Recent Advances in Nanomaterials of Group XIV Elements of Periodic Table in Breast Cancer Treatment
Abstract
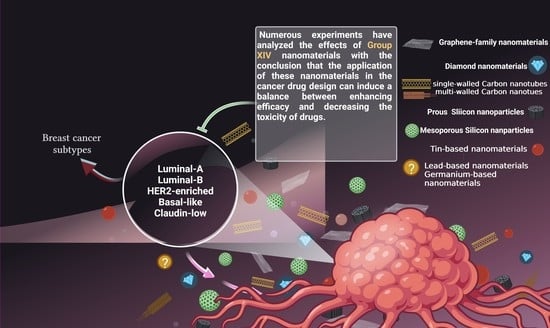
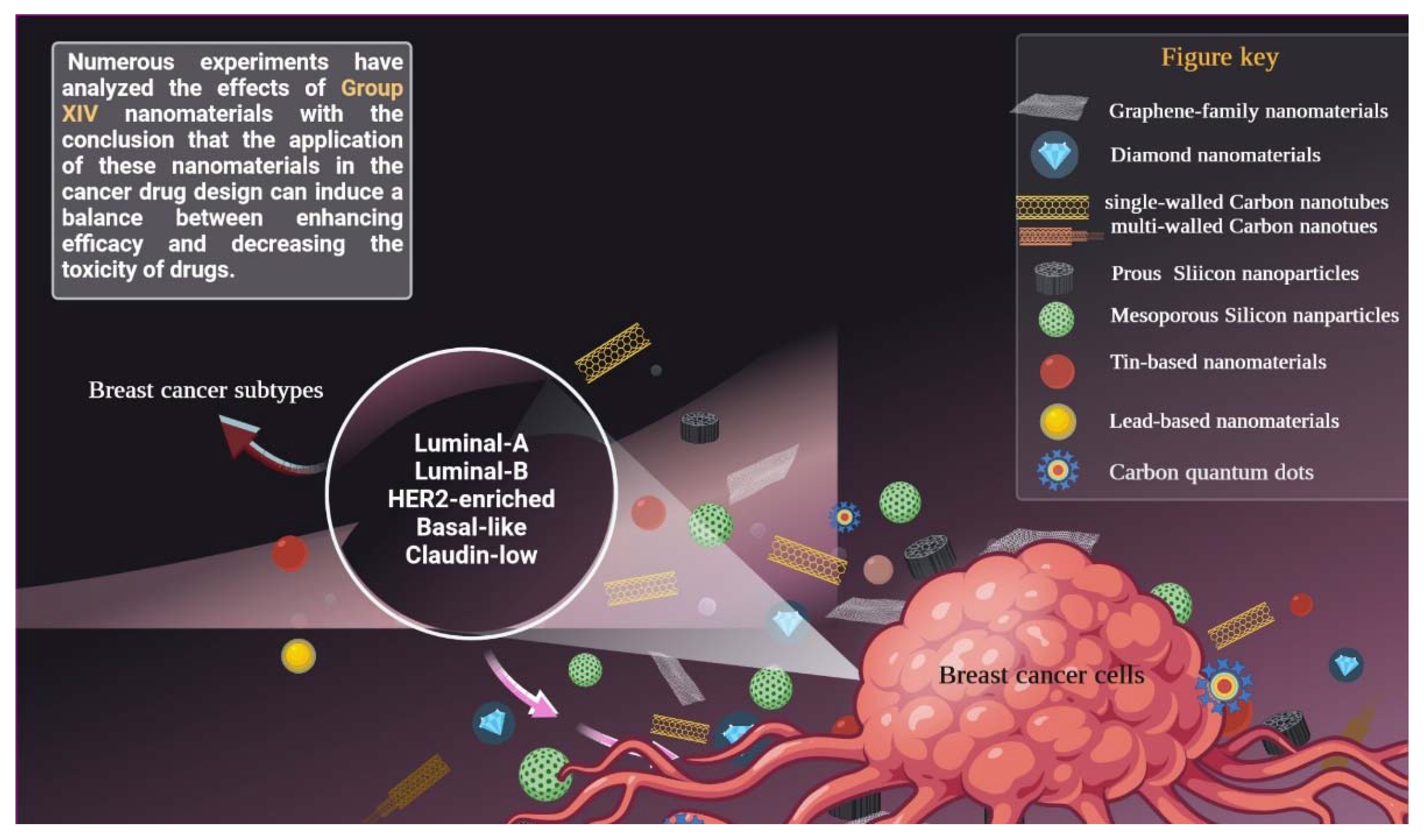
:1. Introduction
2. Breast Cancer Subtypes
3. Breast Cancer Chemotherapy
4. Application of Breast Cancer Cell Lines for Nanopharmaceutical Studies
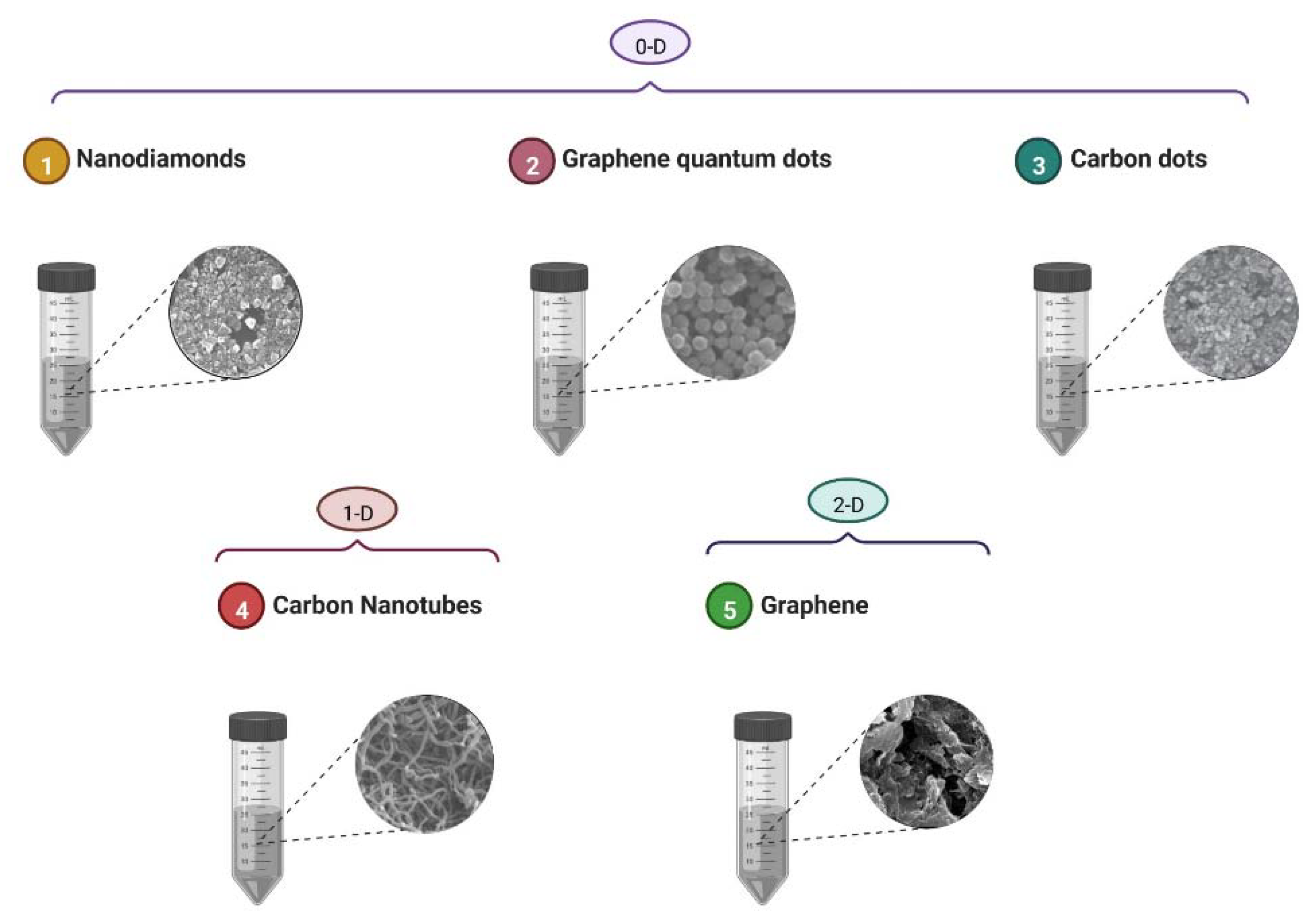
5. Carbon-Based Nanomaterials
5.1. Graphene-Family Nanomaterials
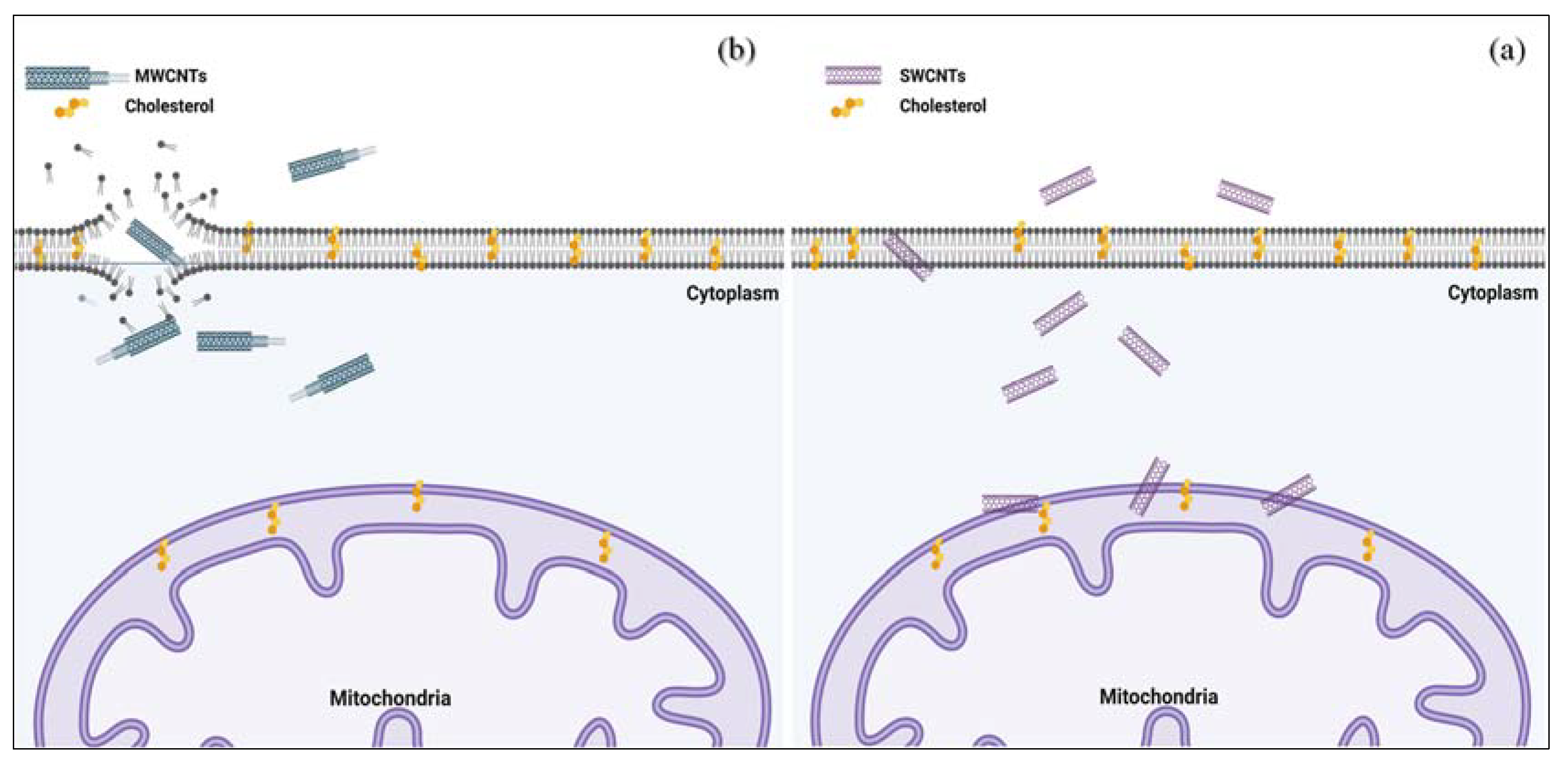
5.2. Carbon Nanotubes
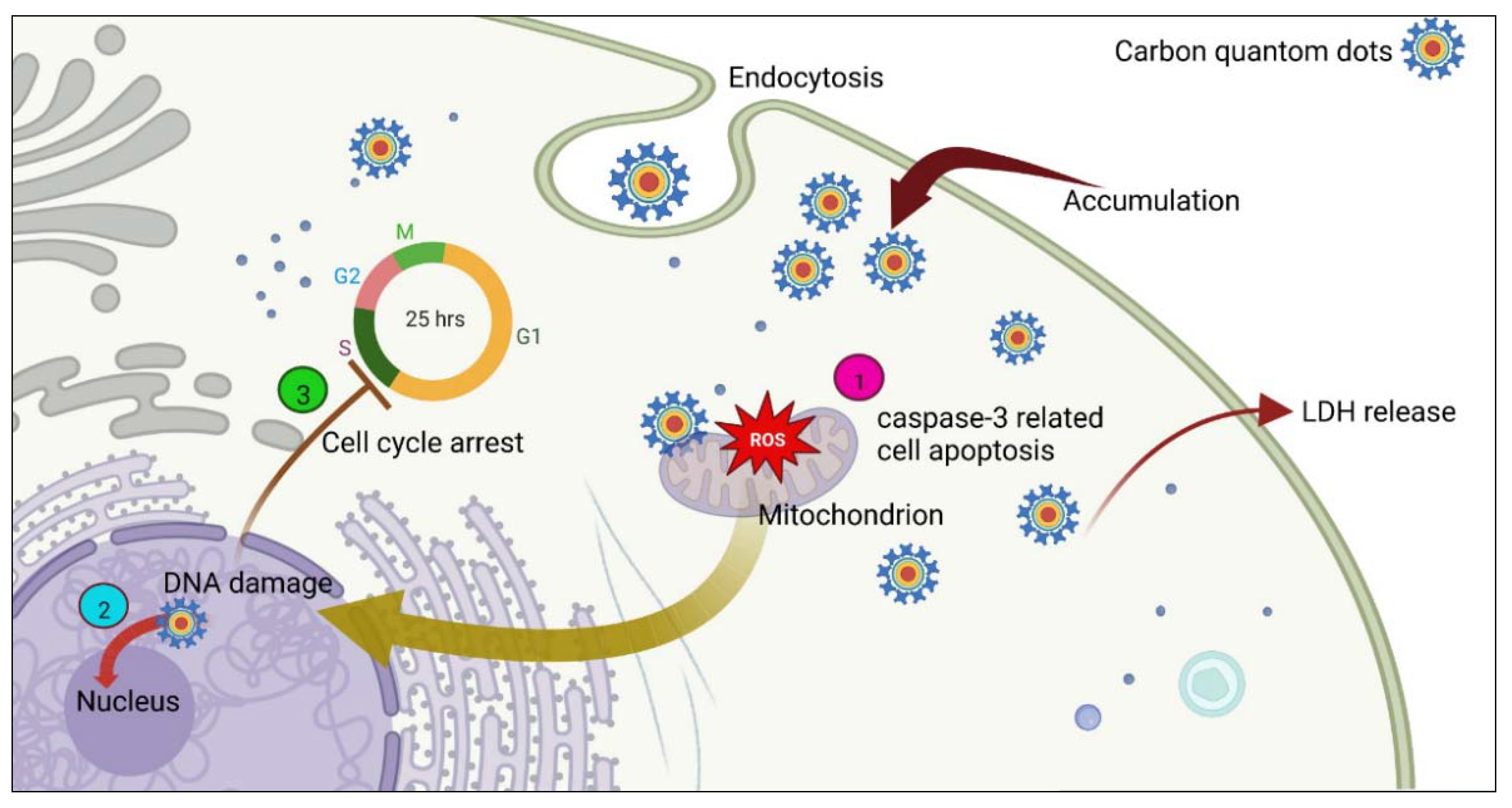
5.3. Carbon Quantum Dots
5.4. Diamond Nanomaterials
6. Silisium (Silicon)-Based Nanomaterials
7. Germanium-Based Nanomaterials
8. Tin-Based Nanomaterials
9. Lead-Based Nanomaterials
10. Mechanisms of Cell Death
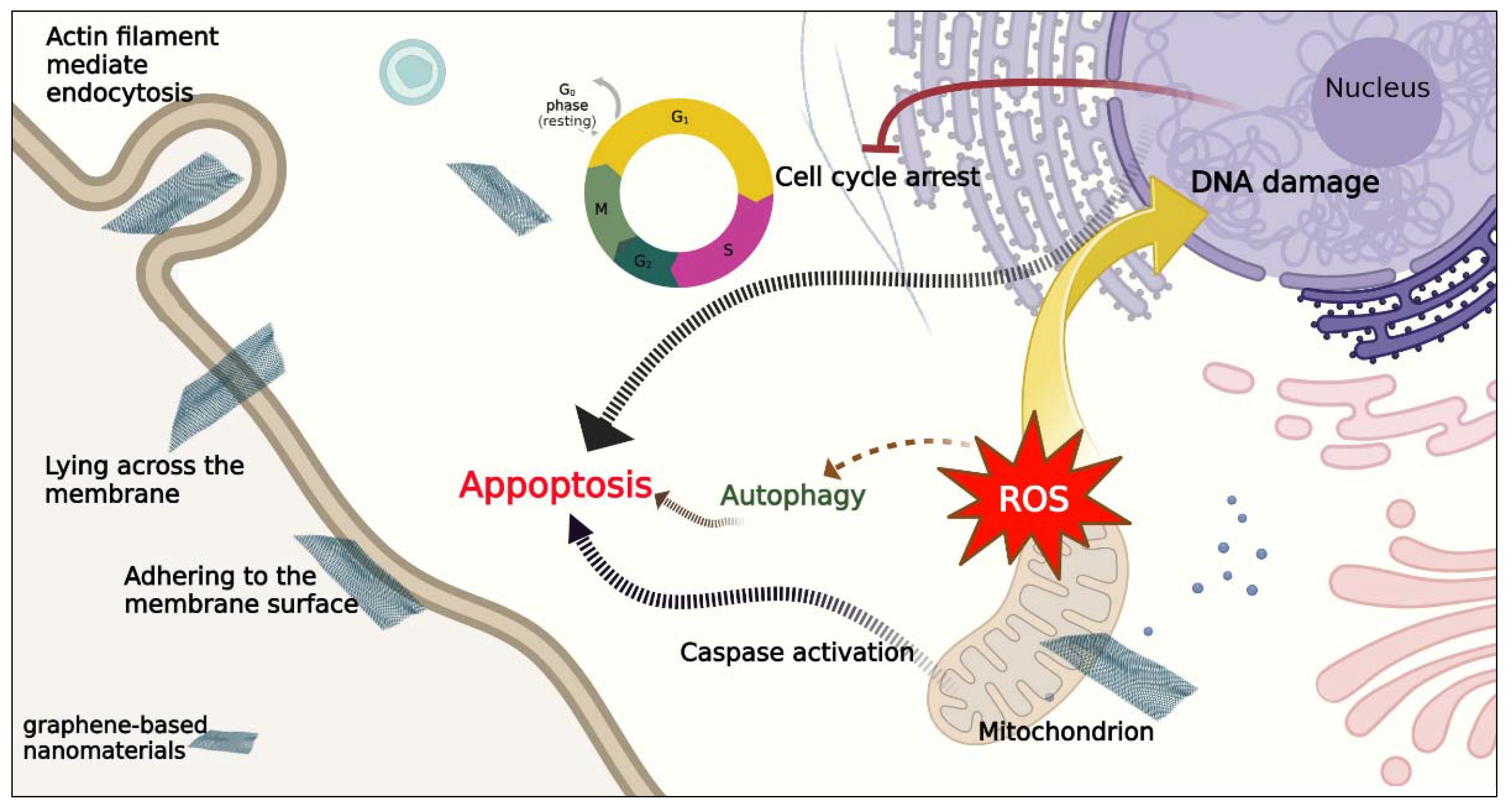
10.1. Most Common Mechanisms of Cell Death Induced by Nanomaterials
10.2. Mechanism of Cell Death Induced by Carbon-Based Nanomaterials
10.3. Mechanism of Cell Death Induced by Silisium-Based Nanomaterials
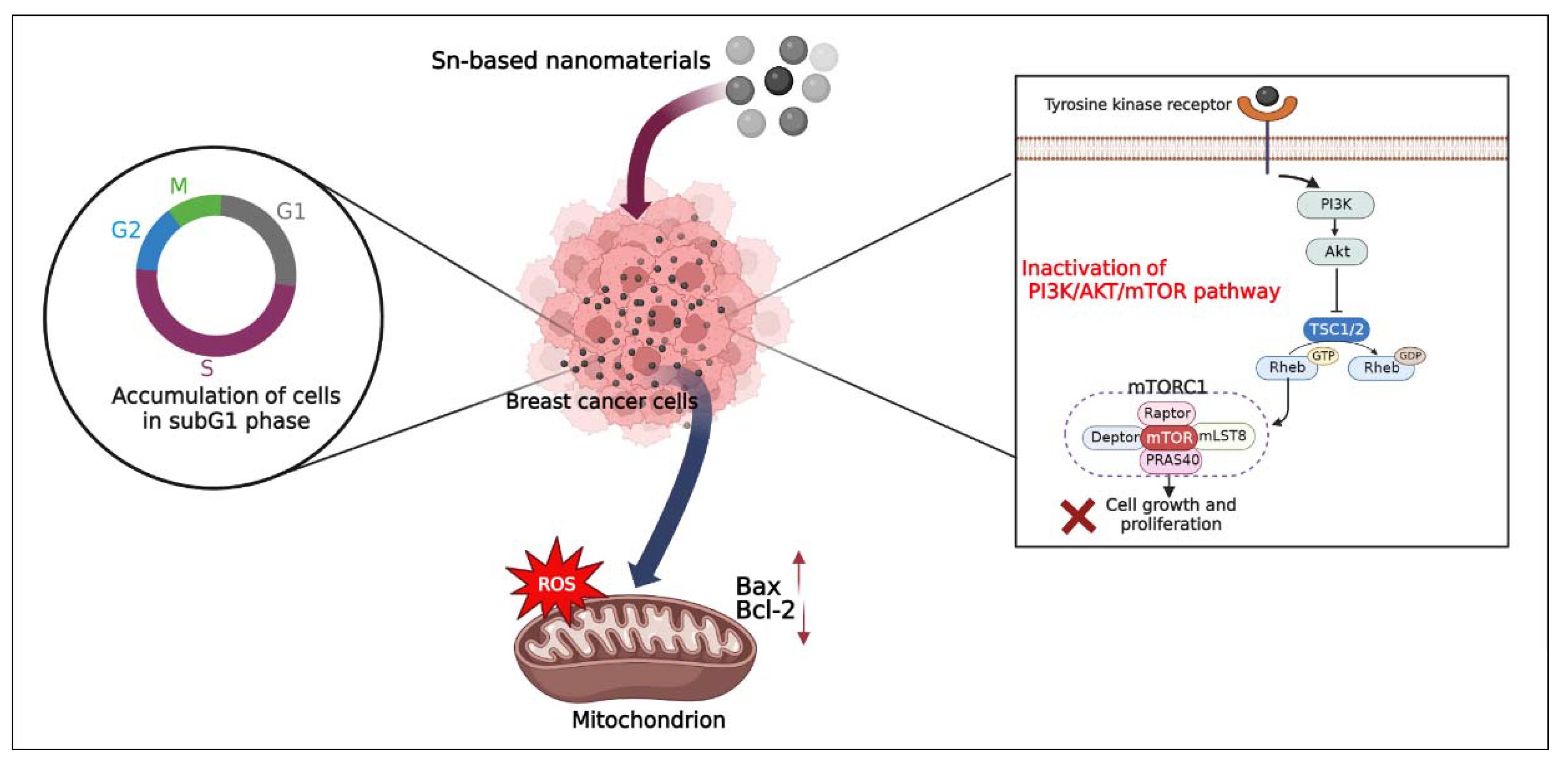
10.4. Mechanism of Cell Death Induced by Tin-Based Nanomaterials
11. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, Z.; Khan, J.A.; Murtaza, S. Nanotechnology: An emerging therapeutic option for breast cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Alharbi, K.S.; Alruwaili, N.K.; Al-Abassi, F.A.; Al-Malki, A.A.L.; Kazmi, I.; Kumar, V.; Kamal, M.A.; Nadeem, M.S.; Aslam, M. Nanomedicine in treatment of breast cancer–A challenge to conventional therapy. Semin. Cancer Biol. 2021, 69, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Lather, V.; Pandita, D. Mesoporous silica nanoparticles: A smart nanosystem for management of breast cancer. Drug Discov. Today 2018, 23, 315–332. [Google Scholar] [CrossRef]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Noor, F.; Noor, A.; Ishaq, A.R.; Farzeen, I.; Saleem, M.H.; Ghaffar, K.; Aslam, M.F.; Aslam, S.; Chen, J.-T. Recent advances in diagnostic and therapeutic approaches for breast cancer: A comprehensive review. Curr. Pharm. Des. 2021, 27, 2344–2365. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Agarwal, P.; Jain, G.K.; Aggarwal, G.; Lather, V.; Pandita, D. Repurposing Drugs as Novel Triple-negative Breast Cancer Therapeutics. Anticancer. Agents Med. Chem. 2022, 22, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019, 84, 689–706. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawy, A.E.-M.; Constantin, C.; Hassan, S.M.; Mostafa, A.M.; Neves, A.F.; De Araújo, T.G.; Neagu, M. Nanomedicine in melanoma: Current trends and future perspectives. In Cutaneous Melanoma: Etiology and Therapy, 1st ed.; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 143–159. [Google Scholar]
- Jain, K.K. Nanopharmaceuticals. The Handbook of Nanomedicine, 1st ed.; Springer: Boston, MA, USA, 2017; pp. 201–271. [Google Scholar]
- Garcia, E.; Shinde, R.; Martinez, S.; Kaushik, A.; Chand, H.S.; Nair, M.; Jayant, R.D. Cell-line-based studies of nanotechnology drug-delivery systems: A brief review. In Nanocarriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery, 1st ed.; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–393. [Google Scholar]
- Crevillen, A.G.; Escarpa, A.; García, C.D. Carbon-based nanomaterials in analytical chemistry. In Carbon-Based Nanomaterials in Analytical Chemistry, 1st ed.; García, C.D., Crevillen, A.G., Escarpa, A., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–36. [Google Scholar]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-based nanomaterials for biomedical applications: A recent study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, I.; Rana, D.; Sarkar, G.; Bhattacharyya, A.; Saha, N.R.; Mondal, S.; Pattanayak, S.; Chattopadhyay, S.; Chattopadhyay, D. Physical and electrochemical characterization of reduced graphene oxide/silver nanocomposites synthesized by adopting a green approach. RSC Adv. 2015, 5, 25357–25364. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Hatamie, S.; Mohamadyar-Toupkanlou, F.; Mirzaei, S.; Ahadian, M.M.; Hosseinzadeh, S.; Soleimani, M.; Sheu, W.-J.; Wei, Z.H.; Hsieh, T.-F.; Chang, W.-C. Cellulose Acetate/Magnetic Graphene Nanofiber in Enhanced Human Mesenchymal Stem Cells Osteogenic Differentiation Under Alternative Current Magnetic Field. SPIN 2019, 9, 1940011. [Google Scholar] [CrossRef]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-conjugated graphene quantum dots as versatile and pH-sensitive nanocarriers for enhanced delivery of curcumin targeting to breast cancer. Mater. Sci. Eng. C 2021, 121, 111809. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical applications of graphene-based structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska, A.; Jaworska, A.; Kasztelan, M.; Berbeć, S.; Pałys, B. Graphene and graphene oxide applications for SERS sensing and imaging. Curr. Med. Chem. 2019, 26, 6878–6895. [Google Scholar] [CrossRef]
