Potential Use of Ascophyllum nodosum as a Biostimulant for Improving the Growth Performance of Vigna aconitifolia (Jacq.) Marechal
Abstract
:1. Introduction
2. Results
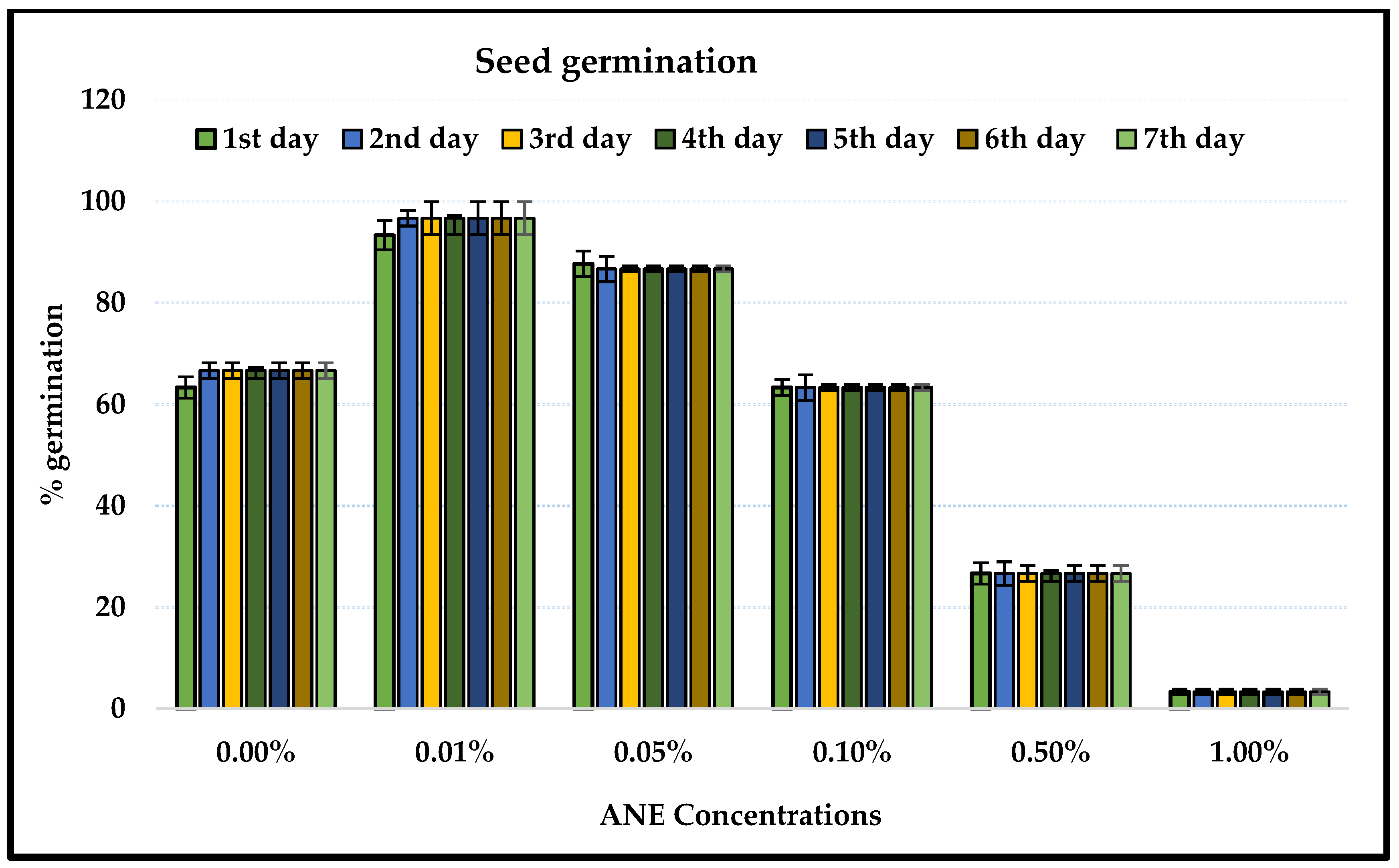
2.1. Ascophyllum Nodosum Extract (ANE) Treatment and Seed Germination
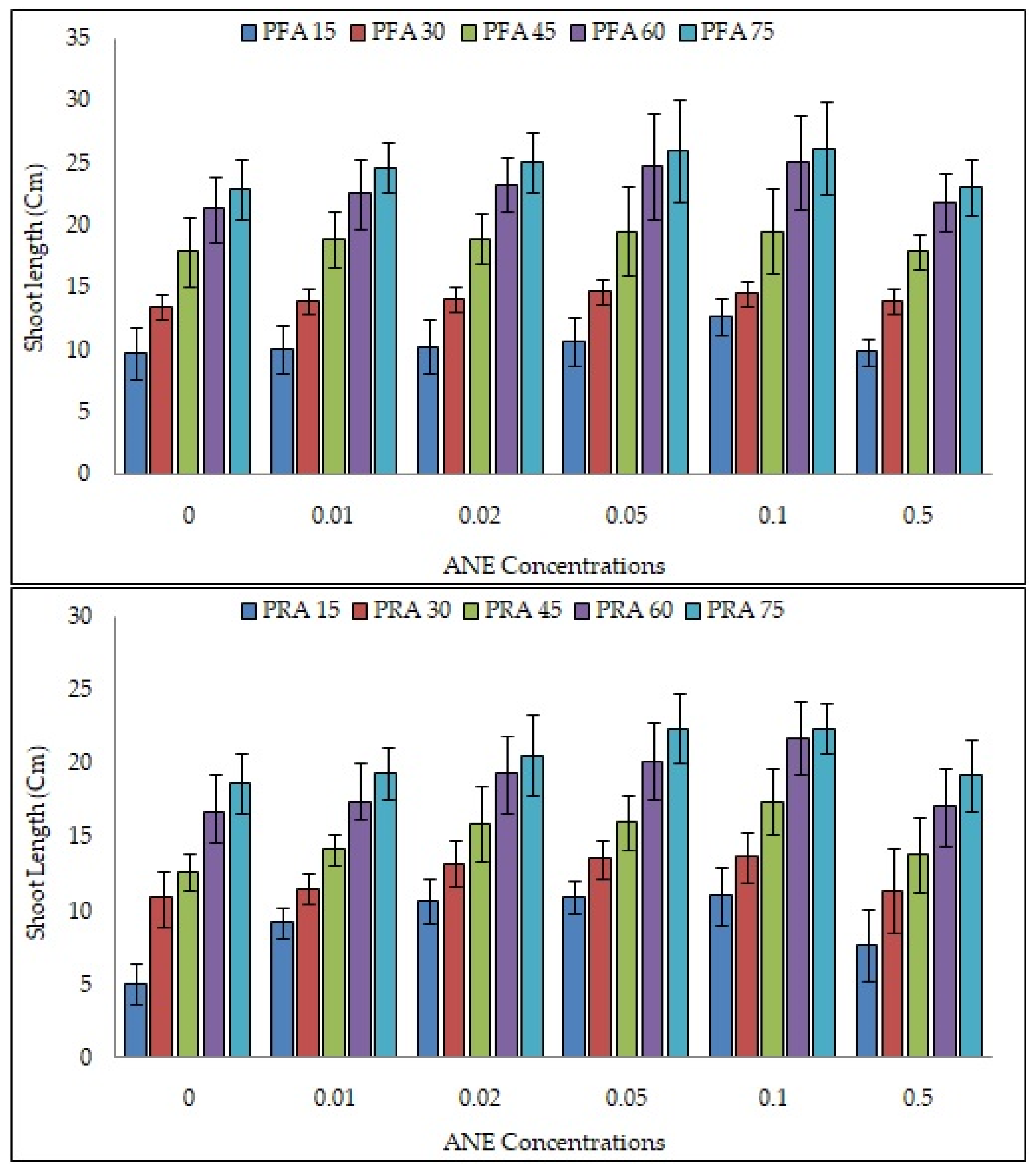
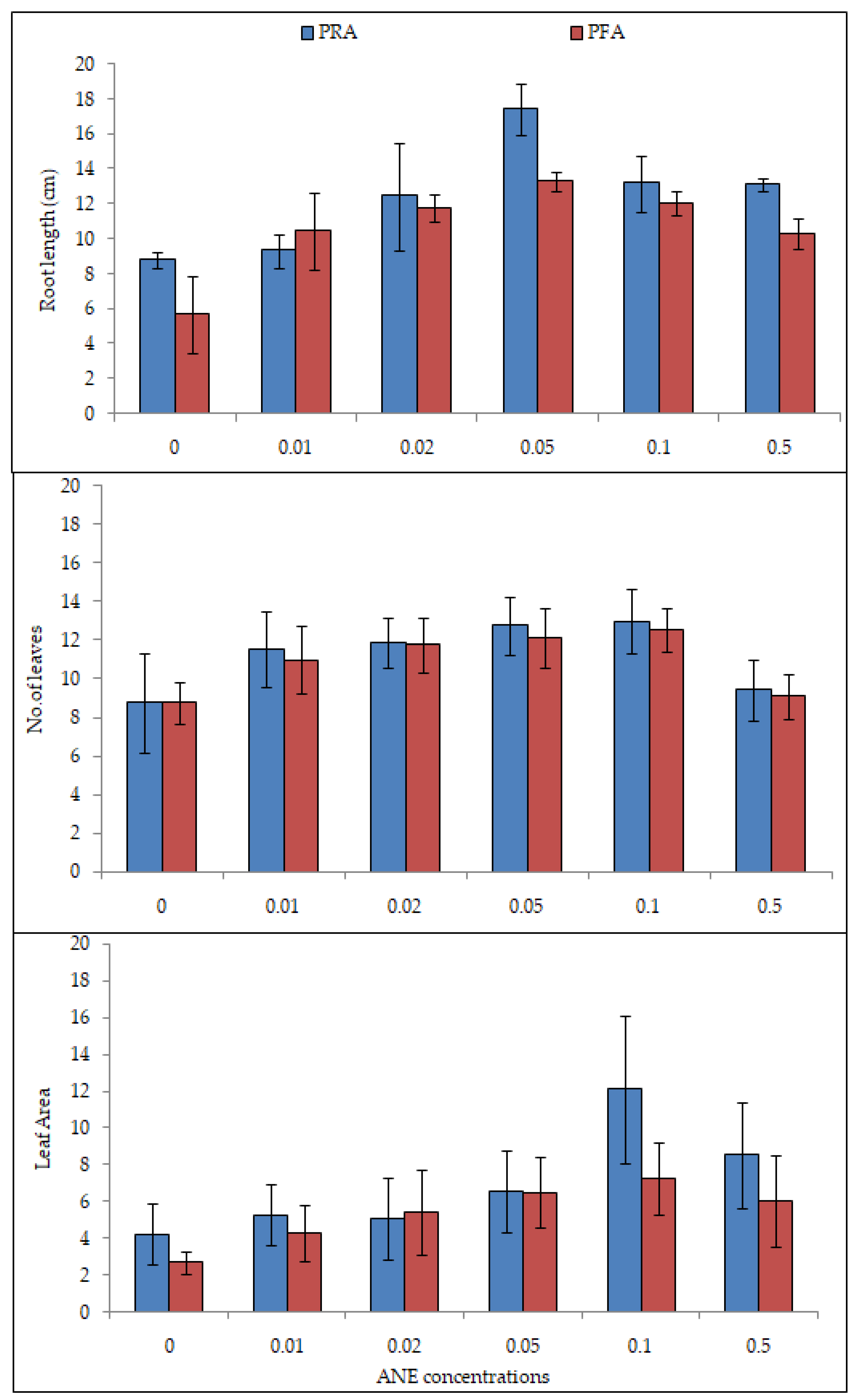
2.2. Efficacy of ANE Treatments on Shoot and Root Lengths of V. aconitifolia
2.3. Effect of ANE Treatments on Leaf Number and Leaf Area of V. aconitifolia at 30th Day
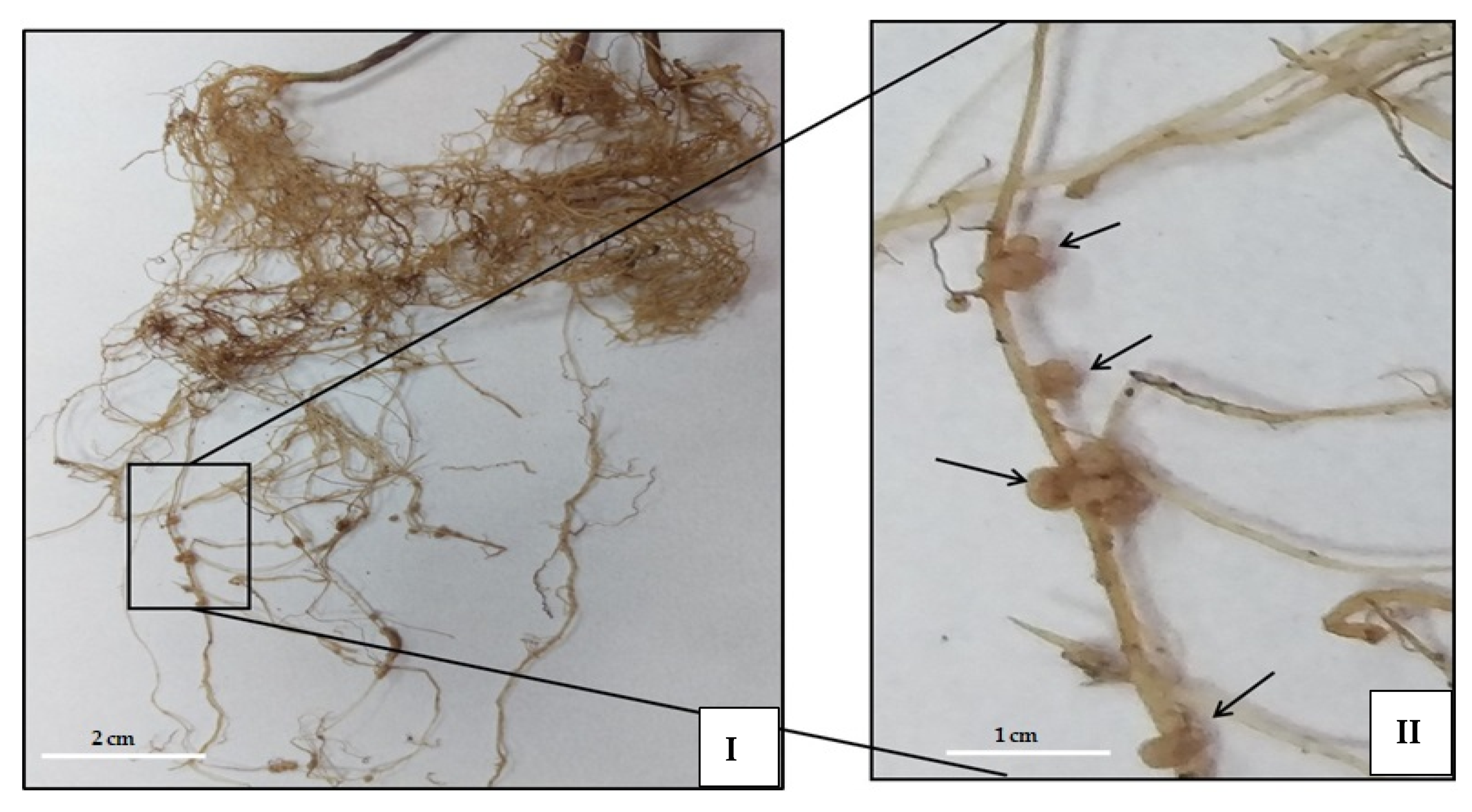
2.4. Effect of ANE Treatments on Nodulation
2.5. Effect in Pot Foliar Application (PFA) and Pot Root Application (PRA) of ANE on Biomass Accumulation
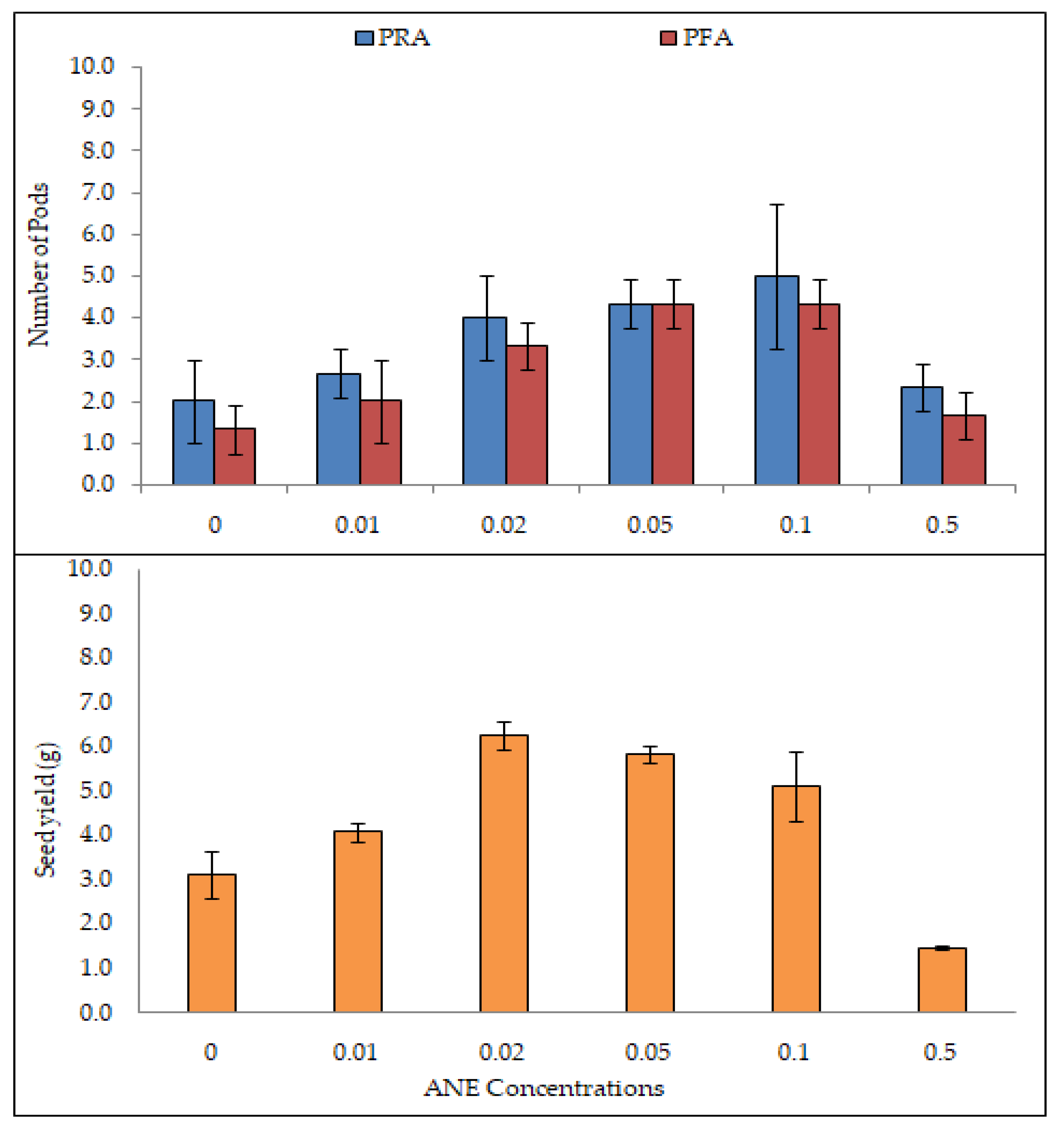
2.6. Effect of ANE on Number of Pods and Seed Yield
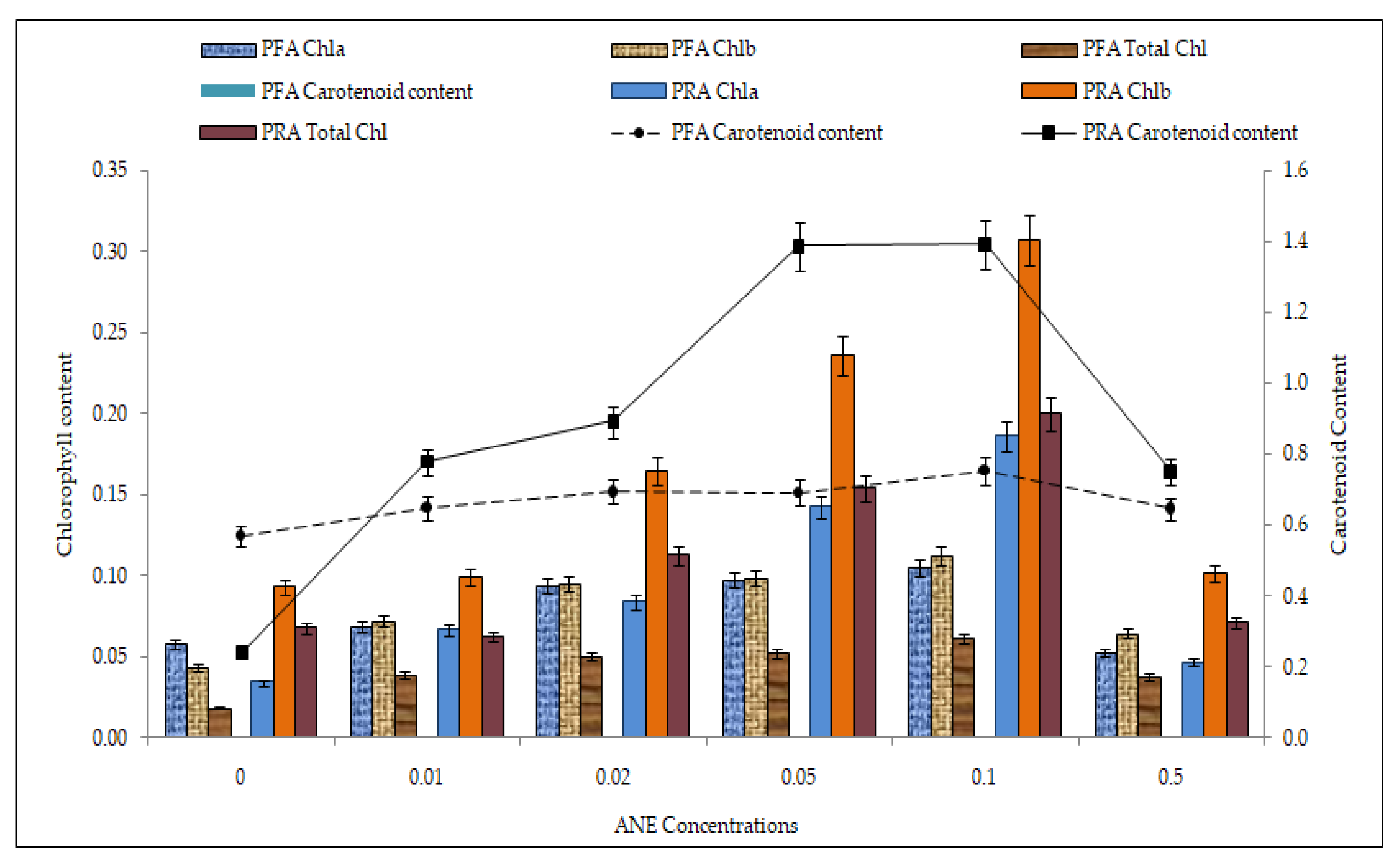
2.7. Efficacy of ANE Treatments on Photosynthetic Pigment Accumulation
3. Discussion
4. Materials and Methods
4.1. Preparation of Ascophyllum Nodosum Extract (ANE) and Seed Germination
4.2. Experimental Design and A. nodosum Extract (ANE) Treatment
4.3. Growth Parameters and Yield Attributes
4.4. Photosynthetic Pigments
4.4.1. Chlorophyll Content Estimation
4.4.2. Estimation of Carotenoid Content
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, R.S.; Singhal, L. Harmful effects of pesticides and their control through cowpathy. Int. J. Cow Sci. 2006, 2, 61–70. [Google Scholar]
- Anandhan, S.; Sorna, K.H. Biorestraining potentials of marine macroalgae collected from Rameshwaram, Tamil Nadu. J. Res. Biol. 2011, 5, 385–392. [Google Scholar]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Psychol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Blunden, G.; Gordon, S.M. Betaines and their sulphonio analogues in marine algae. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress Ltd.: Bristol, UK, 1986; pp. 39–80. [Google Scholar]
