Acute and Rapid Response of Melissa officinalis and Mentha spicata to Saline Reclaimed Water in Terms of Water Relations, Hormones, Amino Acids and Plant Oxylipins
Abstract
:1. Introduction
2. Results
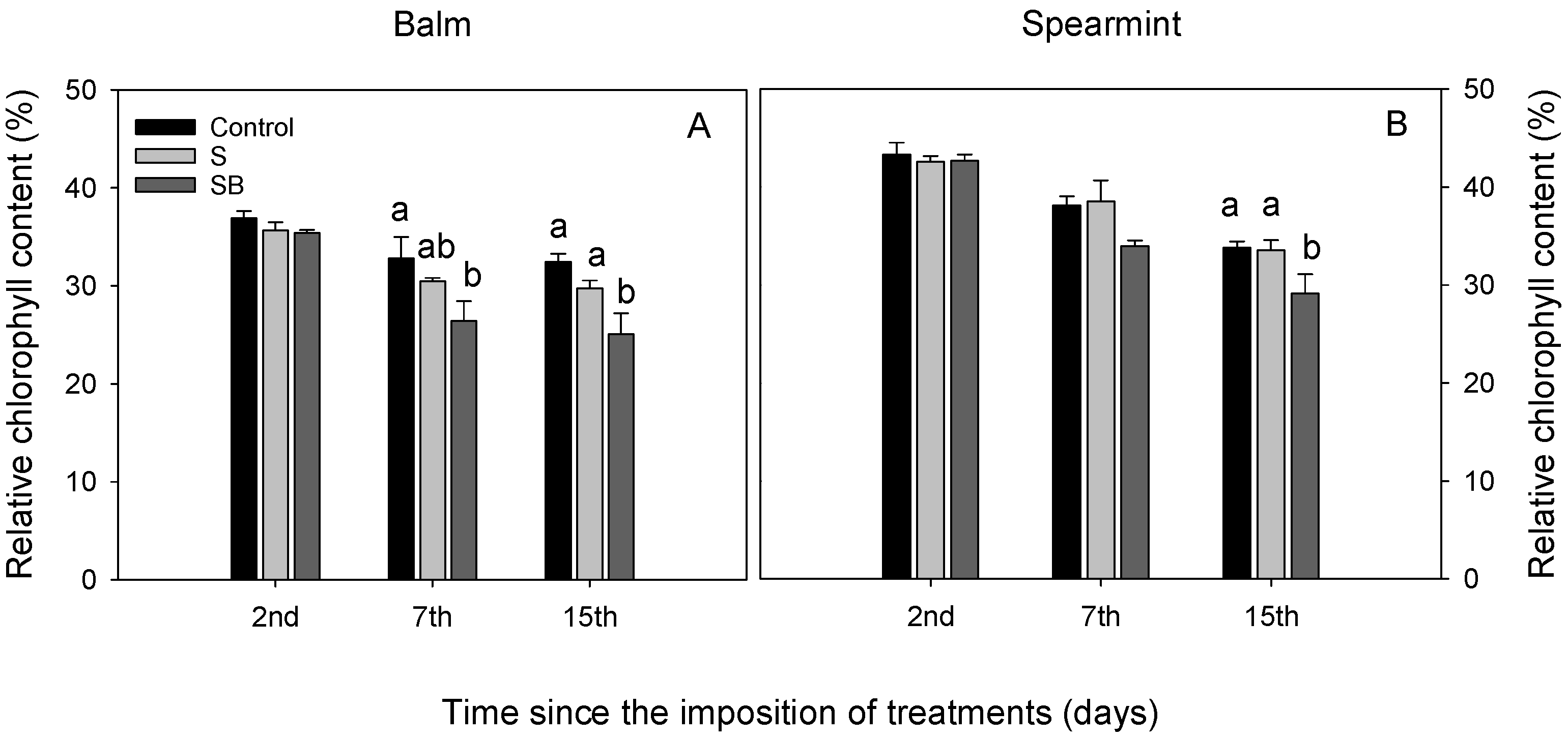
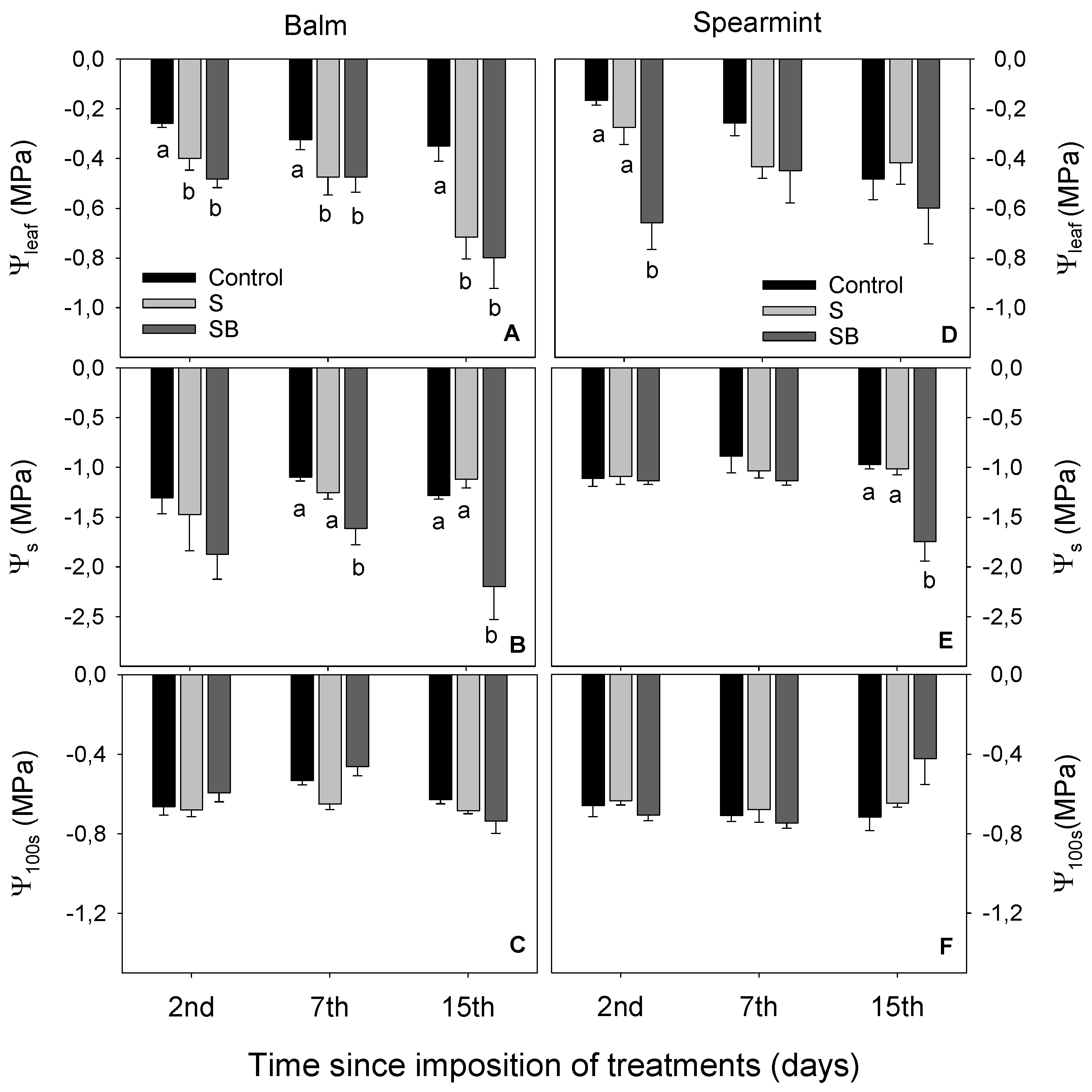
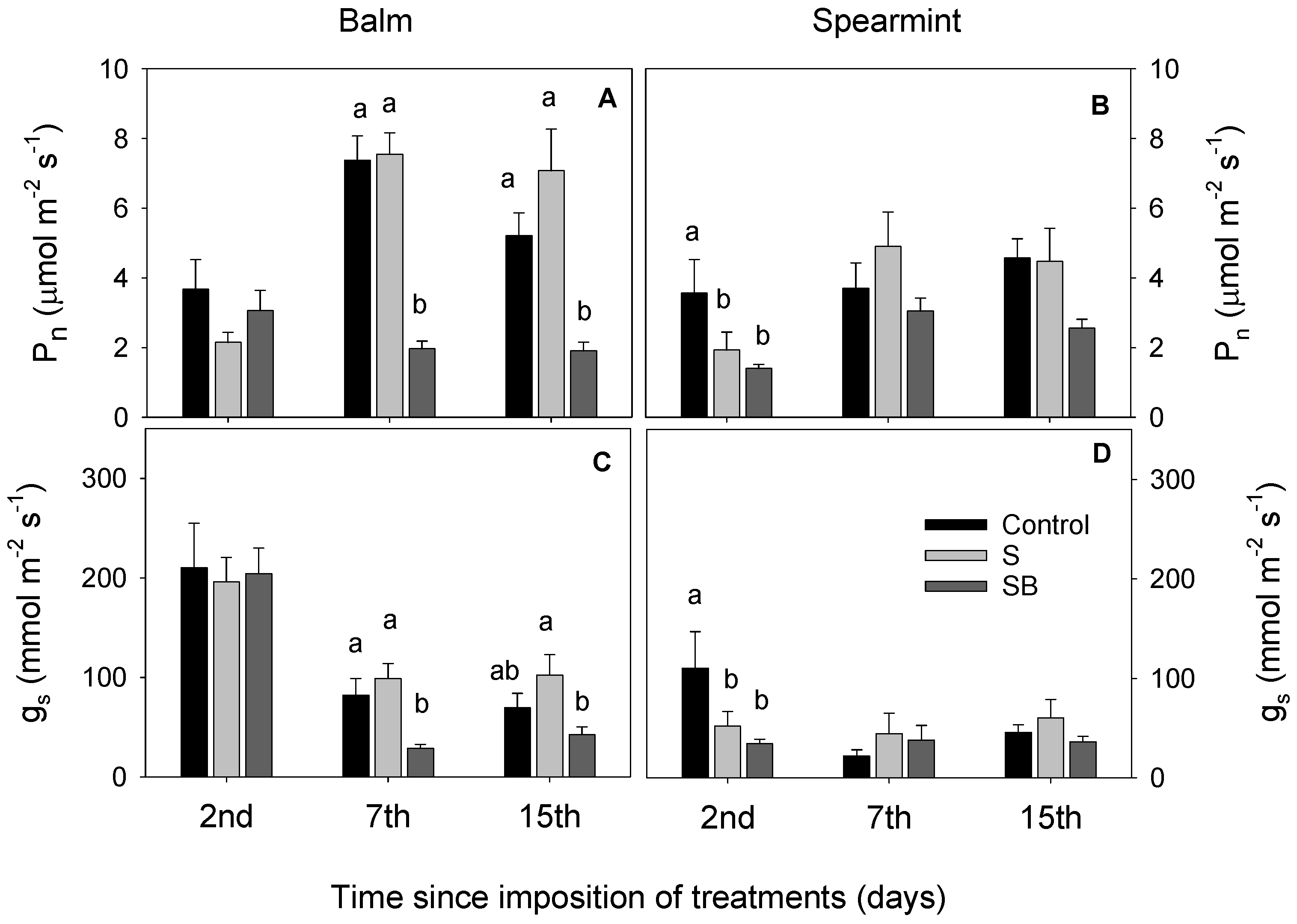
2.1. Relative Chlorophyll Content, Water Relations and Gas Exchange
2.2. Leaf Mineral Content and Plant Growth
2.3. Concentration of Phytohormones, Amino Acids and Phytoprostanes in Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experiment Conditions
4.2. Irrigation Water Treatments and Experimental Design
4.3. Water Relations
4.4. Gas Exchange and Relative Chlorophyll Content
4.5. Chemical and Reagents
4.6. Qualitative and Quantitative Analysis of Phytohormones
4.7. Qualitative and Quantitative Analysis of Amino Acids
4.8. Qualitative and Quantitative Analysis of Phytoprostanes (PhytoPs) and Phytofuranes (PhytoFs)
4.9. Leaf Mineral Content and Plan Growth
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Polcher, J. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.F.; Restrepo, I. Wastewater reuse in agriculture: A review about its limitations and benefits. Sustainability 2017, 9, 1734. [Google Scholar]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. Can batch or semi-batch processes save energy in reverse-osmosis desalination? Desalination 2017, 402, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Bellot, M.J.; Lorente, B.; Ortuño, M.F.; Medina, S.; Gil-Izquierdo, A.; Bañón, S.; Sánchez-Blanco, M.J. Recycled wastewater and reverse osmosis brine use for halophytes irrigation: Differences in physiological, nutritional and hormonal responses of Crithmum maritimum and Atriplex halimus plants. Agronomy 2021, 11, 627. [Google Scholar]
- Corcoran, E. (Ed.) Sick Water? The Central Role of Wastewater Management in Sustainable Development: A Rapid Response Assessment; UNEP/Earthprint: Nairobi, Kenya, 2010. [Google Scholar]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water scarcity and wastewater reuse in crop irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Bhadrachari, G.; Thomas, J.P. Desalination for agriculture: Water quality and plant chemistry, technologies and challenges. Water Sci. Technol. Water Supply 2018, 18, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Gil-Izquierdo, A.; Pedreño, M.A.; Montoro-García, S.; Tárraga-Martínez, M.; Iglesias, P.; Ferreres, F.; Barceló, D.; Núñez-Delicado, E.; Gabaldón, J.A. A sustainable approach by using microalgae to minimize the eutrophication process of Mar Menor lagoon. Sci. Total Environ. 2021, 758, 143163. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Cabrera, R.I. Growth and physiological responses of landscape plants to saline water irrigation: A review. Hortscience 2010, 45, 1605–1609. [Google Scholar] [CrossRef]