- You, P.; Yang, Y.; Wang, M.; Huang, X.; Huang, X. Graphene oxide-based nanocarriers for cancer imaging and drug delivery. Curr. Pharm. Des. 2015, 21, 3215–3222. [Google Scholar] [CrossRef]
- Ribeiro, B.F.; Souza, M.M.; Fernandes, D.S.; do Carmo, D.R.; Machado-Santelli, G.M. Graphene oxide-based nanomaterial interaction with human breast cancer cells. J. Biomed. Mater. Res. A 2020, 108, 863–870. [Google Scholar] [CrossRef]
- Kodous, A.S.; Atta, M.; Abdel-Hamid, G.R.; Ashry, H. Anti-metastatic cancer activity of ultrasonic synthesized reduced graphene oxide/copper composites. Chem. Zvesti. 2022, 76, 373–384. [Google Scholar] [CrossRef]
- Smina, C.; Lalitha, P.; Sharma, S.; Nagabhushana, H. Screening of anti-cancer activity of reduced graphene oxide biogenically synthesized against human breast cancer MCF-7 cell lines. Appl. Nanosci. 2021, 11, 1093–1105. [Google Scholar] [CrossRef]
- Tabrizi, M.; Shahidi, S.-A.; Chekin, F.; Ghorbani-HasanSaraei, A.; Raeisi, S.N. Reduce Graphene Oxide/Fe3O4 Nanocomposite Biosynthesized by Sour Lemon Peel; Using as Electro-catalyst for Fabrication of Vanillin Electrochemical Sensor in Food Products Analysis and Anticancer Activity. Top Catal. 2022, 65, 726–732. [Google Scholar] [CrossRef]
- Liu, H.; Shi, W.; Luo, Y.; Cui, G.; Kong, X.; Han, L.; Zhao, L. Green supported of Au nanoparticles over reduced graphene oxide: Investigation of its cytotoxicity, antioxidant and anti-human breast cancer properties. Inorg. Chem. Commun. 2021, 134, 108918. [Google Scholar] [CrossRef]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Chattopadhyay, D.; Adhikary, A. Folic-Acid-Adorned PEGylated Graphene Oxide Interferes with the Cell Migration of Triple Negative Breast Cancer Cell Line, MDAMB-231 by Targeting miR-21/PTEN Axis through NFκB. ACS Biomater. Sci. Eng. 2018, 5, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Kheiltash, F.; Parivar, K.; Roodbari, N.H.; Sadeghi, B.; Badiei, A. Effects of 8-hydroxyquinoline-coated graphene oxide on cell death and apoptosis in MCF-7 and MCF-10 breast cell lines. Iran J. Basic Med. Sci. 2020, 23, 871–878. [Google Scholar]
- Hekmat, A.; Hatamie, S.; Bakhshi, E. Probing the effects of synthesized silver nanowire/reduced graphene oxide composites on the structure and esterase-like activity of human serum albumin and its impacts on human endometrial stem cells: A new platform in nanomedicine. Nanomed. J. 2021, 8, 42–65. [Google Scholar]
- Athinarayanan, J.; Periasamy, V.S.; Krishnamoorthy, R.; Alshatwi, A.A. Evaluation of antibacterial and cytotoxic properties of green synthesized Cu2O/Graphene nanosheets. Mater. Sci. Eng. C 2018, 93, 242–253. [Google Scholar] [CrossRef]
- Diaz-Diestra, D.; Thapa, B.; Badillo-Diaz, D.; Beltran-Huarac, J.; Morell, G.; Weiner, B.R. Graphene oxide/ZnS: Mn nanocomposite functionalized with folic acid as a nontoxic and effective theranostic platform for breast cancer treatment. Nanomaterials 2018, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Quagliarini, E.; Di Santo, R.; Pozzi, D.; Tentori, P.; Cardarelli, F.; Caracciolo, G. Mechanistic insights into the release of doxorubicin from graphene oxide in cancer cells. Nanomaterials 2020, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Molaparast, M.; Malekinejad, H.; Rahimi, M.; Shafiei-Irannejad, V. Biocompatible functionalized graphene nanosheet for delivery of doxorubicin to breast cancer cells. J. Drug. Deliv. Sci. Technol. 2022, 70, 103234. [Google Scholar] [CrossRef]
- Hatamie, S.; Akhavan, O.; Sadrnezhaad, S.K.; Ahadian, M.M.; Shirolkar, M.M.; Wang, H.Q. Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells. Mater. Sci. Eng. C 2015, 55, 482–489. [Google Scholar] [CrossRef]
- Sanad, M.F.; Shalan, A.E.; Bazid, S.M.; Serea, E.S.A.; Hashem, E.M.; Nabih, S.; Ahsan, M.A. A graphene gold nanocomposite-based 5-FU drug and the enhancement of the MCF-7 cell line treatment. RSC Adv. 2019, 9, 31021–31029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Kaushik, D.; Verma, R.; Bhatt, S.; Sahoo, B.M.; Bhattacharya, T.; Alshehri, S. Quantum Dots: An Emerging Approach for Cancer Therapy. Front. Mater. 2022, 8, 798440. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Mousavi, F. Carbon nanomaterials for electroanalysis in pharmaceutical applications. In Fullerens, Graphenes and Nanotubes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–225. [Google Scholar]
- Kalluri, A.; Debnath, D.; Dharmadhikari, B.; Patra, P. Graphene quantum dots: Synthesis and applications. Methods in Enzymology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 609, pp. 335–354. [Google Scholar]