- Wu, Y.; Jenkins, T.; Blunden, G.; Whapham, C.; Hankins, S.D. The role of betains in alkaline extracts of Ascophyllum nodosum in reduction of Meloidogyne javanica and M. incognita infestations of tomato plants. Fundam. Appl. Nematol. 1997, 20, 99–102. [Google Scholar]
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Chapman and Hall, Ltd.: London, UK, 1980. [Google Scholar] [CrossRef]
- Featonby-Smith, B.C.; Staden, J. Identification and seasonal variation of endogenous cytokinins in Ecklonian maxima. Bot. Mar. 1984, 27, 527–531. [Google Scholar] [CrossRef]
- Crouch, I.J.; VanStaden, J. Evidence for the presence of plant growth regulators in commercial seaweed product. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Erulan, V.; Soundarapandian, P.; Thirumaran, G.; Ananthan, G. Studies on the effect of Sargassum polycystum extract on the growth and biochemical composition of Cajanus cajan (L.) Mill sp. Am.-Eurasian J. Agric. Environ. Sci. 2009, 6, 392–399. [Google Scholar]
- Kavipriya, R.; Dhanalakshmi, P.K.; Jayashree, S.; Thangaraju, N. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotech. 2011, 3, 16–19. [Google Scholar]
- Sathya, B.; Indu, H.; Seenivasan, R.; Geetha, S. Influence of seaweed liquid fertilizer on the growth and biochemical composition of legume crop, Cajanus cajan (L.) Mill sp. J. Phytol. Res. 2010, 2, 50–63. [Google Scholar]
- Thivy, F. Seaweed utilization in India. In Proceedings of the Symposium of Algology; ICAR: New Delhi, India, 1960; p. 345. [Google Scholar]
- Ugarte, R.A.; Craigie, J.S.; Critchley, A.T. Fucoid flora of the rocky intertidal of the Canadian Maritimes: Implications for the future with rapid climate change. In Seaweeds and Their Roles in Globally Changing Environments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: New York, NY, USA, 2021; pp. 73–90. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Verma, N.; Sehrawat, K.D.; Sehrawat, A.R. Studies on the effect of Ascophyllum nodosum extract (ANE) on growth, antioxidant potential and alpha glucosidase inhibition activity of Vigna aconitifolia (RMO 225). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2125–2138. [Google Scholar] [CrossRef]