- Shoukat, E.; Ahmed, M.Z.; Abideen, Z.; Azeem, M.; Ibrahim, M.; Gul, B.; Khan, M.A. Short and long term salinity induced differences in growth and tissue specific ion regulation of Phragmites karka. Flora 2020, 263, 151550. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Baatour, O.; Kaddour, R.; Aidi Wannes, W.; Lachaal, M.; Marzouk, B. Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Phys. Plant. 2010, 32, 45–51. [Google Scholar] [CrossRef]
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Medicinal species as neuroactive agents: Turnera diffusa Willd. Ex Schult is a promising source of MTDLs, inhibiting CNS enzymes and delaying glutamate excitotoxicity in SH-SY5Y cells via oxidative damage. Food Chem. Toxicol. 2017, 106, 466–476. [Google Scholar] [CrossRef]
- Khorasaninejad, S.; Mousavi, A.; Soltanloo, H.; Hemmati, K.; Khalighi, A. The effect of salinity stress on growth parameters, essential oil yield and constituent of peppermint (Mentha piperita L.). World Appl. Sci. J. 2010, 11, 1403–1407. [Google Scholar]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B. Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 295–300. [Google Scholar] [CrossRef]
- Dehghani Bidgoli, R.; Azarnezhad, N.; Akhbari, M.; Ghorbani, M. Salinity stress and PGPR effects on essential oil changes in Rosmarinus officinalis L. Agri. Food Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of salinity and water stress on the essential oil components of rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Collado-González, J.; Durand, T.; Ferreres, F.; Medina, S.; Torrecillas, A.; Gil-Izquierdo, A. Phytoprostanes. Lipid-Technology 2015, 27, 127–130. [Google Scholar] [CrossRef]
- Leung, K.S.; Oger, C.; Guy, A.; Bultel-Poncé, V.; Vigor, C.; Durand, T.; Gil-Izquierdo, A.; Medina, S.; Galano, J.-M.; Lee, J.C.-Y. Alpha-linolenic acid, phytoprostanes and phytofurans in plant, algae and food. Acad. Press 2022, 101, 437–468. [Google Scholar]
- Collado-González, J.; Pérez-López, D.; Memmi, H.; Gijón, M.C.; Medina, S.; Durand, T.; Guy, A.; Galano, J.-M.; Ferreres, F.; Torrecillas, A.; et al. Water deficit during pit hardening enhances phytoprostanes content, a plant biomarker of oxidative stress, in extra virgin olive oil. J. Agric. Food Chem. 2015, 63, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.J. Archetype signals in plants: The phytoprostanes. Curr. Opin. Plant Biol. 2004, 7, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, M.; Domínguez-Perles, R.; Abellán, A.; Bultel-Ponce, V.T.; Durand, T.; Galano, J.M.; Ferreres, F.; Gil-Izquierdo, A. Statement