- Sui, X.; Luo, C.; Wang, C.; Zhang, F.; Zhang, J.; Guo, S. Graphene quantum dots enhance anticancer activity of cisplatin via increasing its cellular and nuclear uptake. Nanomedicine 2016, 12, 1997–2006. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Photodynamic therapy using graphene quantum dot derivatives. J. Solid State Chem. 2020, 282, 121107. [Google Scholar] [CrossRef]
- Ghafary, S.M.; Rahimjazi, E.; Hamzehil, H.; Mousavi, S.M.M.; Nikkhah, M.; Hosseinkhani, S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots. Nanomedicine 2022, 42, 102544. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Q.; Hua, C.; Hu, J.; Chen, B.; Wan, J.; Hu, Z.; Wang, B. pH-responsive nanoparticles loaded with graphene quantum dots and doxorubicin for intracellular imaging, drug delivery and efficient cancer therapy. ChemistrySelect 2019, 4, 6004–6012. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, X.; Ma, K.; Niu, X.; Grothe, J.; Xu, Q.; Liu, L.; Kaskel, S.; Zhu, Y. Metal–organic framework/graphene quantum dot nanoparticles used for synergistic chemo-and photothermal therapy. ACS Omega 2017, 2, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Jin, Z.; Han, C.; Li, J.; Xu, H.; Park, S.; Park, J.-O.; Choi, E.; Xu, K. Graphene quantum dots-decorated hollow copper sulfide nanoparticles for controlled intracellular drug release and enhanced photothermal-chemotherapy. J. Mater. Sci. 2020, 55, 1184–1197. [Google Scholar] [CrossRef]
- Ko, N.; Nafiujjaman, M.; Lee, J.; Lim, H.-N.; Lee, Y.-k.; Kwon, I. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef] [Green Version]
- Khodadadei, F.; Safarian, S.; Ghanbari, N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater. Sci. Eng. C 2017, 79, 280–285. [Google Scholar] [CrossRef]
- Yao, X.; Qian, C.; Zhong, Y.; Sun, S.; Xu, H.; Yang, D. In vivo targeting of breast cancer with peptide functionalized GQDs/hMSN nanoplatform. J. Nanopart. Res. 2019, 21, 263. [Google Scholar] [CrossRef]
- Ko, N.R.; Van, S.Y.; Hong, S.H.; Kim, S.-Y.; Kim, M.; Lee, J.S.; Lee, S.J.; Lee, Y.-k.; Kwon, I.K.; Oh, S.J. Dual pH-and GSH-responsive degradable PEGylated graphene quantum dot-based nanoparticles for enhanced HER2-positive breast cancer therapy. Nanomaterials 2020, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Tade, R.S.; Patil, P.O. Theranostic prospects of graphene quantum dots in breast cancer. ACS Biomater. Sci. Eng. 2020, 6, 5987–6008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, Q.; Jinruan, J.; Fang, J. Biomolecular Interactions and Application of Carbon Nanotubes in Nanomedicine. Austin J. Anal. Pharm. Chem. 2016, 1, 1005. [Google Scholar]
- Zeinabad, H.A.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of single and multi wall carbon nanotubes with the biological systems: Tau protein and PC12 cells as targets. Sci. Rep. 2016, 6, 26508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethuraman, A.; Stroscio, M.A.; Dutta, M. Potential applications of carbon nanotubes in bioengineering. Biological Nanostructures and Applications of Nanostructures in Biology, 1st ed.; Stroscio, M.A., Dutta, M., Eds.; Springer: Boston, MA, USA, 2004; pp. 51–68. [Google Scholar]
- Raza, K.; Kumar, D.; Kiran, C.; Kumar, M.; Guru, S.K.; Kumar, P.; Arora, S.; Sharma, G.; Bhushan, S.; Katare, O. Conjugation of docetaxel with multiwalled carbon nanotubes and codelivery with piperine: Implications on pharmacokinetic profile and anticancer activity. Mol. Pharm. 2016, 13, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Shin, C.; Memic, A.; Shadmehr, S.; Miscuglio, M.; Jung, H.Y.; Jung, S.M.; Bae, H.; Khademhosseini, A.; Tang, X. Aligned carbon nanotube–based flexible gel substrates for engineering biohybrid tissue actuators. Adv. Funct. Mater. 2015, 25, 4486–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. ChemComm 2012, 48, 3911–3926. [Google Scholar] [CrossRef] [PubMed]
- Dizaji, B.F.; Farboudi, A.; Rahbar, A.; Azarbaijan, M.H.; Asgary, M.R. The role of single-and multi-walled carbon nanotube in breast cancer treatment. Ther. Deliv. 2020, 11, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, A.; Hosseini Ghale Noei, S.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 2018, 8, 8375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellucci, S.; Dinicola, S.; Coluccia, P.; Bizzarri, M.; Catizone, A.; Micciulla, F.; Sacco, I.; Ricci, G.; Cucina, A. Multiwalled carbon nanotubes-induced cytotoxic effects on human breast adenocarcinoma cell line. In Proceedings of the Conference: 2012 International Semiconductor Conference (CAS 2012), Sinaia, Romania, 15–17 October 2012; pp. 37–42. [Google Scholar]