- Verma, N.; Sehrawat, K.D.; Kumari, S.; Sehrawat, A.R. Antityrosinase activity and photosynthetic pigments in seaweed treated sprouts of Vigna aconitifolia. Indian J. Agric. Sci. 2019, 89, 1609–1611. [Google Scholar]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Gordon, B.; Jeffrey, N.; Mark Hodges, D. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Rungruangmaitree, R.; Jiraungkoorskul, W. Pea, Pisum sativum, and its anticancer activity. Pharmacol. Rev. 2017, 11, 39–42. [Google Scholar]
- Oboh, G. Antioxidant properties of some commonly consumed and underutilized tropical legumes. Eur. Food Res. Technol. 2006, 224, 61–65. [Google Scholar] [CrossRef]
- Abetz, P.; Young, C.L. The effect of seaweed extract sprays derived from Ascophyllum nodosum on lettuce and cauliflower crops. Bot. Mar. 1983, 26, 487–492. [Google Scholar] [CrossRef]
- Kumar, A.; Vanlalzarzova, B.; Sridhar, S.; Baluswami, M. Effect of liquid seaweed fertilizer of Sargassum wightii grev. on the growth and biochemical content of green gram (Vigna radiate (L.) R. Wilczek). Recent Res. Sci. Technol. 2012, 4, 40–45. [Google Scholar]
- Selvam, G.G.; Sivakumar, K. Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental composition using SEM—Energy dispersive spectroscopic analysis. Asian Pac. J. Reprod. 2013, 2, 119–125. [Google Scholar] [CrossRef]
- Rayorath, P.; Narayanan, J.M.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Ramarajan, S.; Henry, J.L.; Saravana, G.A. Effect of seaweed extracts mediated changes in leaf area and pigment concentration in soybean under salt stress condition. Res. Rev. J. Life Sci. 2013, 3, 17–21. [Google Scholar]
