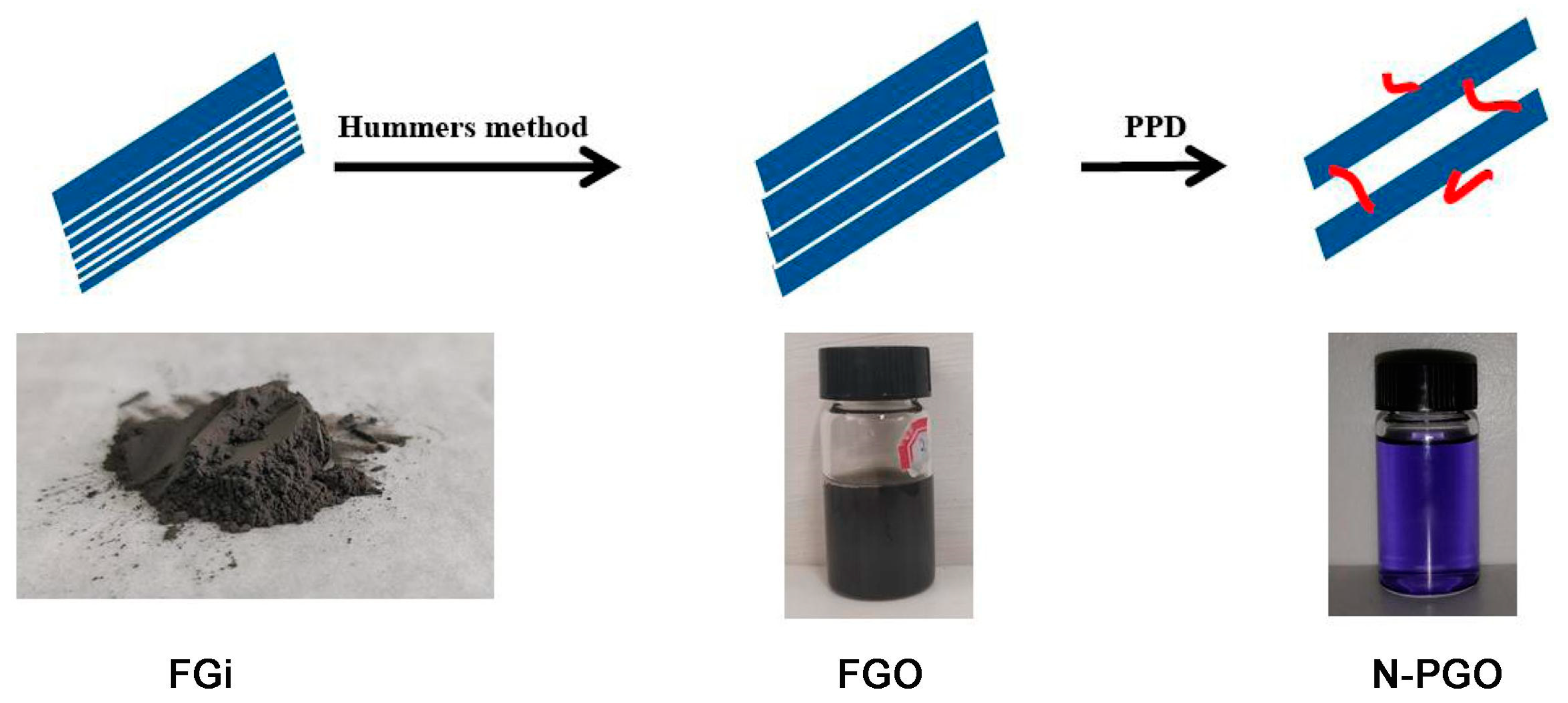
Preparation of Modified Fluorographene Oxide with Interlayer Supporting Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Preparation Methods
2.2.1. Preparation of FGO
2.2.2. Preparation of N-PGO
2.3. Characterization and Corrosion Analysis
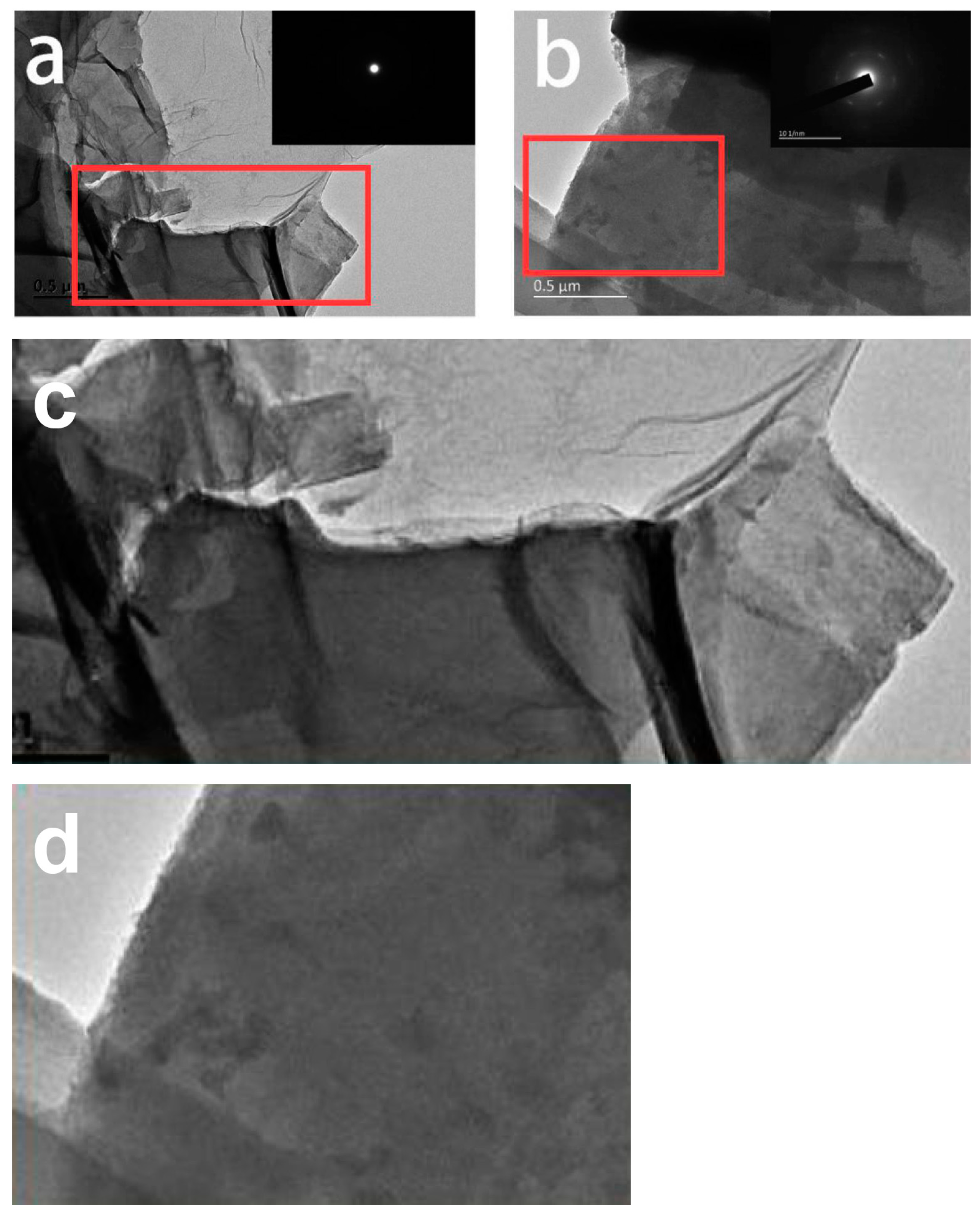
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.N.; Peres, M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Marconcini, P.; Macucci, M. The k.p method and its application to graphene carbon nanotubes and graphene nanoribbons: The Dirac equation. Riv. Nuovo Cimento 2011, 34, 489–584. [Google Scholar]
- Yasser, V.; Elena-Niculina, D.; Fares, A.; Thuan, L.V. Graphene derivatives in bioplastic: A comprehensive review of properties and future perspectives. Chemosphere 2021, 286, 131892. [Google Scholar] [CrossRef]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: From fundamentals to applications. J. Phys. Condens. Matter 2015, 27, 013002. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A two-dimensional counterpart of teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Long, P.; Feng, Y.; Li, Y. Two-dimensional fluorinated graphene: Synthesis, structures, properties and applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.I.; Huang, C.H.; Liao, J.H.; Zhang, W.; Li, L.J.; Lai, C.S.; Su, C.Y. Fluorinated graphene as high performance dielectric materials and the applications for graphene nanoelectronics. Sci. Rep. 2004, 4, 5893. [Google Scholar] [CrossRef]
