1. Introduction
Kiwifruit (
Actinidia chinensis Planch), commonly referred to as “Chinese kiwifruit”, is a woody perennial, dioecious, medicinal plant belonging to the Actinidia Lindl. genus (Actinidiaceae) comprising 75 species and about 125 taxa native to China [
1]. The
A. chinensis Planch. (yellow-fleshed) and
A. chinensis var.
deliciosa (green-fleshed) are categorized as two subspecies under
A. chinensis [
2].
Actinidia chinensis and
A. deliciosa are by far the most important
Actinidia species cultivated and together account for nearly all kiwifruit in international trade [
3].
Actinidia chinensis has a special economic importance owing to its high export quality [
4]. The fruit has achieved great popularity due to its commercial potential [
5]. Kiwifruit is recognized as the most nutritious fruit, rich in vitamins including A, B, C, E, and K, dietary fiber, and phytochemicals such as carotenoids, flavonoids anthocyanins and lutein [
6]. These essential nutrients contribute to its extensive pharmacological properties such as anti-cancerous, anti-diabetic, anti-inflammatory and antioxidant effects, as well as hypoglycemic and hypolipidemic benefits. Therefore, regular consumption of kiwifruit can enhance digestive health and support cardiovascular wellness [
6]. According to FAOSTAT [
7], kiwifruit is one of the most commercially available fruits on the international market with more than 4,348,011 metric tonnes of global kiwifruit production.
The South African kiwifruit (green-fleshed
A. deliciosa ‘Hayward’) industry is gradually emerging, with approximately 200 ha under production in Limpopo, KwaZulu-Natal and Eastern Cape Provinces [
8]. The commercial yield of the well-known and mostly cultivated variety of
A. delicosa ‘Hayward’ in South Africa (SA) is limited to 10–12 t/ha. The variety of
A. delicosa ‘Hayward’ is primarily cultivated and is targeted at local and export markets in the Southern African Development Community (SADC) countries [
8]. Thus, the call to expand kiwifruit production in SA and globally calls for the development of commercial protocols for mass propagation of
A. chinensis that can be used to expand kiwifruit production. Consequently, there is a need for a population of kiwifruit seed plants with natural or controlled crosses. Generally, the presence of dormant embryos in seeds and nonuniformity in seedling growth increase the losses of kiwifruit seed populations. Seeds are a key material both for the propagation of seedlings and breeding new kiwifruit cultivars; nevertheless,
Actinidia has shown a high recalcitrance to seed germination, which leads to poor emergence and seedling establishment. In addition, other limitations include the impossibility of determining the sex of seedlings and genetic differentiation in generative propagation [
9]. Due to the low germination performance of kiwifruit seeds, chemical treatment is required and the seeds must be kept in damp, aerated conditions, alternated between day and night, under low temperatures to increase their germination rate. According to researchers, stratification under cool and moist conditions and/or some chemical treatments can improve the germination rate of kiwifruit seeds [
9,
10]. Germination of kiwifruit is poor and erratic due to dormancy. The
Actinidia seeds are known to have after-ripening (physiological) dormancy, which should be eliminated for successful germination. Physiological dormancy is the most prevalent form of dormancy in temperate seeds, which occurs when internal chemical or hormonal factors inhibit a seed from germinating, even when external conditions are favorable [
11]. Previous studies suggested that low temperature and gibberellic acid can overcome kiwifruit seed dormancy [
11,
12,
13]. Additionally, germination is a complex process that cannot be fully understood by studying one or two factors; thus, it is necessary to adopt a multidimensional strategy, combining the analysis of multiple factors to achieve optimal germination percentages. The intrinsic molecular mechanisms determining dormancy can have an embryo and/or a coat component that can interact to determine the degree of ‘whole-seed’ dormancy [
11,
14]. Different methods such as stratification (CS), scarification, fluctuating temperatures and gibberellic acid (GA
3) application can be used to promote and achieve higher germination and seedling establishment. Although some studies demonstrated that GA
3 can promote kiwifruit seed germination [
9,
13,
15,
16], other research suggests that CS is more effective in overcoming kiwifruit seed dormancy [
11,
14,
17]. Hsieh et al. [
11] achieved 80% germination in freshly harvested kiwifruit seeds using alternating temperatures (25/15 °C). Other studies highlighted the influence of both fluctuating and constant temperatures, as well as far-red and red light, on the germination of stratified ‘Hayward’ seeds. They also observed that fluctuating temperatures (20/30 °C) positively contributed to breaking kiwifruit seed dormancy. However, while red light had no effect on germination, far-red light exhibited an inhibitory effect [
12].