- Divya, K.; Roja, N.M.; Padal, S.B. Influence of seaweed liquid fertilizer of ulva lactuca on the seed germination, growth, productivity of Abelmoschus esculentus (L.). Int. J. Pharmacol. Res. 2015, 5, 344–346. [Google Scholar]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Influence of Seaweed liquid fertilizers on growth, biochemical and yield parameters of Cluster bean plant. J. Green Bioenergy 2015, 1, 19–32. [Google Scholar]
- Tandon, S.; Dubey, A. Effects of Biozyme (Ascophyllum nodosum) Biostimulant on Growth and Development of Soybean (Glycine Max (L.) Merill). Commun. Soil Sci Plant Anal. 2015, 46, 845–858. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000. [Google Scholar]
- Craigie, J.S. Seaweed extracts stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Sangeetha, V.; Thevanathan, R. Biofertilizer potential of traditional and panchagavya amended with seaweed extract. J. Am. Sci. 2010, 6, 61–67. [Google Scholar]
- Badri, D.V.; Weir, T.L.; Van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant–microbe interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Gandhiyappan, K.; Perumal, P. Growth promoting effect of seaweed liquid fertilizer (Enteromorpha intestinalis) on the sesame crop plant. Seaweed Res. Util. 2001, 23, 23–25. [Google Scholar]
- Moore, K.K. Using seaweed compost to grow bedding plants. BioCycle 2004, 45, 43–44. [Google Scholar]
- Sehrawat, A.R.; Verma, N.; Sehrawat, K.D.; Pandey, D. Biological potential of Ascophyllum nodosum extract on rhizobial diversity in nodules of Moth bean Vigna aconitifolia Jacq. via amplified ribosomal DNA restriction analysis. J. Environ. Biol. 2021, 42. [Google Scholar]
- Ishii, T.; Aikawa, J.; Kirino, S.; Kitabayashi, H.; Matsumoto, I.; Kadoya, K. Effects of alginate oligosaccharide and polyamines on hyphal growth of vesicular-arbuscular mycorrhizal fungi and their infectivity of citrus roots. In Proceedings of the 9th International Society of Citriculture Congress, Orlando, FL, USA, 3–7 December 2000; pp. 1030–1032. [Google Scholar]