of Foliar Fertilization Impact on Yield, Composition, and Oxidative Biomarkers in Rice. J. Agric. Food Chem. 2019, 67, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Collado-González, J.; Cano-Lamadrid, M.; Pérez-López, D.; Carbonell-Barrachina, A.A.; Centeno, A.; Medina, S.; Griñán, I.; Guy, A.; Galano, J.M.; Durand, T.; et al. Effects of deficit irrigation, rootstock and roasting on the contents of fatty acids, phytoprostanes, and phytofurans in pistachio kernels. J. Agric. Food Chem. 2020, 68, 8915–8924. [Google Scholar] [CrossRef]
- Medina, S.; Gil-Izquierdo, A.; Durand, T.; Ferreres, F.; Domínguez-Perles, R. Structural/functional matches and divergences of phytoprostanes and phytofurans with bioactive human oxylipins. Antioxidants 2018, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Campillo, M.; Medina, S.; Fanti, F.; Gallego-Gomez, J.I.; Simonelli Muñoz, A.; Bultel-Poncé, V.; Durand, T.; Galano, J.-M.; Tomás-Barberán, F.A.; Gil-Izquierdo, A.; et al. Phytoprostanes and phytofurans modulate COX-2-linked inflammation markers in LPS-stimulated THP-1 monocytes by lipidomics workflow. Free Rad. Biol. Med. 2021, 167, 335–347. [Google Scholar] [CrossRef]
- Bensabah, F.; Lamiri, A.; Naja, J. Effect of purified wastewater from the city of Settat (Morocco) on the quality of Lippia citriodora essential oil and infusion. J. Saudi Soc. Agric. Sci. 2015, 14, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Elsokkary, I.H.; Aboukila, A.F. Beneficial additive values of wastewater irrigation of two aromatic plants grown in low fertile soil. Water Sci. 2020, 34, 132–142. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Piñero, M.C.; Houdusse, F.; Garcia-Mina, J.M.; Garnica, M.; Del Amor, F.M. Regulation of hormonal responses of sweet pepper as affected by salinity and elevated CO2 concentration. Physiol. Plant. 2014, 151, 375–389. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Maestre-Valero, J.F.; González-Ortega, M.J.; Martínez-Álvarez, V.; Martin-Gorriz, B. The role of reclaimed water for crop irrigation in southeast Spain. Water Supply 2019, 19, 1555–1562. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Stasińska-Jakubas, M.; Wójciak, M.; Sowa, I.; Matraszek-Gawron, R. NaCl-Induced Elicitation Alters Physiology and Increases Accumulation of Phenolic Compounds in Melissa officinalis L. Int. J. Mol. Sci. 2021, 22, 6844. [Google Scholar] [CrossRef]
- Murkute, A.A.; Sharma, S.; Singh, S.K. Citrus in terms of soil and water salinity: A Review. J. Sci. Ind. Res. 2005, 22, 6844. [Google Scholar]
- Ghorbanpour, M.; Varma, A. (Eds.) Heavy metal-mediated changes in growth and phytochemicals of edible and medicinal plants. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 189–214. [Google Scholar]