It is also imperative to develop commercial protocols for mass propagation of
A. chinensis, which can be used in the expansion of the SA kiwifruit production. In vitro culture is another method of mass plant production that can be used for plants that are difficult to propagate conventionally [
18]. Studies on in vitro propagation of
A. chinensis species for higher multiplication rate are limited. The first micropropagation protocol by nodal culture for
Actinidia species was proposed by Harada [
19] and it has been improved in recent years [
20]. In vitro propagation of kiwifruit has also been achieved through shoot regeneration [
21], organogenesis [
22] and plant regeneration [
23]. Explants used as initial plant material included whole leaf [
24], endosperm [
25], shoot tips [
26], shoot meristems [
20], whole buds and nodal segments [
24]. However, most of these studies have primarily focused on
A. deliciosa [
22,
27],
A. polygama [
20], and others with limited research on the in vitro seed culture of
A. chinensis [
28]. This is due to the fact that the treatments needed to break dormancy of kiwifruit seeds can increase the risk of contamination [
20,
29]. Akbaş et al. [
13] studied the effect of the 6-benzylaminopurine (BAP; 0.5–4.0 mg L
−1) on shoot proliferation of
A. deliciosa using seeds. The optimal results were observed with 0.5 mg L
−1 BAP, yielding an average of 4.7 ± 1.08 shoots per explant on the 4th week of culture. The in vitro-developed shoots were successfully rooted on MS medium supplemented with 1.0 mg L
−1 α-naphthaleneacetic acid, and the resulting plantlets were successfully acclimatized to in vivo conditions. The present study was carried to investigate the effects of CS and GA
3, both individually and in combination, on breaking dormancy and promoting germination of
A. chinensis seeds using in vitro methods and to assess the effect of BAP on in vitro seedling development, multiple shoot induction and rooting of
A. chinensis. 4. Materials and Methods
4.1. Plant Material
Actinidia chinensis seeds were extracted from physiologically matured fruits harvested from uniform, healthy and mature kwifruit plants under commercial cultivation at Nooyenskopje Farm at Magoebaskoof (23°53′13″ S, 29°56′13″ E), Tzaneen, in Limpopo Province of South Africa.
4.2. Sterilisation of Seeds
Extracted seeds of A. chinensis were first washed with soapy water, rinsed under running tap water for 5–10 min (min) and then followed by a rinsing with distilled water (dH2O). Seeds were surface sterilised in 70% ethanol for 1 min and then in 50% commercial bleach JIK® (Waterfall City, Midrand, South Africa) solution containing 1.75% active sodium hypochlorite (NaOCl) with a few drops of Tween 20 for 30 min. After each sterilisation procedure, seeds were rinsed few times with sterile dH2O to remove the disinfectant solution.
4.3. Culture Media
Full-strength Murashige and Skoog [
40] medium (MS) containing 3% sucrose (
w/
v) and 0.3% (
w/
v) gelrite was used for in vitro germination experiments. The seeds were also cultured on sterile moist filter paper (Whatman No. 1) bridges in Sigma
® (St. Louis, MO, USA) culture bottles. All aseptic procedures (media preparation, seed sterilisation and inoculation) were conducted in a plant tissue culture laboratory under a plant tissue culture laminar flow cabinet.