- Salah, E.l.; Din, R.A.; Elbakry, A.A.; Ghazi, S.M.; Abdel Hamid, O.M. Effect of seaweed extract on the growth and yield of faba bean (Vicia faba L.). Egypt. J. Phycol. 2008, 9, 25–38. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Lorenc, F.; Peskova, V.; Modlinger, R.; Podrázský, V.; Baláš, M.; Kleinová, D. Effect of Bio-Algeen Preparation on Growth and Mycorrhizal Characteristics of Norway spruce Seedlings. J. Forest. Sci. 2016, 62, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Sharkawy Mahmoud, E.l.; Talaat, E.l.; Rania, A.l.; Ali, M. Effect of plant growth stimulants on alfalfa response to salt stress. Agric. Sci. 2017, 8, 267–291. [Google Scholar]
- Pise, N.M.; Sabale, A.B. Effect of seaweed concentrates on the growth and biochemical constituents of Trigonella foenum-graecum L. J. Phytol. 2010, 2, 50–56. [Google Scholar]
- Fornes, F.; Sanchez-Perale, M.; Guardiola, J.L. Effect of a seaweed extract on the productivity of ‘de Nules’ clementine mandarin and navelina orange. Bot. Mar. 2002, 45, 486–489. [Google Scholar] [CrossRef]
- Abdel-Hafeez, A.A. Effect of pre-harvest spraying with seaweed extract “Acadian” and active dry yeast on “Le Conte” pear (Pyrus leconte, Rehd) fruit quality and cold storability. Ann. Agric. Sci. Moshtohor 2005, 43, 1915–1935. [Google Scholar]
- Abhilash, E.S.; Jacob, J.; Parayil, S.P. Effect of Sea Weed (Caulerpa racemosa) extract on biochemical variations, growth and yield of Vigna mungo. Asia Pacific J. Environ. Ecol. Sustain. Dev. 2013, 1, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Jadhao Ganesh, R.; Chaudhary, D.R.; Khadse, V.A.; Zodape, S.T. Utilization of seaweeds in enhancing productivity and quality of black gram [Vigna Mungo (L.) Hepper] for sustainable agriculture. Indian J. Nat. Prod. Resour. 2015, 6, 16–22. [Google Scholar]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231–234. [Google Scholar] [CrossRef]
- Kumari, S.; Phogat, D.; Sehrawat, K.D.; Choudhary, R.; Rajput, V.D.; Ahlawat, J.; Karunakaran, R.; Minkina, T.; Sehrawat, A.R. The effect of Ascophyllum nodosum extract on the nutraceutical antioxidant potential of Vigna radiata sprout under salt stress. Plants 2021, 10, 1216. [Google Scholar] [CrossRef]