- Aktsoglou, D.C.; Kasampalis, D.S.; Sarrou, E.; Tsouvaltzis, P.; Chatzopoulou, P.; Martens, S.; Siomos, A.S. Improvement of the quality in hydroponically grown fresh aromatic herbs by inducing mild salinity stress is species-specific. Folia Hort. 2021, 33, 265–274. [Google Scholar] [CrossRef]
- Mohamadiyeh, Z.; Abedi, B.; Moghaddam, M.; Samiei, L. Effect of salin stress on morphological caracteristics of Mint (Mentha spicata L.). In Proceedings of the 3rd National Congress on Medicinal Plants, Mashhad, Iran, 14–15 May 2014. [Google Scholar]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Alterations of endogenous hormonal levels in plants under drought and salinity. Am. J. Plant Sci. 2016, 7, 1357. [Google Scholar] [CrossRef] [Green Version]
- Atia, A.; Barhoumi, Z.; Debez, A.; Hkiri, S.; Abdelly, C.; Smaoui, A.; Haouari, C.C.; Gouia, H. Plant hormones: Potent targets for engineering salinity tolerance in plants. In Salinity Responses and Tolerance in Plants; Springer: Cham, Switzerland, 2018; Volume 1, pp. 159–184. [Google Scholar]
- Nishiyama, R.; Le, D.T.; Watanabe, Y.; Matsui, A.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS ONE 2012, 7, e32124. [Google Scholar] [CrossRef] [Green Version]
- Llanes, A.; Masciarelli, O.; Ordoñez, R.; Isla, M.I.; Luna, V. Differential Growth Responses to Sodium Salts Involve Different ABA Catabolism and Transport in the Halophyte Prosopis strombulifera. Biol. Plant. 2014, 58, 80–88. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; Freidit, F.N.; Leung, J. An Update on Abscisic Acid Signaling in Plants and More. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwinska, J.; Kadzinski, L.; Banasiuk, R.; Gwizdek-Wisniewska, A.; Olry, A.; Banecki, B.; Ihnatowicz, A. Identification of QTLs affecting scopolin and scopoletin biosynthesis in Arabidopsis thaliana. BMC Plant Boil. 2014, 14, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Kun-Ming, C.; Hai-Jun, G.; Guo-Cang, C.; Cheng-Lie, Z. ACC and MACC Biosynthesis and Ethylene Production in Water-Stressed Spring Wheat. Acta Bot. Sin. 2002, 44, 775–781. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Collado-González, J.; Cruz, Z.N.; Rodríguez, P.; Galindo, A.; Ferreres, F.; Medina, S.; Romojaro, F.; Egea, I.; Torrecillas, A.; Gil-Izquierdo, A. Effects of water deficit during maturation on amino acids and jujube fruit eating quality. Maced. J. Chem. Chem. Eng. 2014, 33, 105–119. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef]
- Yonny, M.E.; Rodríguez Torresi, A.; Cuyamendous, C.; Reversat, G.; Oger, C.; Galano, J.M.; Nazareno, M.A. Thermal stress in melon plants: Phytoprostanes and phytofurans as oxidative stress biomarkers and the effect of antioxidant supplementation. J. Agric. Food Chem. 2016, 64, 8296–8304. [Google Scholar] [CrossRef]