- Chiaretti, M.; Mazzanti, G.; Bosco, S.; Bellucci, S.; Cucina, A.; Le Foche, F.; Carru, G.; Mastrangelo, S.; Di Sotto, A.; Masciangelo, R. Carbon nanotubes toxicology and effects on metabolism and immunological modification in vitro and in vivo. J. Condens. Matter. Phys. 2008, 20, 474203. [Google Scholar] [CrossRef]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaik, A.S.; Shaik, A.P.; Bammidi, V.K.; Al Faraj, A. Effect of polyethylene glycol surface charge functionalization of SWCNT on the in vitro and in vivo nanotoxicity and biodistribution monitored noninvasively using MRI. Toxicol. Mech. Methods 2019, 29, 233–243. [Google Scholar] [CrossRef]
- Ünlü, A.; Meran, M.; Dinc, B.; Karatepe, N.; Bektaş, M.; Güner, F.S. Cytotoxicity of doxrubicin loaded single-walled carbon nanotubes. Mol. Biol. Rep. 2018, 45, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Wang, J.; Fan, L.; Zhu, W.; Cai, D. Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Die Pharmazie-Int. J. Pharm. Sci. 2019, 74, 83–90. [Google Scholar]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J Control Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Chen, L.; Li, Q.; Du, J.; Gao, Y.; Zhang, L.; Yang, Y. Antitumor effects of carbon nanotube-drug complex against human breast cancer cells. Exp. Ther. Med. 2018, 16, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Wang, H.; Ji, D. Carbon nanotubes (CNT)-loaded ginsenosides Rb3 suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer. Aging 2021, 13, 17177. [Google Scholar] [CrossRef]
- Badea, M.A.; Prodana, M.; Dinischiotu, A.; Crihana, C.; Ionita, D.; Balas, M. Cisplatin loaded multiwalled carbon nanotubes induce resistance in triple negative breast cancer cells. Pharmaceutics 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shahawy, A.A.; Elnagar, N.; Zohery, M.; Abd Elhafeez, M.S.; El-Dek, S. Smart nanocarrier-based chitosan@ silica coated carbon nanotubes composite for breast cancer treatment approach. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 910–922. [Google Scholar] [CrossRef]
- Biagiotti, G.; Pisaneschi, F.; Gammon, S.T.; Machetti, F.; Ligi, M.C.; Giambastiani, G.; Tuci, G.; Powell, E.; Piwnica-Worms, H.; Pranzini, E. Multiwalled carbon nanotubes for combination therapy: A biodistribution and efficacy pilot study. J. Mater. Chem. B 2019, 7, 2678–2687. [Google Scholar] [CrossRef]
- Sharma, S.; Naskar, S.; Kuotsu, K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization and in vitro toxicity in human breast cancer. Carbon Lett. 2020, 30, 435–447. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, Y.; Xu, B.; Zhang, S.; Liu, J.; Zhang, T.; Jin, L.; Song, S.; Zhang, H. Rapidly clearable MnCo2O4@ PAA as novel nanotheranostic agents for T1/T2 bimodal MRI imaging-guided photothermal therapy. Nanoscale 2021, 13, 16251–16257. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Zhang, J.; Gao, M.; Wu, D.; Yang, Y.; Liu, R. A facile hydrothermal approach towards photoluminescent carbon dots from amino acids. J. Colloid. Interface Sci. 2015, 439, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Vinothini, K.; Ramesh, T.; Rajan, M.; Ramu, A. Combined photodynamic-chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Deliv. 2020, 27, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots-quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin delivery to breast cancer cells with transferrin-targeted carbon quantum dots: An in vitro and in silico study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Naik, K.; Chaudhary, S.; Ye, L.; Parmar, A.S. A Strategic Review on Carbon Quantum Dots for Cancer-Diagnostics and Treatment. Front. Bioeng. Biotechnol. 2022, 10, 882100. [Google Scholar] [CrossRef] [PubMed]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A sustainable green synthesis of functionalized biocompatible carbon quantum dots from Aloe barbadensis Miller and its multifunctional applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, J.; Song, Y.; Zhao, H.; Wei, Z.; Li, X.; Jin, Y.; Yang, B.; Jiang, J. Synthesis of ginsenoside Re-based carbon dots applied for bioimaging and effective inhibition of cancer cells. Int. J. Nanomed. 2018, 13, 6249–6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkan, E.; Barati, A.; Rahmanpanah, M.; Hosseinzadeh, L.; Moradi, S.; Hajialyani, M. Green synthesis of carbon dots derived from walnut oil and an investigation of their cytotoxic and apoptogenic activities toward cancer cells. Adv. Pharm. Bull. 2018, 8, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Şimşek, S.; Şüküroğlu, A.A.; Yetkin, D.; Özbek, B.; Battal, D.; Genç, R. DNA-damage and cell cycle arrest initiated anti-cancer potency of super tiny carbon dots on MCF7 cell line. Sci. Rep. 2020, 10, 13880. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chitanda, J.M.; Michel, D.; Maley, J.; Borondics, F.; Yang, P.; Verrall, R.E.; Badea, I. Lysine-functionalized nanodiamonds: Synthesis, physiochemical characterization, and nucleic acid binding studies. Int. J. Nanomed. 2012, 7, 3851–3866. [Google Scholar]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar]