- Sloger, C. Symbiotic effectiveness and N2-fixation in nodulated soybean. Plant Physiol. 1969, 44, 1666–1668. [Google Scholar] [CrossRef] [Green Version]

| Percent Germination on 3rd Day | |||||
|---|---|---|---|---|---|
| Source | SS | df | MS | F | p |
| Treatment | 8706.67 | 4.00 | 2176.67 | 23.32 | <0.0001 |
| Error | 933.33 | 10.00 | 93.33 | ||
| Total | 9640.00 | 14.00 | |||
| 15th Day PRA | 15th Day PFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 253.88 | 5 | 50.78 | 18.80 | 2.6 × 10−10 | 53.92 | 5 | 10.78 | 3.24 | NS |
| Within Groups | 129.63 | 48 | 2.70 | 159.76 | 48 | 3.33 | ||||
| Total | 383.51 | 53 | 213.69 | 53 | ||||||
| 60th Day PRA | 60th Day PFA | |||||||||
| Between Groups | 176.21 | 5 | 35.24 | 6.64 | 9 × 10−5 | 103.21 | 5 | 20.64 | 2.19 | NS |
| Within Groups | 254.90 | 48 | 5.31 | 453.16 | 48 | 9.44 | ||||
| Total | 431.10 | 53 | 556.37 | 53 | ||||||
| PRA | PFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 146.32 | 5 | 29.26 | 11.33 | 0.0003 | 104.47 | 5 | 20.89 | 10.67 | 0.0004 |
| Within Groups | 30.99 | 12 | 2.58 | 23.50 | 12 | 1.96 | ||||
| Total | 177.31 | 17 | 127.97 | 17 | ||||||
| PRA | PFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 114.22 | 5 | 22.84 | 12.31 | <0.0001 | 137.43 | 5 | 27.49 | 8.43 | <0.0001 |
| Within Groups | 89.11 | 48 | 1.86 | 156.44 | 48 | 3.26 | ||||
| Total | 203.33 | 53 | 293.87 | 53 | ||||||
| PRA | PFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | Df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 278.50 | 5 | 55.70 | 1.68 | 0.21 | 69.33 | 5 | 13.87 | 1.92 | 0.16 |
| Within Groups | 398.00 | 12 | 33.17 | 86.67 | 12 | 7.22 | ||||
| Total | 676.50 | 17 | 156.00 | 17 | ||||||
| ANOVA: PRA | |||||||||||||||
| Shoot Organic Content | Shoot Moisture Content | Shoot Moisture Content | |||||||||||||