- Ueda, A.; Yamamoto-Yamane, Y.; Takabe, T. Salt stress enhances proline utilization in the apical region of barley roots. Biochem. Biophys. Res. Commun. 2007, 355, 61–66. [Google Scholar] [CrossRef]
- Wang, C.; He, R.; Lu, J.; Zhang, Y. Selection and regeneration of Vitis vinifera Chardonnay hydroxyproline-resistant calli. Protoplasma 2018, 255, 1413–1422. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Rasouli, F.; Rabbi, B.; Falakboland, Z.; Yong, M.; Chen, Z.H.; Zhou, M.; Shabala, S. Stomatal traits as a determinant of superior salinity tolerance in wild barley. J. Plant Physiol. 2020, 245, 153108. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci. Hort. 2013, 156, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Gucci, R.; Xiloyannis, C.; Flore, J.A. Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol. Plant. 1991, 83, 497–505. [Google Scholar] [CrossRef]
- Vigor, C.; Balas, L.; Guy, A.; Bultel-Poncé, V.; Reversat, G.; Galano, J.M.; Durand, T.; Oger, C. Isoprostanoids, Isofuranoids and Isoketals–From Synthesis to Lipidomics. Eur. J. Org. Chem. 2022, 2022, e202101523. [Google Scholar] [CrossRef]
- Cerrillo, I.; Fernández-Pachón, M.S.; Collado-González, J.; Herrero-Martín, G.; Berná, G.; Escudero-López, B.; Ferreres, F.; Gil-Izquierdo, A. Effect of Fermentation and Subsequent Pasteurization Processes on Amino Acids Composition of Orange Juice. Plant Foods Hum. Nutr. 2015, 70, 153–159. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, A.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Medina, S.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Ferreres, F.; Gil-Izquierdo, A. New UHPLC-QqQ-MS/MS method for quantitative and qualitative determination of free phytoprostanes in foodstuffs of commercial olive and sunflower oils. Food Chem. 2015, 178, 212–220. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Abellán, A.; León, D.; Ferreres, F.; Guy, A.; Oger, C.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, A. Sorting out the phytoprostane and phytofuran profile in vegetable oils. Food Res. Int. 2018, 107, 619–628. [Google Scholar] [CrossRef]
- Medina, S.; Gil-Izquierdo, A.; Abu-Reidah, I.; Durand, A.; Guy, T.; Galano, J.M.; Domínguez-Perles, R. Evaluation of Phoenix dactylifera edible parts and by-products as a source of phytoprostanes and phytofurans. J. Agric. Food Chem. 2020, 68, 8942–8950. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.F.; Morrow, J.D.; Roberts, L.J., II. A nomenclature system for the isoprostanes. Prostaglandins 1997, 53, 63. [Google Scholar] [CrossRef] [PubMed]