- Balek, L.; Buchtova, M.; Bosakova, M.K.; Varecha, M.; Foldynova-Trantirkova, S.; Gudernova, I.; Vesela, I.; Havlik, J.; Neburkova, J.; Turner, S. Nanodiamonds as “artificial proteins”: Regulation of a cell signalling system using low nanomolar solutions of inorganic nanocrystals. Biomaterials 2018, 176, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.; Salavati, F.; Hesami Tackallou, S. The effects of paclitaxel in the combination of diamond nanoparticles on the structure of human serum albumin (HSA) and their antiproliferative role on MDA-MB-231cells. Protein J. 2020, 39, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Application of glycosylation in targeted drug delivery. Eur. J. Med. Chem. 2019, 182, 111612. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, M.; Lai, H.; Lu, H.; Lalevée, J.; Barner-Kowollik, C.; Stenzel, M.H.; Xiao, P. Delivery of amonafide from fructose-coated nanodiamonds by oxime ligation for the treatment of human breast cancer. Biomacromolecules 2018, 19, 481–489. [Google Scholar] [CrossRef]
- Lai, H.; Lu, M.; Chen, F.; Lalevee, J.; Stenzel, M.; Xiao, P. Amphiphilic polymer coated nanodiamonds: A promising platform to deliver azonafide. Polym. Chem. 2019, 10, 1904–1911. [Google Scholar] [CrossRef]
- Daniluk, K.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Strojny, B.; Szczepaniak, J.; Bałaban, J.; Sosnowska, M.; Chwalibog, A.; Sawosz, E. Use of selected carbon nanoparticles as melittin carriers for MCF-7 and MDA-MB-231 human breast cancer cells. Materials 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.-S.; Ho, Y.; Lin, Y.-W.; Raj, E.N.; Liu, K.-K.; Chen, C.; Zhou, X.-Z.; Lu, K.-P.; Chao, J.-I. Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel-and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019, 86, 395–405. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Xu, Y.-H.; Wang, C.; An, H.-C.; Xu, H.-Z.; Li, K.; Komatsu, N.; Zhao, L.; Chen, X. Doxorubicin-polyglycerol-nanodiamond conjugate is a cytostatic agent that evades chemoresistance and reverses cancer-induced immunosuppression in triple-negative breast cancer. J. Nanobiotechnol. 2019, 17, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.J.; Lee, C.Y.; Lin, Y.C.; Lin, C.Y.; Perevedentseva, E.; Hung, S.F.; Cheng, C.L. Phagocytosis and immune response studies of Macrophage-Nanodiamond Interactions in vitro and in vivo. J. Biophotonics 2017, 10, 1315–1326. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond particles: Properties and perspectives for bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Chen, M.; Zuo, X.; Xu, Q.; Wang, R.; Fan, S.; Wu, H. Investigating the interaction of nanodiamonds with human serum albumin and induced cytotoxicity. J. Spectrosc. 2019, 2019, 4503137. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Hong, S.-F.; Huang, K.-J.; Chiang, I.-T.; Lee, C.-Y.; Tseng, Y.-T.; Cheng, C.-L. Nanodiamond internalization in cells and the cell uptake mechanism. J. Nanopart. Res. 2013, 15, 1834. [Google Scholar] [CrossRef]
- He, Y.; Fan, C.; Lee, S.-T. Silicon nanostructures for bioapplications. Nano Today 2010, 5, 282–295. [Google Scholar] [CrossRef]
- Ouhibi, A.; Raouafi, A.; Lorrain, N.; Guendouz, M.; Raouafi, N.; Moadhen, A. Functionalized SERS substrate based on silicon nanowires for rapid detection of prostate specific antigen. Sens. Actuators B Chem. 2021, 330, 129352. [Google Scholar] [CrossRef]
- Paredes, K.O.; Díaz-García, D.; Mena-Palomo, I.; Marciello, M.; Chamizo, L.L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a theranostic platform based on fibrous silica nanoparticles for the enhanced treatment of triple-negative breast cancer promoted by a combination of chemotherapeutic agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, R.; Liu, W.; Shen, P.; Tang, Y.; Luo, J.; Zhang, W.; Jia, G.; Wang, Y.; Zhao, S. Preparation of chitosan-silicon dioxide/BCSG1-siRNA nanoparticles to enhance therapeutic efficacy in breast cancer cells. Mol. Med. Rep. 2018, 17, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Su, Y. Silicon nano-biotechnology, 1st ed.; Springer: Boston, MA, USA, 2014; pp. 1–38. [Google Scholar]
- Zhang, Y.; Xu, J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R Soc. Open Sci. 2018, 5, 170986. [Google Scholar] [CrossRef] [Green Version]