4.4. Treatment of Seeds and Germination
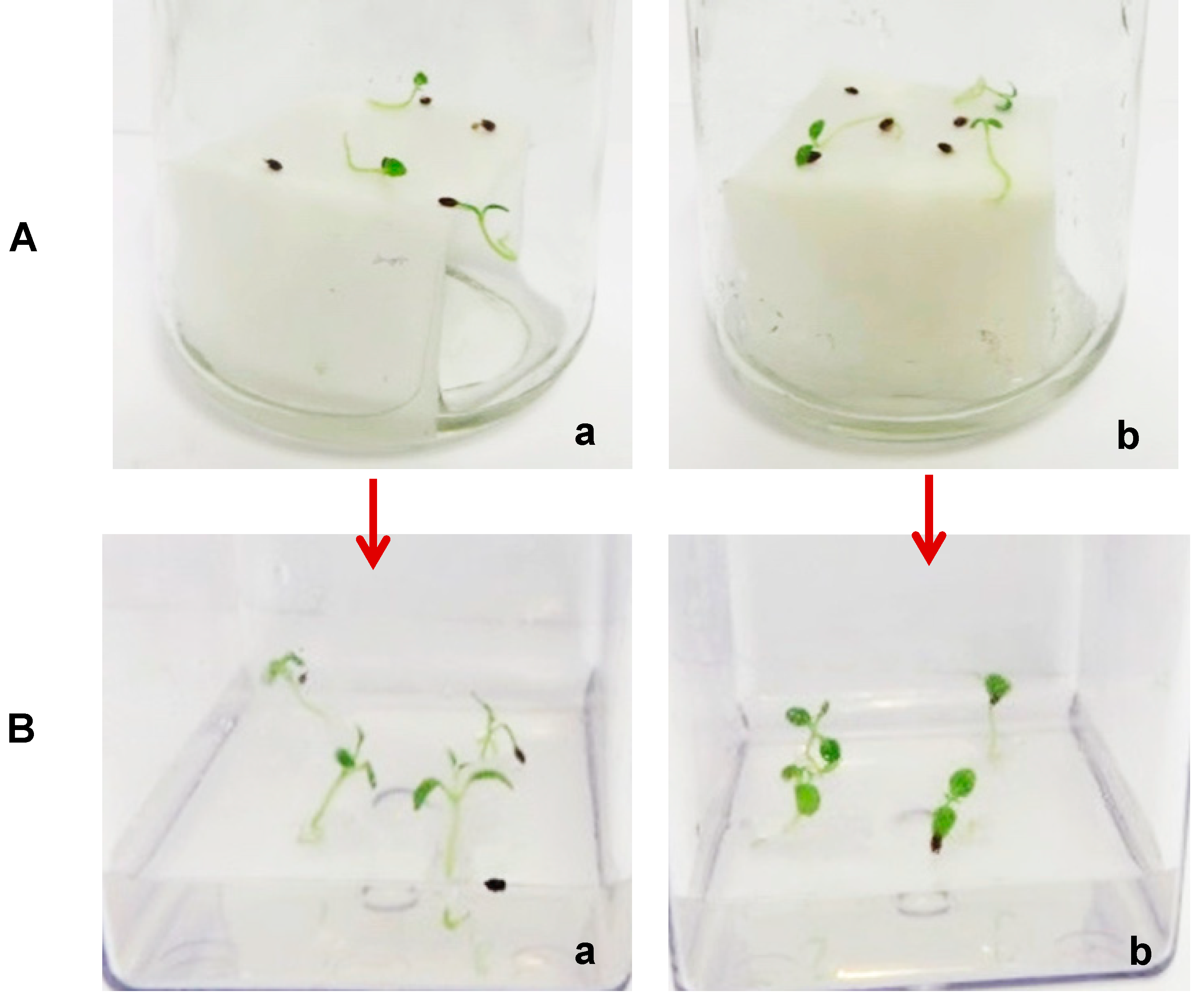
4.4.1. Cold Stratification
Sterilised seeds of A. chinensis were aseptically cultured on sterile moist filter paper bridges and cold stratified (CS) at 4 °C for 28 and 42 days. The durations of CS were selected based on the preliminary results from in vivo germination of A. chinensis. Non-stratified (NS) seeds were used as controls. Batches of CS and NS seeds from A. chinensis were then germinated on plant growth regulator-free (PGR-free) MS medium and on sterile filter paper bridges moistened with dH2O. All seed cultures were kept under controlled growth conditions for germination.
4.4.2. Gibberellic Acid (GA3)
An initial study was conducted, where NS seeds of A. chinensis were imbibed in culture bottles for 24 h in different GA3 concentrations (100, 1000 and 2000 ppm) on an orbital shaker (50 rpm). Seeds imbibed in dH2O and dry seeds (no imbibed) served as controls. After imbibition, seeds from all GA3 treatments and controls were surface sterilised as described above and were aseptically inoculated on PGR-free MS media for germination. In another experiment, NS sterilised seeds were aseptically inoculated on sterile moist filter paper bridges placed in Sigma® culture bottles and dipped in solutions containing five GA3 concentrations (500, 1000, 1500, 2000 and 2500 ppm). The seed cultures were kept under controlled growth conditions. Germinated seeds from these treatments were transferred into PGR-free MS media for seedling development under controlled growth conditions.