| Mineral Content in Leaves (g 100 g−1 of Dry Weight) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | K+ | Cl− | Na+ | P | |||||||||||||||
| Control | 0.80 | ± | 0.08 | 4.99 | ± | 0.36 | 4.72 | ± | 0.24 | 0.24 | ± | 0.04 | 0.90 | ± | 0.06 | a | |||
| Balm | S | 0.70 | ± | 0.17 | 4.68 | ± | 0.43 | 4.23 | ± | 0.07 | 0.20 | ± | 0.10 | 0.72 | ± | 0.08 | ab | ||
| SB | 0.49 | ± | 0.04 | 4.58 | ± | 0.16 | 4.64 | ± | 0.38 | 0.23 | ± | 0.05 | 0.62 | ± | 0.02 | b | |||
| P | ns | ns | ns | ns | * | ||||||||||||||
| Control | 1.04 | ± | 0.16 | 4.40 | ± | 0.59 | 4.49 | ± | 0.40 | b | 0.19 | ± | 0.03 | b | 0.69 | ± | 0.07 | a | |
| Spearmint | S | 0.86 | ± | 0.06 | 3.85 | ± | 0.15 | 4.42 | ± | 0.20 | b | 0.18 | ± | 0.02 | b | 0.57 | ± | 0.03 | b |
| SB | 1.12 | ± | 0.17 | 5.20 | ± | 0.81 | 6.19 | ± | 0.26 | a | 0.56 | ± | 0.14 | a | 0.74 | ± | 0.13 | a | |
| P | ns | ns | ** | * | * | ||||||||||||||
| Biomass Parameters | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf Number | Leaf Area (cm2) | Leaf DW (g) | Root DW (g) | ||||||||||||||
| Control | 386.3 | ± | 27.6 | a | 178.1 | ± | 6.9 | a | 11.0 | ± | 0.9 | a | 9.3 | ± | 1.5 | a | |
| Balm | S | 308.0 | ± | 44.0 | b | 164.2 | ± | 12.1 | a | 11.2 | ± | 1.0 | a | 7.0 | ± | 2.0 | a |
| SB | 109.0 | ± | 9.0 | c | 53.5 | ± | 10.3 | b | 7.5 | ± | 0.4 | b | 4.3 | ± | 0.2 | b | |
| P | ** | ** | * | * | |||||||||||||
| Control | 392.3 | ± | 77.6 | 136.5 | ± | 7.3 | a | 12.5 | ± | 2.12 | b | 7.7 | ± | 0.8 | b | ||
| Spearmint | S | 239.3 | ± | 40.2 | 122.5 | ± | 5.8 | a | 19.7 | ± | 1.24 | a | 8.2 | ± | 0.2 | b | |
| SB | 346.7 | ± | 91.4 | 51.59 | ± | 16.5 | b | 12.2 | ± | 1.34 | b | 10.3 | ± | 1.1 | a | ||
| P | ns | ** | ** | * | |||||||||||||
| Phytohormones (ng g−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | S | SB | P | |||||||||||
| ACC | 18.20 | ± | 2.08 | b | 15.85 | ± | 1.21 | b | 180.05 | ± | 17.06 | a | *** | |
| TZ | 33.33 | ± | 8.01 | b | 21.39 | ± | 2.11 | b | 64.66 | ± | 11.71 | a | ** | |
| TZ-rib | 0.27 | ± | 0.04 | b | 0.22 | ± | 0.03 | b | 0.56 | ± | 0.04 | a | *** | |
| Balm | TZ-glc | 5.59 | ± | 0.54 | a | 4.99 | ± | 0.46 | a | 2.07 | ± | 0.09 | b | *** |
| ABA | 1.07 | ± | 0.13 | a | 0.67 | ± | 0.05 | b | 1.25 | ± | 0.07 | a | * | |
| SA | 7.25 | ± | 1.52 | a | 2.17 | ± | 0.26 | b | 6.88 | ± | 0.45 | a | ** | |
| SC | 33.32 | ± | 4.48 | 47.28 | ± | 7.99 | 25.21 | ± | 3.99 | ns | ||||
| Spearmint | ||||||||||||||
| ACC | 42.89 | ± | 6.94 | b | 66.28 | ± | 2.57 | a | 77.33 | ± | 8.94 | a | * | |
| TZ | 95.22 | ± | 5.03 | a | 64.27 | ± | 6.62 | b | 63.69 | ± | 11.57 | b | * | |
| TZ-rib | 0.32 | ± | 0.11 | 0.50 | ± | 0.04 | 0.69 | ± | 0.15 | |||||