- Kakaei, K.; Balavandi, A. Hierarchically porous fluorine-doped graphene nanosheets as efficient metal-free electrocatalyst for oxygen reduction in gas diffusion electrode. J. Colloid Interf. Sci. 2017, 490, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, W.C.; Manga, K.K.; Ang, P.K.; Lu, J.; Liu, Y.P.; Lim, C.T.; Loh, K.P. Fluorinated graphene for promoting neuro-induction of stem cells. Adv. Mater. 2012, 24, 4285–4290. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Zhu, Y.; Che, J.; Xiao, Y. Two-dimensional transparent hydrophobic coating based on liquid-phase exfoliated graphene fluoride. Carbon 2013, 63, 49–156. [Google Scholar] [CrossRef]
- Hou, K.; Gong, P.; Wang, J.; Yang, Z.; Ma, L.; Yang, S. Construction of highly ordered fluorinated graphene composite coatings with various fluorine contents for enhanced lubrication performance. Tribol. Lett. 2015. [Google Scholar] [CrossRef]
- Ye, X.; Liu, X.; Yang, Z.; Wang, Z.; Wang, H.; Wang, J.; Yang, S. Tribological properties of fluorinated graphene reinforced polyimide composite coatings under different lubricated conditions. Compos. Part A Appl. Sci. 2016, 81, 282–288. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Long, P.; Gao, Y.; Qin, C.; Cao, C.; Feng, Y.; Feng, W. Hydrothermal preparation of fluorinated graphene hydrogel for high-performance supercapacitors. J. Power Sources 2016, 312, 146–155. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Chen, W.; Su, Y.; Peng, J.; Dong, Y.; Zhao, X.; Jiang, Z. Engineering a robust, versatile amphiphilic membrane surface through forced surface segregation for ultralow flux-decline. Adv. Funct. Mater. 2011, 21, 191–198. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotech. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, J.; He, G.; Zhong, D.; Chen, J.; Shi, L.; Zhou, X.H.; Jiang, H.J. Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. Electroanal. Chem. 2012, 672, 40–44. [Google Scholar] [CrossRef]