4.4.3. Combination of Cold Stratification and GA3
Sterilised and CS seeds for 28 and 42 days were aseptically inoculated on filter paper bridges in Sigma® culture bottles containing five GA3 solutions (500, 1000, 1500, 2000 and 2500 ppm). The seed cultures were also kept under controlled growth conditions. Germinated seeds from these treatments were transferred into PGR-free MS media for seedling development.
4.5. Seedling Development
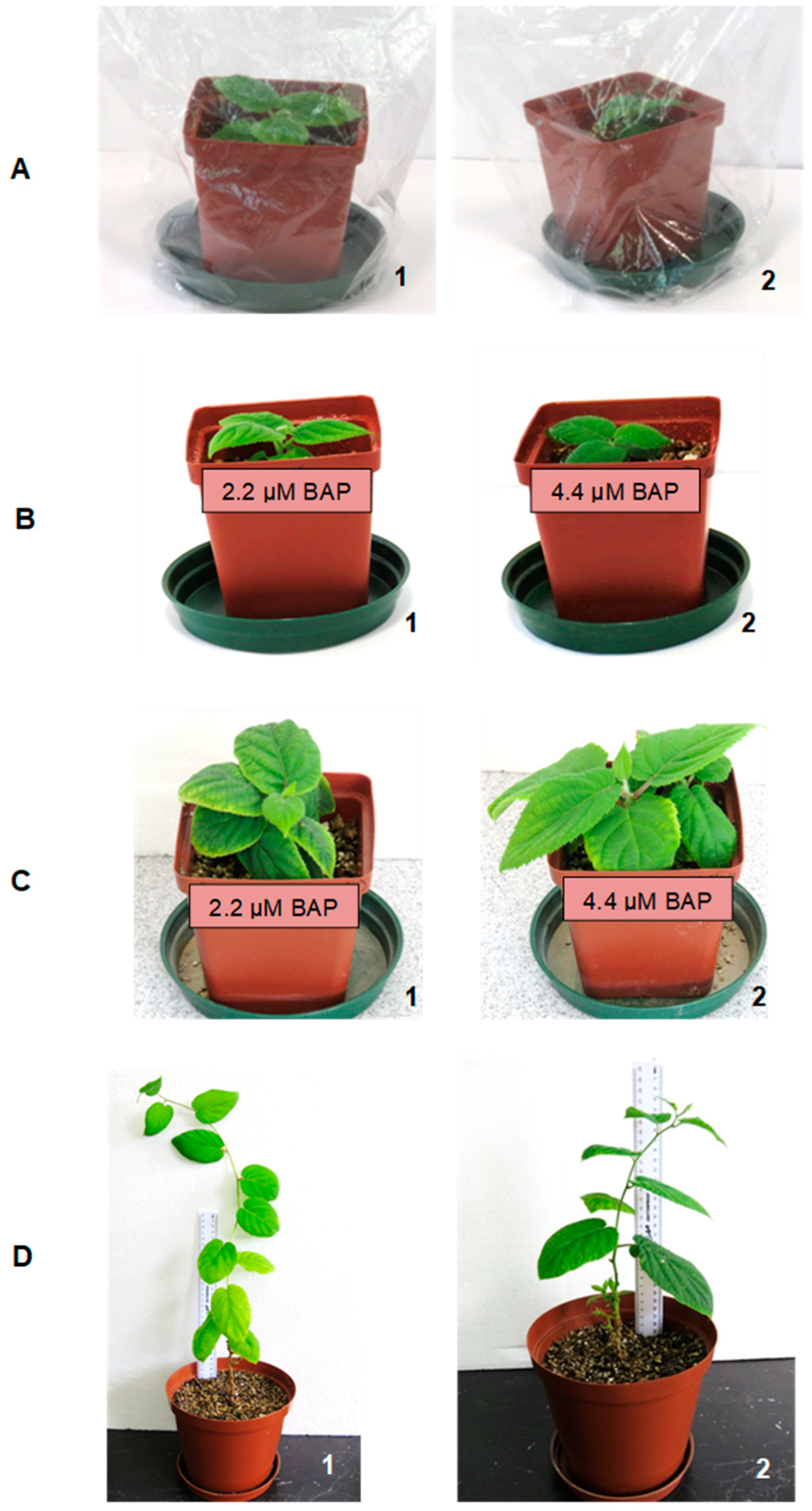
After 32 days, germinated seeds cultured on the MS medium from the combined stratification durations (28 and 42 days) and 500 ppm GA3-treated seeds on the filter paper-bridges were gently removed under sterile conditions and transferred onto the plant growth regulator (PGR)-free MS medium and MS media supplemented with 2.2 µM and 4.4 µM BAP for seedling development. The concentrations of PGR-BAP were selected based on the preliminary results from seedling development of A. chinensis.