| Spearmint | TZ-glc | 10.66 | ± | 0.24 | a | 5.13 | ± | 0.56 | b | 4.27 | ± | 0.55 | b | ** |
| ABA | 1.07 | ± | 0.10 | b | 1.95 | ± | 0.18 | a | 1.68 | ± | 0.18 | a | *** | |
| SA | 4.17 | ± | 0.64 | 5.53 | ± | 1.90 | 5.90 | ± | 1.21 | |||||
| SC | 6.36 | ± | 0.85 | b | 9.44 | ± | 0.66 | a | 6.29 | ± | 0.21 | b | * | |
| Amino Acids (µg g−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | S | SB | P | |||||||||||
| Ser | 67.7 | ± | 3.8 | 78.7 | ± | 15.3 | 103.8 | ± | 31.5 | ns | ||||
| Asp | 79.1 | ± | 11.1 | b | 128.2 | ± | 6.2 | a | 119.1 | ± | 12.0 | a | * | |
| Arg | 54.1 | ± | 8.4 | 50.4 | ± | 9.6 | 26.9 | ± | 7.0 | ns | ||||
| Balm | P-Hyp | 5.6 | ± | 0.7 | 5.4 | ± | 0.6 | 5.7 | ± | 0.7 | ns | |||
| Met-His | 430.1 | ± | 18.3 | 445.9 | ± | 18.5 | 437.6 | ± | 18.0 | ns | ||||
| Thr | 13.0 | ± | 2.0 | 13.7 | ± | 0.6 | 12.6 | ± | 2.9 | ns | ||||
| Ala | 16.1 | ± | 1.4 | 17.4 | ± | 0.9 | 16.8 | ± | 1.0 | ns | ||||
| Leu | 5.5 | ± | 0.6 | 6.4 | ± | 0.7 | 6.3 | ± | 0.9 | ns | ||||
| Ser | 61.5 | ± | 8.6 | b | 401.1 | ± | 48.1 | b | 81.9 | ± | 15.4 | a | ** | |
| Asp | 160.5 | ± | 11.0 | 200.0 | ± | 35.3 | 147.9 | ± | 10.3 | ns | ||||
| Arg | 46.1 | ± | 4.8 | 65.5 | ± | 13.7 | 37.4 | ± | 6.4 | ns | ||||
| Spearmint | P-Hyp | 5.6 | ± | 1.2 | b | 9.6 | ± | 0.9 | a | 9.4 | ± | 1.5 | a | * |
| Met-His | 413.9 | ± | 8.5 | b | 433.7 | ± | 12.9 | ab | 478.4 | ± | 20.5 | a | * | |
| Thr | 14.4 | ± | 1.9 | ab | 18.2 | ± | 3.1 | a | 10.0 | ± | 2.1 | b | * | |
| Ala | 15.1 | ± | 0.8 | 16.6 | ± | 0.8 | 13.2 | ± | 1.6 | ns | ||||
| Leu | 5.7 | ± | 0.9 | b | 10.4 | ± | 0.7 | a | 12.0 | ± | 1.5 | a | ** | |
| Phytoprostanes and Phytofurans (µg 100 g−1 F.W.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Control | S | SB | P | ||||||||||
| Balm | 9-F1t-PhytoP | 0.016 | ± | 0.007 | 0.014 | ± | 0.002 | 0.025 | ± | 0.004 | ns | |||
| 9-epi-9-F1t-PhytoP | 0.023 | ± | 0.007 | 0.025 | ± | 0.007 | 0.056 | ± | 0.011 | ns | ||||
| ent-16-epi-16-F1t-PhytoP + ent-16-F1t-PhytoP | 0.001 | ± | 0.001 | 0.002 | ± | 0.002 | 0.001 | ± | 0.001 | ns | ||||
| Total PhytoP | 0.041 | ± | 0.017 | 0.041 | ± | 0.014 | 0.077 | ± | 0.012 | ns | ||||
| ent-9(RS)-12-epi-ST-Δ10-13-PhytoF | 400.2 | ± | 23,56 | a | 287.6 | ± | 29,32 | b | 358.0 | ± | 28,94 | ab | * | |
| ent-16(RS)-13-epi-ST-Δ14-9-PhytoF | 0.107 | ± | 0.117 | 0.144 | ± | 0.158 | 0.144 | ± | 0.157 | ns | ||||
| ent-16(RS)-9-epi-ST-Δ14-10-PhytoF | 2.902 | ± | 0.559 | 2.801 | ± | 0.382 | 3.188 | ± | 0.261 | ns | ||||
| Total PhytoF | 403.29 | ± | 23.81 | a | 290.04 | ± | 29.57 | b | 360.76 | ± | 29.13 | ab | * | |
| Spearmint | 9-F1t-PhytoP | 0.043 | ± | 0.009 | b | 0.087 | ± | 0.015 | a | 0.018 | ± | 0.006 | b | * |
| 9-epi-9-F1t-PhytoP | 0.105 | ± | 0.012 | 0.199 | ± | 0.039 | 0.121 | ± | 0.048 | ns | ||||