| Source | SS | Df | MS | F | p | SS | df | MS | F | p | SS | df | MS | F | p |
| B/w Grps | 0.96 | 5 | 0.19 | 9.94 | 0.0006 | 83.7 | 5 | 16.74 | 51.7 | <0.0001 | 2582.74 | 5 | 516.55 | 6.64 | 0.003 |
| Within Grps | 0.23 | 12 | 0.02 | 3.89 | 12 | 0.32 | 933.17 | 12 | 77.76 | ||||||
| Total | 1.20 | 17 | 87.6 | 17 | 3515.91 | 17 | |||||||||
| ANOVA: PFA | |||||||||||||||
| Shoot Organic Content | Shoot Moisture Content | Shoot Moisture Content | |||||||||||||
| Source | SS | df | MS | F | p | SS | df | MS | F | p | SS | df | MS | F | p |
| B/w Grps | 3.96 | 5 | 0.79 | 26.7 | <0.001 | 29.55 | 5 | 5.91 | 51.36 | <0.0001 | 586.48 | 5 | 117.3 | 8.66 | 0.001 |
| Within Grps | 0.36 | 12 | 0.03 | 1.38 | 12 | 0.12 | 162.48 | 12 | 13.54 | ||||||
| Total | 4.32 | 17 | 30.93 | 17 | 748.96 | 17 | |||||||||
| ANOVA: PRA | |||||||||||||||
| Root Organic Content | Root Moisture Content | Root Moisture Content | |||||||||||||
| Source | SS | Df | MS | F | p | SS | df | MS | F | p | SS | df | MS | F | p |
| B/w Grps | 0.15 | 5 | 0.03 | 21.8 | <0.003 | 0.06 | 5 | 0.01 | 3.01 | 0.054 | 1311.44 | 5 | 262.29 | 7.12 | 0.003 |
| Within Grps | 0.02 | 12 | 0.00 | 0.05 | 12 | 0.0 | 442.20 | 12 | 36.85 | ||||||
| Total | 0.17 | 17 | 0.11 | 17 | 1753.64 | 17 | |||||||||
| ANOVA: PFA | |||||||||||||||
| Root Organic Content | Root Moisture Content | Root Moisture Content | |||||||||||||
| Source | SS | df | MS | F | p | SS | df | MS | F | p | SS | df | MS | F | p |
| B/w Grps | 0.10 | 5 | 0.02 | 10.7 | 0.0004 | 0.23 | 5 | 0.05 | 4.09 | 0.02 | 4132.56 | 5 | 826.5 | 3.54 | 0.03 |
| Within Grps | 0.02 | 12 | 0.002 | 0.13 | 12 | 0.01 | 2802.22 | 12 | 233.5 | ||||||
| Total | 0.13 | 17 | 0.36 | 17 | 6934.78 | 17 | |||||||||
| PRA | PFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | Df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 22.28 | 5 | 4.46 | 4.46 | 0.02 | 27.17 | 5 | 5.43 | 12.23 | 0.0002 |
| Within Groups | 12.00 | 12 | 1 | 5.33 | 12 | 0.44 | ||||