- Lohiya, G.; Katti, D.S. Mesoporous Silica Nanoparticle-Based Combination of Niclosamide and Doxorubicin: Effect of Treatment Regimens on Breast Cancer Subtypes. ACS Appl. Bio Mater. 2021, 4, 7811–7824. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, S.; Hu, Y.; Yang, F.; Huo, Q.; Xie, N. Tumour-targeted and redox-responsive mesoporous silica nanoparticles for controlled release of doxorubicin and an siRNA against metastatic breast cancer. Int. J. Nanomed. 2021, 16, 1961–1976. [Google Scholar] [CrossRef]
- Konoplyannikov, M.; Eremina, A.; Kargina, Y.V.; Le-Deygen, I.; Kharin, A.Y.; Bazylenko, T.Y.; Yusubalieva, G.; Revkova, V.; Matchuk, O.; Zamulaeva, I. Mesoporous silicon nanoparticles loaded with salinomycin for cancer therapy applications. Microporous Mesoporous Mater. 2021, 328, 111473. [Google Scholar] [CrossRef]
- Mohan Viswanathan, T.; Krishnakumar, V.; Senthilkumar, D.; Chitradevi, K.; Vijayabhaskar, R.; Rajesh Kannan, V.; Senthil Kumar, N.; Sundar, K.; Kunjiappan, S.; Babkiewicz, E. Combinatorial Delivery of Gallium (III) Nitrate and Curcumin Complex-Loaded Hollow Mesoporous Silica Nanoparticles for Breast Cancer Treatment. Nanomater 2022, 12, 1472. [Google Scholar] [CrossRef]
- Landgraf, M.; Lahr, C.A.; Kaur, I.; Shafiee, A.; Sanchez-Herrero, A.; Janowicz, P.W.; Ravichandran, A.; Howard, C.B.; Cifuentes-Rius, A.; McGovern, J.A. Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis. Biomaterials 2020, 240, 119791. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, L.; Deng, Y.; Zhang, C.; Zhang, Y.; Xiong, S.; Ding, C.; Zhao, J.; Liao, C.; Gong, D. A review of public and environmental consequences of organic germanium. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1384–1409. [Google Scholar] [CrossRef]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in effect of germanium or germanium compounds on animals—A review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef] [Green Version]
- Keith, L.S.; Faroon, O.M.; Maples-Reynolds, N.; Fowler, B.A. Germanium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, N., Fowler, B., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 799–816. [Google Scholar]
- Ma, Y.-H.; Huang, C.-P.; Tsai, J.-S.; Shen, M.-Y.; Li, Y.-K.; Lin, L.-Y. Water-soluble germanium nanoparticles cause necrotic cell death and the damage can be attenuated by blocking the transduction of necrotic signaling pathway. Toxicol. Lett. 2011, 207, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Bezuidenhout, M.; Liu, P.; Singh, S.; Kiely, M.; Ryan, K.M.; Kiely, P.A. Promoting cell proliferation using water dispersible germanium nanowires. PLoS ONE 2014, 9, e108006. [Google Scholar] [CrossRef] [PubMed]
- McVey, B.F.; Prabakar, S.; Gooding, J.J.; Tilley, R.D. Solution synthesis, surface passivation, optical properties, biomedical applications, and cytotoxicity of silicon and germanium nanocrystals. ChemPlusChem 2017, 82, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zong, M.; Xu, D.; Chen, Z.; Yang, J.; Yao, H.; Wei, C.; Chen, Y.; Lin, H.; Shi, J. Freestanding germanene nanosheets for rapid degradation and photothermal conversion. Mater. Today Nano 2021, 15, 100119. [Google Scholar] [CrossRef]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic nanoparticle inks for 3D printing of electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem Mater 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Zou, X.; Yang, F.; Sun, X.; Qin, M.; Zhao, Y.; Zhang, Z. Functionalized nano-adsorbent for affinity separation of proteins. Nanoscale Res. Lett. 2018, 13, 165. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Ravoof, T.B.; Page, A.J. Cytotoxicity of Tin (IV)-based compounds: A review. Polyhedron 2021, 198, 115069. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Zhao, X.; Song, S.; Qian, B. Exploring the anticancer effects of tin oxide nanoparticles synthesized by pulsed laser ablation technique against breast cancer cell line through downregulation of PI3K/AKT/mTOR signaling pathway. Arab. J. Chem. 2021, 14, 103212. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A. Oxidative stress mediated cytotoxicity of tin (IV) oxide (SnO2) nanoparticles in human breast cancer (MCF-7) cells. Colloids Surf. B 2018, 172, 152–160. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, N.; Mehrotra, T.; Pervaiz, N.; Agrawal, L.; Tripathi, S.; Jha, A.; Poullikkas, T.; Kumar, R.; Ledwani, L. In vitro and in silico molecular docking studies of Rheum emodi-derived diamagnetic SnO2 nanoparticles and their cytotoxic effects against breast cancer. New J. Chem. 2021, 45, 1695–1711. [Google Scholar] [CrossRef]