- Gan, L.; Guo, H.; Wang, Z.; Li, X.; Peng, W.; Wang, J.; Huang, S.; Su, M. A facile synthesis of graphite/silicon/graphene spherical composite anode for lithium-ion batteries. Electrochim. Acta 2013, 104, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Guo, S.; Zhai, Y.; Dong, S. Layer-by-layer self-assembly for constructing a graphene/platinum nanoparticle three-dimensional hybrid nanostructure using ionic liquid as a linker. Langmuir 2010, 26, 7614–7618. [Google Scholar] [CrossRef]
- Park, J.; Jin, T.; Liu, C.; Li, G.; Yan, M. Three-dimensional graphene-TiO2 nanocomposite photocatalyst synthesized by covalent attachment. ACS Omega 2016, 1, 351–356. [Google Scholar] [CrossRef]
- Badri, A.; Whittaker, M.R.; Zetterlund, P.B. Modification of graphene/graphene oxide with polymer brushes using controlled/living radical polymerization. J. Polym. Sci. Pol. Chem. 2012, 50, 2981–2992. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Vermisoglou, E.C.; Jakubec, P.; Bakandritsos, A.; Pykal, M.; Talande, S.; Kupka, V.; Zboril, R.; Otyepka, M. Chemical tuning of specific capacitance in functionalized fluorographene. Chem. Mater. 2019, 31, 4698–4709. [Google Scholar] [CrossRef]
- Zaoralová, D.; Hrubý, V.; Šedajová, V.; Mach, R.; Kupka, V.; Ugolotti, J.; Bakandritsos, A.; Medved, M.; Otyepka, M. Tunable synthesis of nitrogen doped graphene from fluorographene under mild conditions. ACS Sustain. Chem. Eng. 2020, 8, 4764–4772. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y. Layer-by-layer (LBL) assembly of graphene with p-phenylenediamine (PPD) spacer for high performance supercapacitor applications. RSC Adv. 2014, 4, 19908–19915. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.; Song, B.; Moon, K.S.; Hu, N.; Liao, G.; Shi, T.; Wong, C. Mechanistic investigation of the graphene functionalization using p-phenylenediamine and its application for supercapacitors. Nano Energy 2015, 17, 160–170. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Romero-Aburto, R.; Narayanan, T.N.; Nagaoka, Y.; Hasumura, T.; Mitcham, T.M.; Fukuda, T.; Cox, P.J.; Bouchard, R.R.; Maekawa, T.; Kumar, D.S.; et al. Fluorinated graphene oxide: A new multimodal material for biological applications. Adv. Mater. 2013, 25, 5632–5637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathkar, A.; Narayanan, T.N.; Alemany, L.B.; Cox, P.; Nguyen, P.; Gao, G.; Chang, P.; Romero-Aburto, R.; Mani, S.A.; Ajayan, P.M. Synthesis of fluorinated graphene oxide and its amphiphobic properties. Part. Part. Syst. Charact. 2013, 30, 266–272. [Google Scholar] [CrossRef]
- Zhu, W.T.; Wu, C.J.; Chang, Y.X.; Cheng, H.C.; Yu, C.B. Solvent-free preparation of hydrophilic fluorinated graphene oxide modified with amino-groups. Mater. Lett. 2019, 237, 1–4. [Google Scholar] [CrossRef]
- Liu, L.Q.; Shi, K.Q.; Lu, Y.L.; Yu, C.B. Graphene oxide used as the modifier to prepare silica-encapsulated waterborne flaky aluminium for enhanced anticorrosive property. Micro Nano Lett. 2020, 15, 728–731. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lin, Y.Y.; Lin, C.H.; Chan, C.C.; Huang, Y.C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Yan, W.Y.; Zhou, Q.; Chen, X.; Yang, Y.; Zhang, Y.; Huang, X.J.; Wu, Y.C. Size-controlled TiO2 nanocrystals with exposed {001} and {101} facets strongly linking to graphene oxide via p-phenylenediamine for efficient photocatalytic degradation of fulvic acids. J. Hazard. Mater. 2016, 314, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energ. Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, H.B.; Hu, Q.H.; Li, W.J.; Jiang, Z.G.; Yu, Z.Z.; Dasari, A. Functionalization and reduction of graphene oxide with p-phenylene diamine for electrically conductive and thermally stable polystyrene composites. ACS Appl. Mater. Int. 2012, 4, 1948–1953. [Google Scholar] [CrossRef] [PubMed]



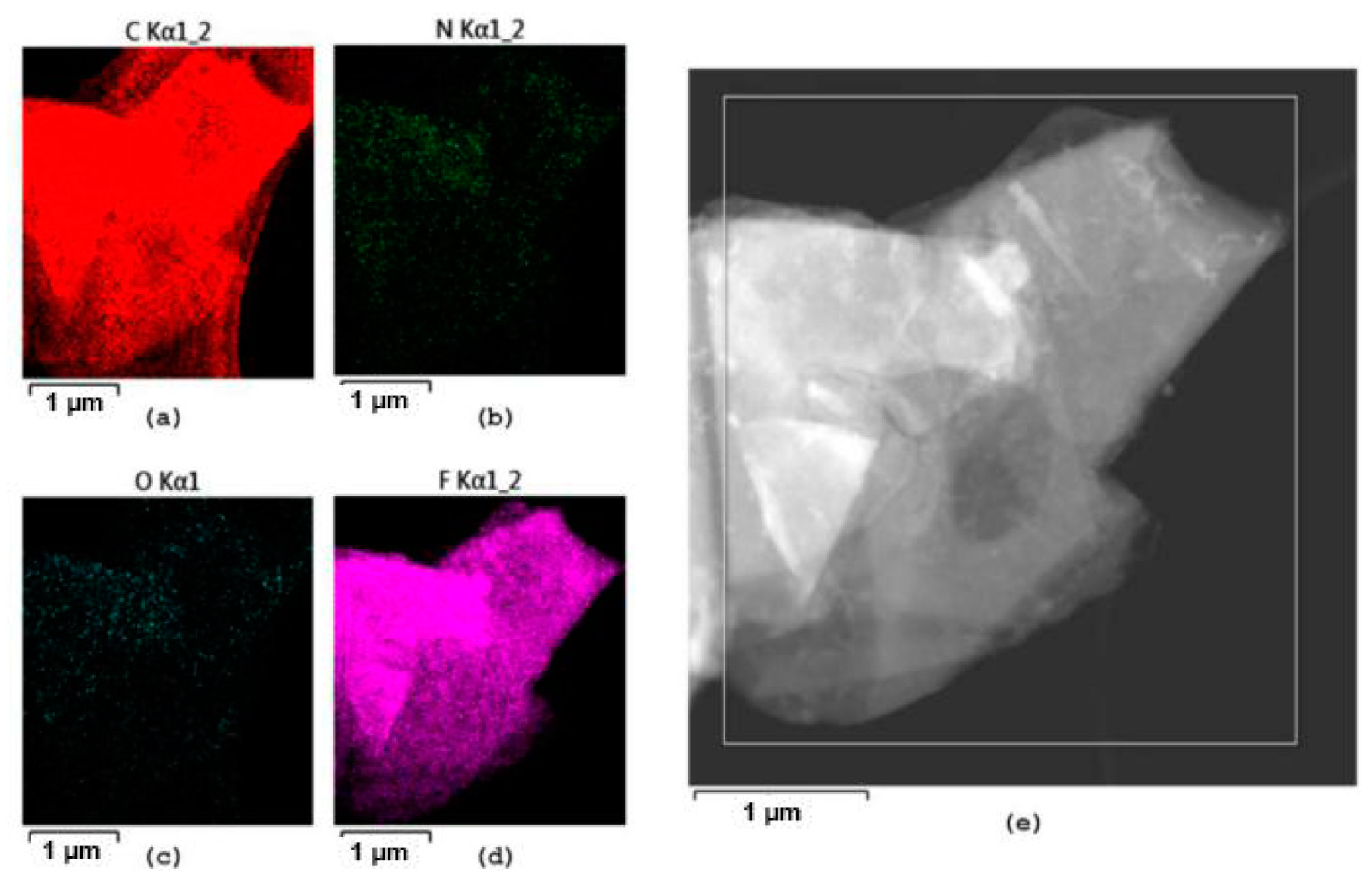
| Samples | Element Compositions (%) | |||
|---|---|---|---|---|
| C1s | O1s | N1s | F1s | |
| FGi | 64.42 | 0 | 0 | 35.58 |
| FGO | 61.49 | 8.67 | 0 | 29.84 |
| N-PGO | 69.37 | 4.68 | 8.81 | 17.14 |
| Samples | Z-Average (r. nm) | Zeta Potential (mV) |
|---|---|---|
| FGi | - | - |
| FGO | 438.9 | −15.3 |
| N-PGO | 293.3 | −38.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Shi, K.; Ning, J.; Liu, J. Preparation of Modified Fluorographene Oxide with Interlayer Supporting Structure. Polymers 2021, 13, 3126. https://doi.org/10.3390/polym13183126
Yu C, Shi K, Ning J, Liu J. Preparation of Modified Fluorographene Oxide with Interlayer Supporting Structure. Polymers. 2021; 13(18):3126. https://doi.org/10.3390/polym13183126
Chicago/Turabian StyleYu, Chengbing, Kaiqin Shi, Jinyan Ning, and Jun Liu. 2021. "Preparation of Modified Fluorographene Oxide with Interlayer Supporting Structure" Polymers 13, no. 18: 3126. https://doi.org/10.3390/polym13183126