| Total | 34.28 | 17 | 32.5 | 17 | ||||||
| Total Chl: PRA | Total Chl: PFA | |||||||||
| Source | SS | Df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 0.05 | 5 | 0.01 | 117,220.8 | <0.0001 | 0.003 | 5 | 0.001 | 5602.03 | <0.0001 |
| Within Groups | 0.00 | 12 | 0.00 | 0.000 | 12 | 0.000 | ||||
| Total | 0.05 | 17 | 0.003 | 17 | ||||||
| Total Carotenoid: PRA | Total Carotenoid: PFA | |||||||||
| Source | SS | Df | MS | F | p | SS | df | MS | F | p |
| Between Groups | 2.87 | 5 | 0.57 | 484,561.80 | <0.0001 | 0.06 | 5 | 0.01 | 14,375.03 | <0.0001 |
| Within Groups | 0.00 | 12 | 0.00 | 0.00 | 12 | 0.00 | ||||
| Total | 2.87 | 17 | 0.06 | 17 | ||||||
| T Test | PFAVs PRA | ||
|---|---|---|---|
| T | df | p | |
| Chla | −720.465 | 2 | <0.0001 |
| Chlb | −667.701 | 2 | <0.0001 |
| Total Chl | −493.448 | 2 | <0.0001 |
| Carotenoid | −627.176 | 2 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, N.; Sehrawat, K.D.; Mundlia, P.; Sehrawat, A.R.; Choudhary, R.; Rajput, V.D.; Minkina, T.; van Hullebusch, E.D.; Siddiqui, M.H.; Alamri, S. Potential Use of Ascophyllum nodosum as a Biostimulant for Improving the Growth Performance of Vigna aconitifolia (Jacq.) Marechal. Plants 2021, 10, 2361. https://doi.org/10.3390/plants10112361
Verma N, Sehrawat KD, Mundlia P, Sehrawat AR, Choudhary R, Rajput VD, Minkina T, van Hullebusch ED, Siddiqui MH, Alamri S. Potential Use of Ascophyllum nodosum as a Biostimulant for Improving the Growth Performance of Vigna aconitifolia (Jacq.) Marechal. Plants. 2021; 10(11):2361. https://doi.org/10.3390/plants10112361
Chicago/Turabian StyleVerma, Nidhi, Krishnan D. Sehrawat, Poonam Mundlia, Anita R. Sehrawat, Ravish Choudhary, Vishnu D. Rajput, Tatiana Minkina, Eric D. van Hullebusch, Manzer H. Siddiqui, and Saud Alamri. 2021. "Potential Use of Ascophyllum nodosum as a Biostimulant for Improving the Growth Performance of Vigna aconitifolia (Jacq.) Marechal" Plants 10, no. 11: 2361. https://doi.org/10.3390/plants10112361