- Khan, S.A.; Kanwal, S.; Rizwan, K.; Shahid, S. Enhanced antimicrobial, antioxidant, in vivo antitumor and in vitro anticancer effects against breast cancer cell line by green synthesized un-doped SnO2 and Co-doped SnO2 nanoparticles from Clerodendrum inerme. Microb. Pathogen 2018, 125, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Shi, C.; Hu, Y.; Xu, Z.; Wang, R. Anti-breast carcinoma effects of green synthesized tin nanoparticles from Calendula officinalis leaf aqueous extract inhibits MCF7, Hs 319. T, and MCF10 cells proliferation. J. Exp. Nanosci. 2022, 17, 351–361. [Google Scholar] [CrossRef]
- Dudev, T.; Grauffel, C.; Lim, C. How Pb2+ binds and modulates properties of Ca2+-signaling proteins. Inorg. Chem. 2018, 57, 14798–14809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Saran, R.; Liu, J. Metal sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. [Google Scholar] [CrossRef] [Green Version]
- Gui, S.; Huang, Y.; Zhu, Y.; Jin, Y.; Zhao, R. Biomimetic Sensing System for Tracing Pb2+ Distribution in Living Cells Based on the Metal–Peptide Supramolecular Assembly. ACS Appl. Mater. Interfaces 2019, 11, 5804–5811. [Google Scholar] [CrossRef]
- Chiu, T.-Y.; Yang, D.-M. Intracellular Pb2+ content monitoring using a protein-based Pb2+ indicator. Toxicol. Sci. 2012, 126, 436–445. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Liu, C.; Luo, Y.; Chen, J.; Fu, Y.; Xu, Y.; Wu, H.; Li, X.; Wang, H. Relationships Between Biological Heavy Metals and Breast Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 838762. [Google Scholar] [CrossRef]
- Amiri, A.; Mohammadi, M.; Shabani, M. Synthesis and toxicity evaluation of lead oxide (PbO) nanoparticles in rats. eJBio 2016, 12, 110–114. [Google Scholar]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Jan, S.A.; Shinwari, Z.K.; Maaza, M. Bioinspired synthesis of pure massicot phase lead oxide nanoparticles and assessment of their biocompatibility, cytotoxicity and in-vitro biological properties. Arab. J. Chem. 2020, 13, 916–931. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
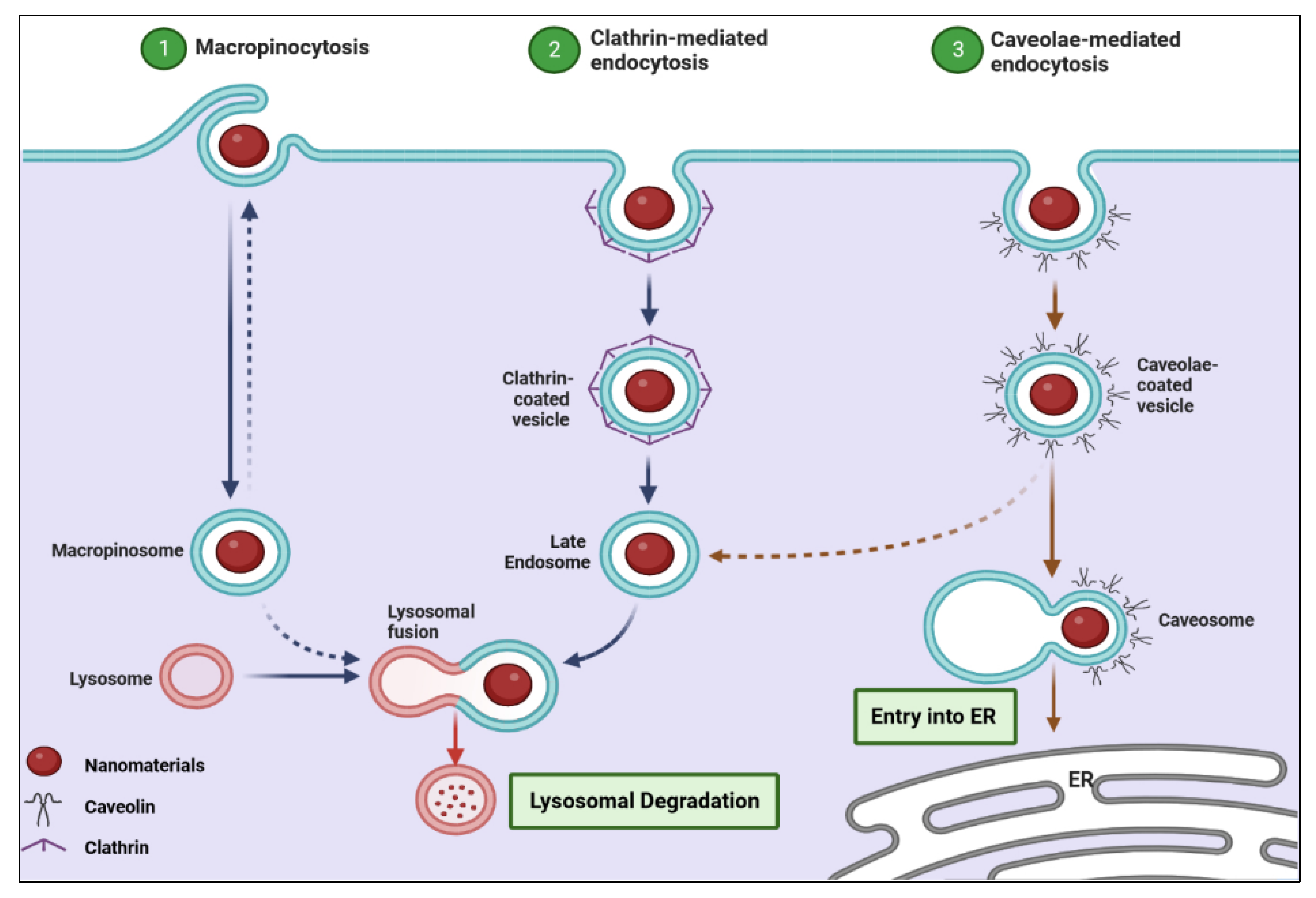
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Häfeli, U.O.; Mahmoudi, M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS ONE 2012, 7, e29997. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. React Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Hirano, S.; Kanno, S.; Furuyama, A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol. Appl. Pharmacol. 2008, 232, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz Jr, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.; Saboury, A.A. Protein kinase inhibitors and cancer targeted therapy. In Protein Kinase Inhibitors From Discovery to Therapeutics, 1st ed.; Hassan, M.I., Noor, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–70. [Google Scholar]
- Ali, S.; Alam, M.; Hassan, M.I. Kinase inhibitors: An overview. In Protein Kinase Inhibitors From Discovery to Therapeutics, 1st ed.; Hassan, M.I., Noor, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–22. [Google Scholar]
- Boutouja, F.; Stiehm, C.M.; Platta, H.W. mTOR: A cellular regulator interface in health and disease. Cells 2019, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Achazi, K.; Schulz, A.; Mülhaupt, R.; Thierbach, S.; Rühl, E.; Adeli, M.; Haag, R. Combination of surface charge and size controls the cellular uptake of functionalized graphene sheets. Adv. Funct. Mater. 2017, 27, 1701837. [Google Scholar] [CrossRef]
- Shaheen, F.; Aziz, M.H.; Fatima, M.; Khan, M.A.; Ahmed, F.; Ahmad, R.; Ahmad, M.A.; Alkhuraiji, T.S.; Akram, M.W.; Raza, R. In Vitro cytotoxicity and morphological assessments of GO-ZnO against the MCF-7 Cells: Determination of singlet oxygen by chemical trapping. Nanomaterials 2018, 8, 539. [Google Scholar] [CrossRef]
- Adil, S.F.; Shaik, M.R.; Nasr, F.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Kuniyil, M.; Almutairi, A.; Alwarthan, A.; Siddiqui, M.R.H. Enhanced apoptosis by functionalized highly reduced graphene oxide and gold nanocomposites in MCF-7 breast cancer cells. ACS Omega 2021, 6, 15147–15155. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, S.; Maheshwari, V.; Liu, J. DNA adsorbed on graphene and graphene oxide: Fundamental interactions, desorption and applications. Curr. Opin. Colloid Interface Sci. 2016, 26, 41–49. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, Q.; Ouyang, S. Direct and indirect genotoxicity of graphene family nanomaterials on DNA—A review. Nanomaterials 2021, 11, 2889. [Google Scholar] [CrossRef] [PubMed]
- Krętowski, R.; Cechowska-Pasko, M. The Reduced Graphene Oxide (rGO) Induces Apoptosis, Autophagy and Cell Cycle Arrest in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9285. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Guo, L.; Zhang, F.; Liu, H.; Zhang, J.; Zheng, J.; Zhang, J.; Guo, S. Graphene quantum dots downregulate multiple multidrug-resistant genes via interacting with their C-rich promoters. Adv. Healthc. Mater. 2017, 6, 1700328. [Google Scholar] [CrossRef] [PubMed]