4.6. Seed and Seedling Culture Growth Conditions
All in vitro seed and seedling cultures were kept in a growth room at 24 ± 2 °C and 16 h photoperiod of 50–60 µmol m−2 s−1 light intensity for germination and seedling development.
4.7. Explant Preparation
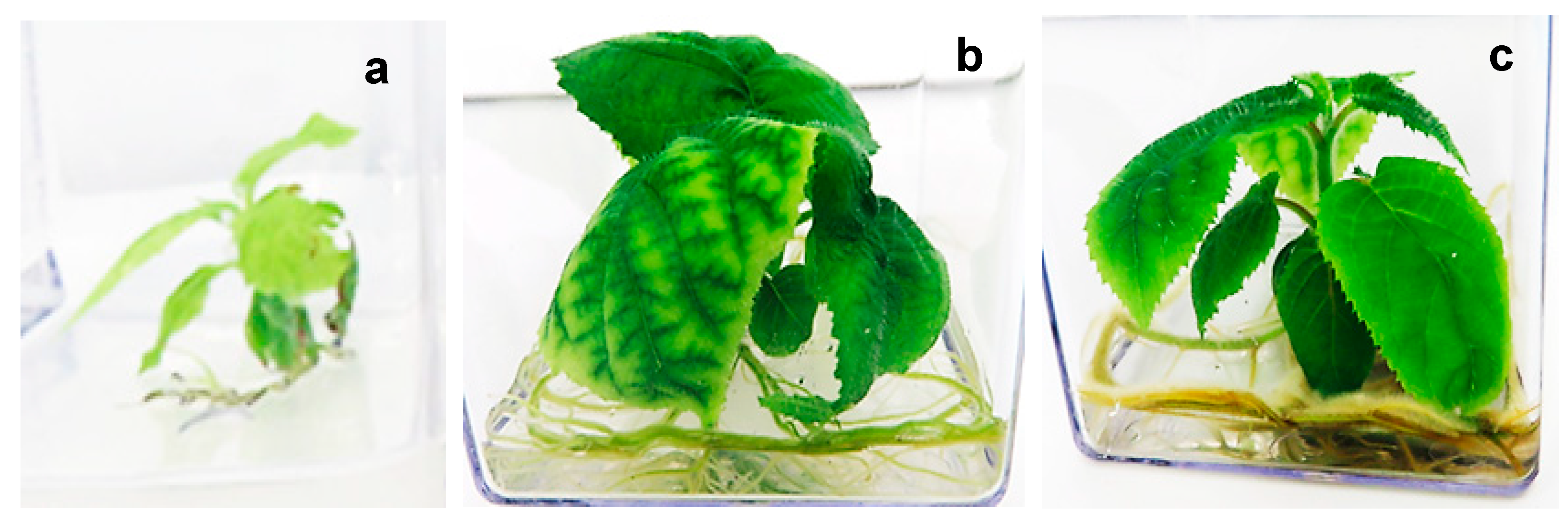
In vitro-developed seedlings of A. chinensis were used as an aseptic explant source for shoot multiplication culture. Shootlet explants were excised from the in vitro raised seedlings, which were dissected into two parts, namely apical shoot explants (+A) and basal shoot explants (−A).