| ent-16-epi-16-F1t-PhytoP + Ent-16-F1t-PhytoP | 0.001 | ± | 0.001 | 0.001 | ± | 0.001 | 0.000 | ± | 0.000 | ns | ||||
| Total PhytoP | 0.117 | ± | 0.03 | ab | 0.247 | ± | 0.075 | a | 0.096 | ± | 0.045 | b | * | |
| ent-9(RS)-12-epi-ST-Δ10-13-PhytoF | 299.26 | ± | 17.93 | a | 273.42 | ± | 19.46 | a | 191.71 | ± | 24.05 | b | ** | |
| ent-16(RS)-13-epi-ST-Δ14-9-PhytoF | 0.000 | ± | 0.000 | b | 0.159 | ± | 0.087 | a | 0.000 | ± | 0.000 | b | * | |
| ent-16(RS)-9-epi-ST-Δ14-10-PhytoF | 2.286 | ± | 0.327 | 1.654 | ± | 0.223 | 1.728 | ± | 0.411 | ns | ||||
| Total PhytoF | 301.54 | ± | 19.66 | a | 274.905 | ± | 19.661 | a | 193.44 | ± | 24.206 | b | ** | |
| Physicochemical Analysis | C | S | SB |
|---|---|---|---|
| pH | 8.6 | 8.52 | 8.43 |
| CE (dS m−1) | 1.14 | 1.55 | 4.37 |
| B (mg L−1) | 0.06 | 0.12 | 0.22 |
| Ca (mg L−1) | 39.26 | 48.84 | 127.90 |
| Fe (mg L−1) | <0.01 | 0.09 | 0.01 |
| K (mg L−1) | 9.57 | 29.25 | 75.15 |
| Mg (mg L−1) | 39.35 | 34.45 | 87.57 |
| Mn (mg L−1) | 0.36 | 0.32 | 0.68 |
| Na (mg L−1) | 172.00 | 219.20 | 828.60 |
| P (mg L−1) | <0.01 | 1.58 | 12.09 |
| S (mg L−1) | 95.90 | 79.58 | 338.90 |
| Zn (mg L−1) | 0.35 | 0.04 | 0.05 |
| Ni (mg L−1) | <0.01 | <0.01 | 0.01 |
| Cl− (mg L−1) | 214.62 | 317.87 | 1066.08 |
| NO2− (mg L−1) | <0.1 | <0.1 | <0.1 |
| Br− (mg L−1) | 0.21 | 0.33 | 32.22 |
| NO3− (mg L−1) | 13.08 | 26.87 | 41.29 |
| PO43− (mg L−1) | <1.0 | <1.0 | <1.0 |
| SO42− (mg L−1) | 338.33 | 273.18 | 1067.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Bellot, M.J.; Lorente, B.; Medina, S.; Gil-Izquierdo, Á.; Durand, T.; Galano, J.-M.; Vicente-Sánchez, S.; Ortuño, M.F.; Sánchez-Blanco, M.J. Acute and Rapid Response of Melissa officinalis and Mentha spicata to Saline Reclaimed Water in Terms of Water Relations, Hormones, Amino Acids and Plant Oxylipins. Plants 2022, 11, 3427. https://doi.org/10.3390/plants11243427
Gómez-Bellot MJ, Lorente B, Medina S, Gil-Izquierdo Á, Durand T, Galano J-M, Vicente-Sánchez S, Ortuño MF, Sánchez-Blanco MJ. Acute and Rapid Response of Melissa officinalis and Mentha spicata to Saline Reclaimed Water in Terms of Water Relations, Hormones, Amino Acids and Plant Oxylipins. Plants. 2022; 11(24):3427. https://doi.org/10.3390/plants11243427
Chicago/Turabian StyleGómez-Bellot, María José, Beatriz Lorente, Sonia Medina, Ángel Gil-Izquierdo, Thierry Durand, Jean-Marie Galano, Sergio Vicente-Sánchez, María Fernanda Ortuño, and María Jesús Sánchez-Blanco. 2022. "Acute and Rapid Response of Melissa officinalis and Mentha spicata to Saline Reclaimed Water in Terms of Water Relations, Hormones, Amino Acids and Plant Oxylipins" Plants 11, no. 24: 3427. https://doi.org/10.3390/plants11243427