- Knopfová, L.; Beneš, P.; Pekarčíková, L.; Hermanová, M.; Masařík, M.; Pernicová, Z.; Souček, K.; Šmarda, J. c-Myb regulates matrix metalloproteinases 1/9, and cathepsin D: Implications for matrix-dependent breast cancer cell invasion and metastasis. Mol. Cancer 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.; Chang, S.; Dai, Y.; Yu, D.; Chen, D. Cell response to carbon nanotubes: Size-dependent intracellular uptake mechanism and subcellular fate. Small 2010, 6, 2362–2366. [Google Scholar] [CrossRef]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Ge, M.; Zhou, G.; Sun, W.; Liu, D.; Liang, X.-J.; Zhang, J. A distinct endocytic mechanism of functionalized-silica nanoparticles in breast cancer stem cells. Sci. Rep. 2017, 7, 16236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, D.; Kim, H.; Nam, K.; Oh, S.; Son, S.-H.; Shin, I. Cytotoxic effect of nano-SiO2 in human breast cancer cells via modulation of EGFR signaling cascades. Anticancer Res. 2017, 37, 6189–6197. [Google Scholar] [PubMed]
- Wu, Y.-L.; Putcha, N.; Ng, K.W.; Leong, D.T.; Lim, C.T.; Loo, S.C.J.; Chen, X. Biophysical responses upon the interaction of nanomaterials with cellular interfaces. Acc. Chem. Res. 2013, 46, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Cauda, V. Multimodal decorations of mesoporous silica nanoparticles for improved cancer therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed]
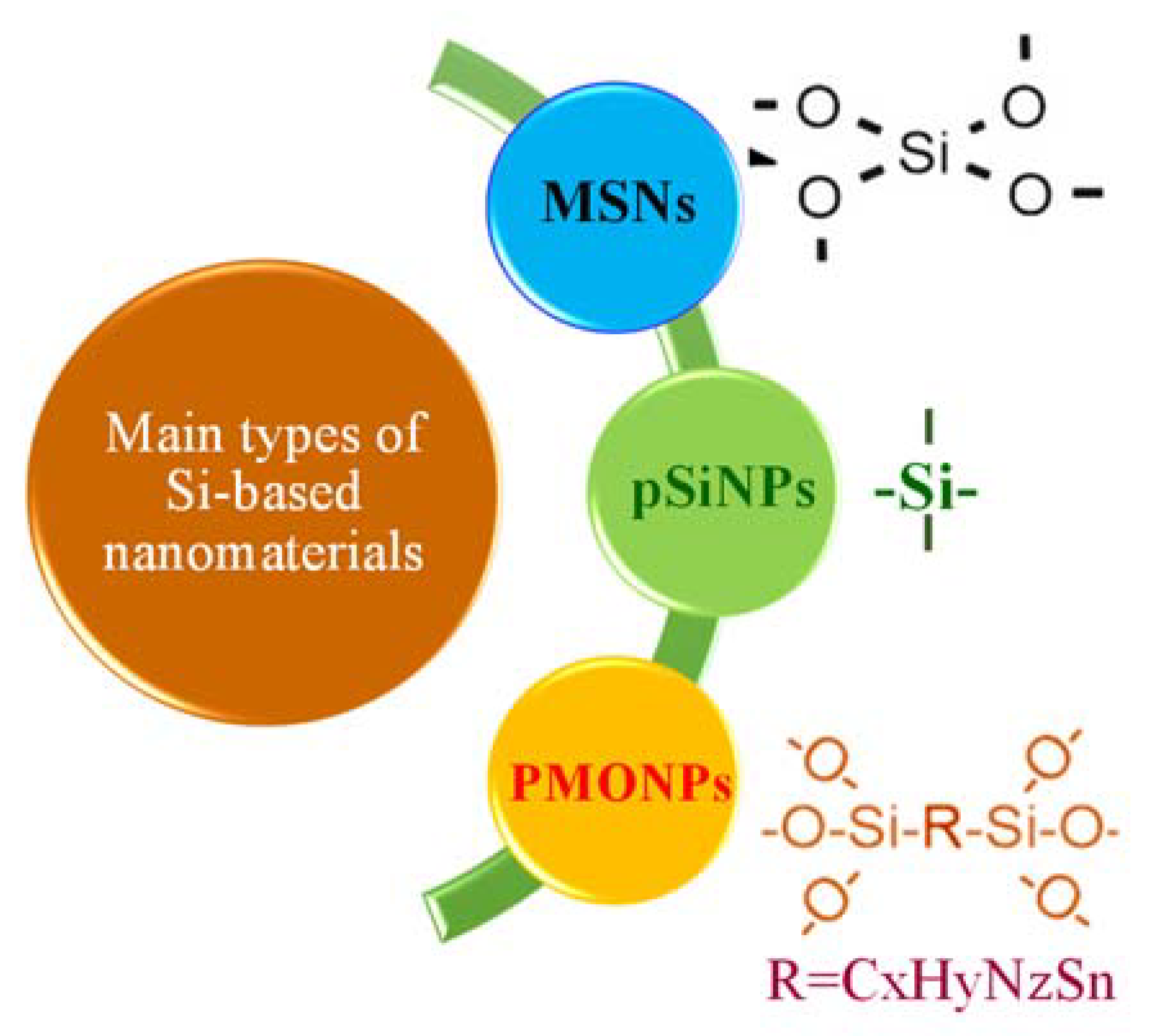

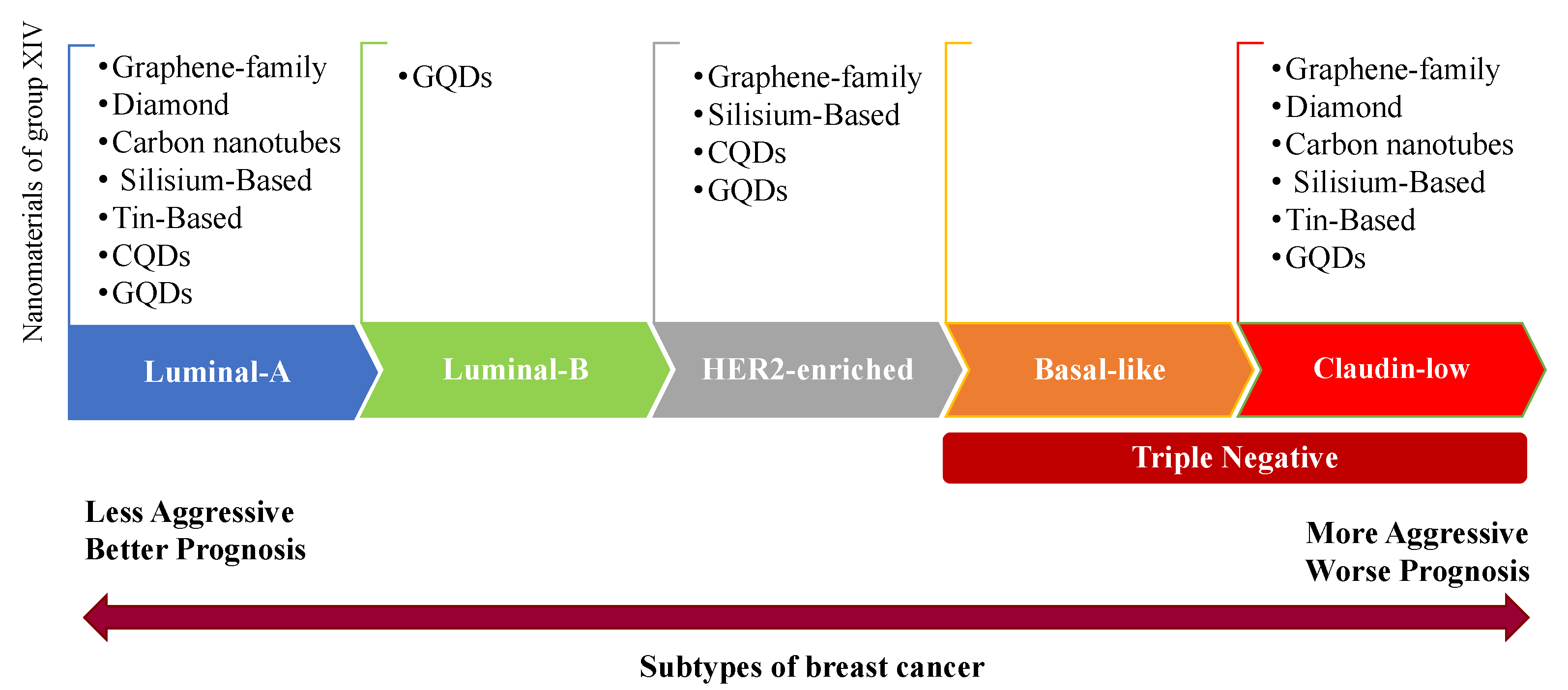
| Molecular Subtype | Luminal A | Luminal B | HER2-Enriched | Basal-Like | Claudin-Low | |
|---|---|---|---|---|---|---|
| cell lines | MCF-7 MDA-MB-134 MDA-MB-175 MDA-MB-415 T-47D | BT-474 MDA-MB-330 MDA-MB-361 | AU-565 HCC-1008 HH-315 HH-375 KPL-4 MDA-MB-453 SK-BR-3 SK-BR-5 SUM190PT UACC-893 | BT-20 CAL-148 HCC1143 KPL-3C MDA-MB-468 SUM229PE | BT-549 MDA-MB-157 MDA-MB-231 SK-BR-7 SUM102PT SUM1315M02 SUM149PT SUM159PT | |
| molecular signatures | ER 1 | P § | P | N | N | N |
| PR 2 | N/P # | N/P | N | N | N | |
| HER2 3 | N ‡ | P | P | N | N | |
| Material | Anticancer Agent/Drugs | Cellular/Animal Models | Outcomes | Ref. |
|---|---|---|---|---|
| SWCNTs | PEG-β-estradiol-Lobaplatin | MCF-7 C57BL/6 mice | - Inhibition of MCF-7 cell growth - Reduction of Lobaplatin side effects - No histopathological variations in organs | [67] |
| CNTs | Ginsenoside Rg3 | MDA-MB-231 BT-549 | - Apoptosis induction in cells - Suppressed the PD-1/PD-L1 pathway | [68] |
| MWCNTs | Cisplatin | MDA-MB-231 | - Reduction of cells growth - Significant reduction of P53 and caspase-3 expression - Downregulation of NF-κB in cells | [69] |
| Mesoporous silica-Chitosan-DOX | MCF-7 Rats | - DOX released at pH 4.0 - Higher plasma concentration of DOX - A lower volume of distribution and clearance | [70] | |
| DOX/Metformin | MCF-7 4T1 | - Marginal beneficial impact on 4T1 cells | [71] | |
| PEGylated Carboplatin | MDA-MB-231 | - No significant impact on the viability of cells - Exhibit pH-responsive drug activity in a sustained way particularly at pH 6.8 | [72] |
| Precursors | Size (nm) | Cellular Models | Outcomes | Ref. |
| Aloe barbadensis miller | 3.2 | MCF-7 | - Apoptosis induction in cells | [79] |
| Ginsenoside Re | 4.6 | MCF-7 | - Increase in LDH release - Increase in ROS levels - Caspase-3-related cell apoptosis | [80] |
| Walnut Oil | 12 | MCF-7 | - Caspase-3-related cell apoptosis | [81] |
| Nerium Oleander | 2.05 | MCF-7 | - Influence on the cell cycle - DNA damage | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hekmat, A.; Saso, L.; Lather, V.; Pandita, D.; Kostova, I.; Saboury, A.A. Recent Advances in Nanomaterials of Group XIV Elements of Periodic Table in Breast Cancer Treatment. Pharmaceutics 2022, 14, 2640. https://doi.org/10.3390/pharmaceutics14122640
Hekmat A, Saso L, Lather V, Pandita D, Kostova I, Saboury AA. Recent Advances in Nanomaterials of Group XIV Elements of Periodic Table in Breast Cancer Treatment. Pharmaceutics. 2022; 14(12):2640. https://doi.org/10.3390/pharmaceutics14122640
Chicago/Turabian StyleHekmat, Azadeh, Luciano Saso, Viney Lather, Deepti Pandita, Irena Kostova, and Ali Akbar Saboury. 2022. "Recent Advances in Nanomaterials of Group XIV Elements of Periodic Table in Breast Cancer Treatment" Pharmaceutics 14, no. 12: 2640. https://doi.org/10.3390/pharmaceutics14122640
APA StyleHekmat, A., Saso, L., Lather, V., Pandita, D., Kostova, I., & Saboury, A. A. (2022). Recent Advances in Nanomaterials of Group XIV Elements of Periodic Table in Breast Cancer Treatment. Pharmaceutics, 14(12), 2640. https://doi.org/10.3390/pharmaceutics14122640