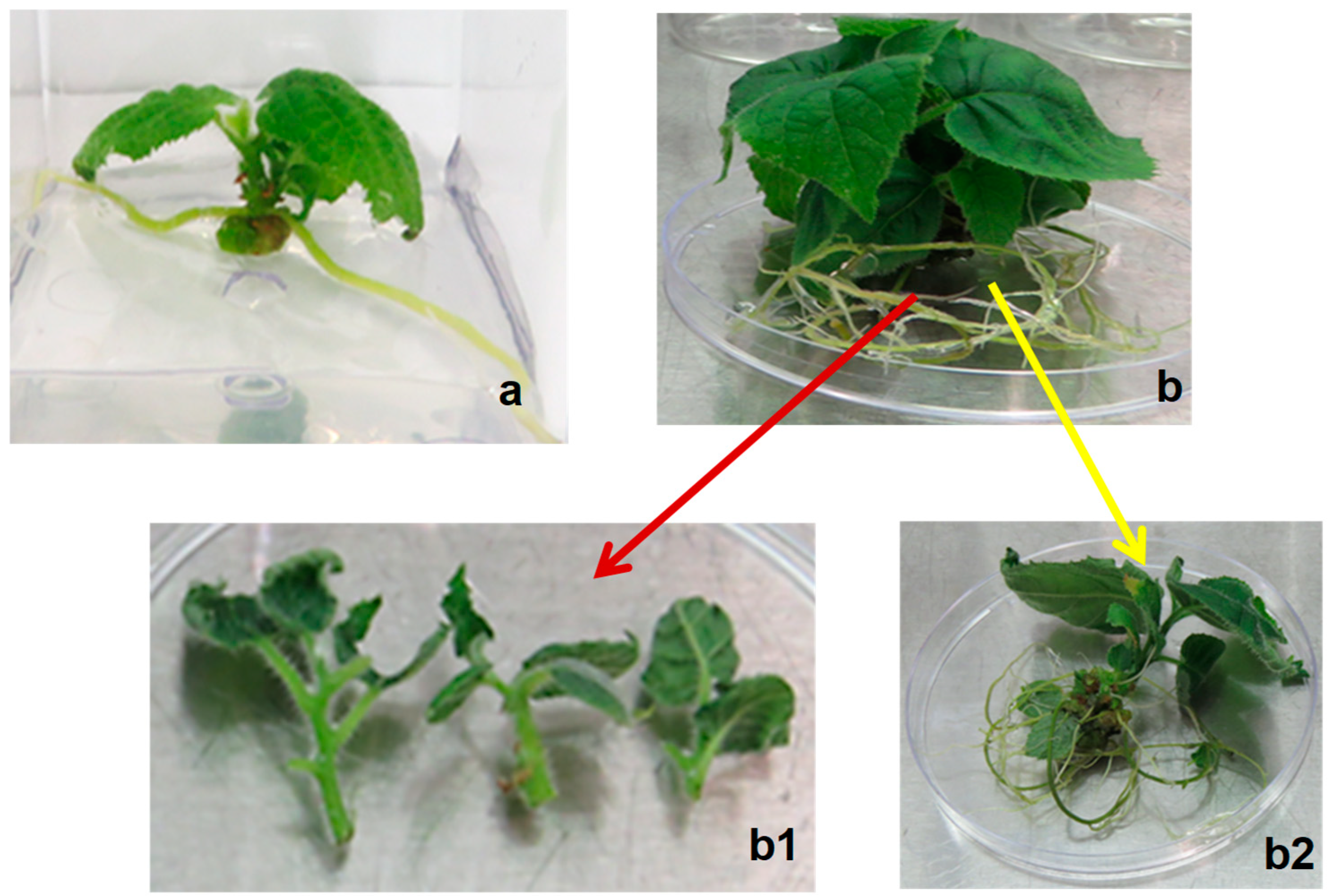
4.8. Shoot Induction and Multiplication
Apical (+A) and basal (−A) shoot explants from seedlings were then cultured in MS medium supplemented with 2.2 µM and 4.4 µM BAP for shoot induction and multiplication for 4 weeks. Explants cultured on PGR-free MS medium were used as a control. All cultures were kept in a culture room at 24 ± 2 °C and 16 h photoperiod of 50–60 µmol m−2 s−1 light intensity.
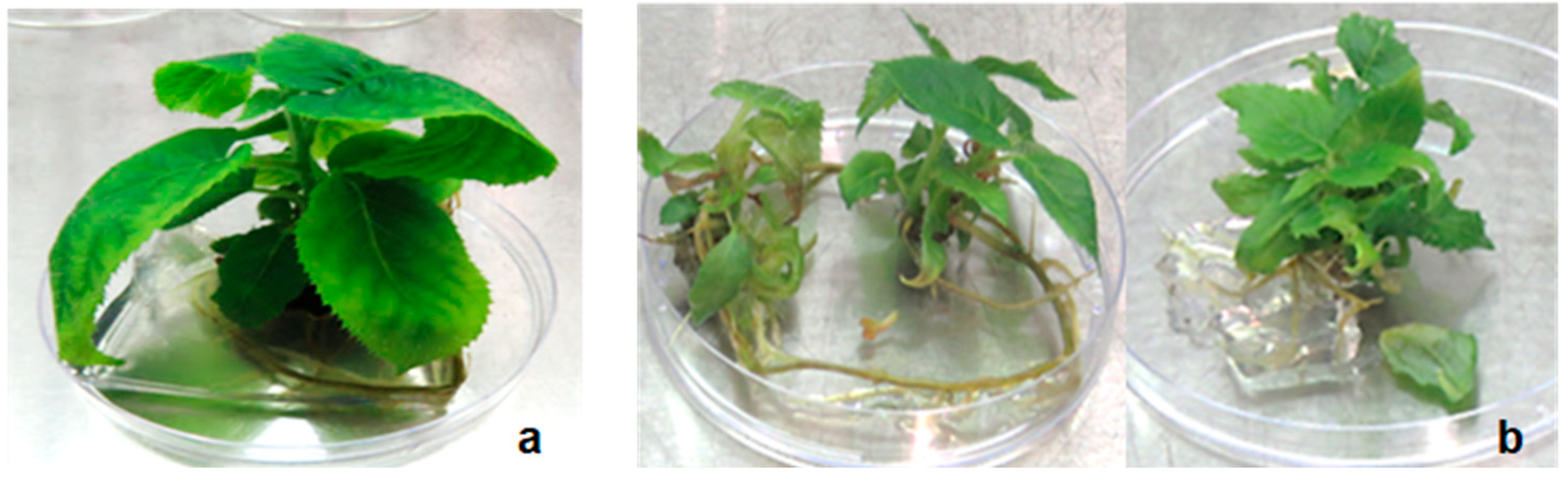
4.9. Root Induction, Acclimatisation and Transplanting of Plantlets
The in vitro multiplied shoots were excised from the mother plant and rooted on a PGR-free MS medium. Successfully rooted plantlets were removed gently from the culture medium and carefully washed with dH2O to remove excess agar. Plantlets with developed adventitious roots were then transferred to 5 cm diameter plastic pots filled with moist vermiculite and kept under controlled conditions at 24 ± 2 °C and 16 h photoperiod of 150–200 µmol m−2 s−1 light intensity. The plantlets were covered with transparent plastic bags, which were gradually opened daily until complete removal to ensure adaptation of plants from in vitro to in vivo conditions. Once the plantlets had fully acclimatised, they were transferred to a greenhouse for further hardening and plant development in vivo.
4.10. Experimental Design
All seed germination treatments (CS and GA3) and the controls were arranged in a completely randomized design (CRD) with 5 replications, each consisting of 5 seeds (n = 25). Similarly, all seedling treatments (2.2 µM and 4.4 µM BAP) were arranged in a CRD, with each treatment replicated 5 times, with 3 explants per culture vessel.
4.11. Data Collection and Analysis
4.11.1. Seed Germination
Radicle emergence from the testa was considered as an indicator for successful germination. In all treatments and the controls, the numbers of germinated seeds were recorded every 7 days until no further germination was observed. Mean germination time (MGT) was calculated using the following equation of Ellis and Roberts [
41]:
where n is the number of seeds that germinate on day D, and D is the number of days from initiation to completion of the germination process. The germination percentage (GP) was calculated as described in the Association of Official Seeds Analysts [
42] using the following equation:
Fisher’s least significant difference test was used to separate the means at the probability level of 5%. Germination variables with significant (p ≤ 0.05) treatment means were subjected to lines of the best fit.
4.11.2. Shoot Culture
After 21 days of incubation under controlled conditions, the numbers of newly sprouted axillary shoots were recorded weekly. The number of surviving plantlets after the acclimatisation in vivo was recorded and the survival percentage was calculated.