Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases
Abstract
:1. Introduction
2. Urinary Bladder as a Temporary Reservoir with Impermeable Epithelium
3. Role of Nano Materials in IDD
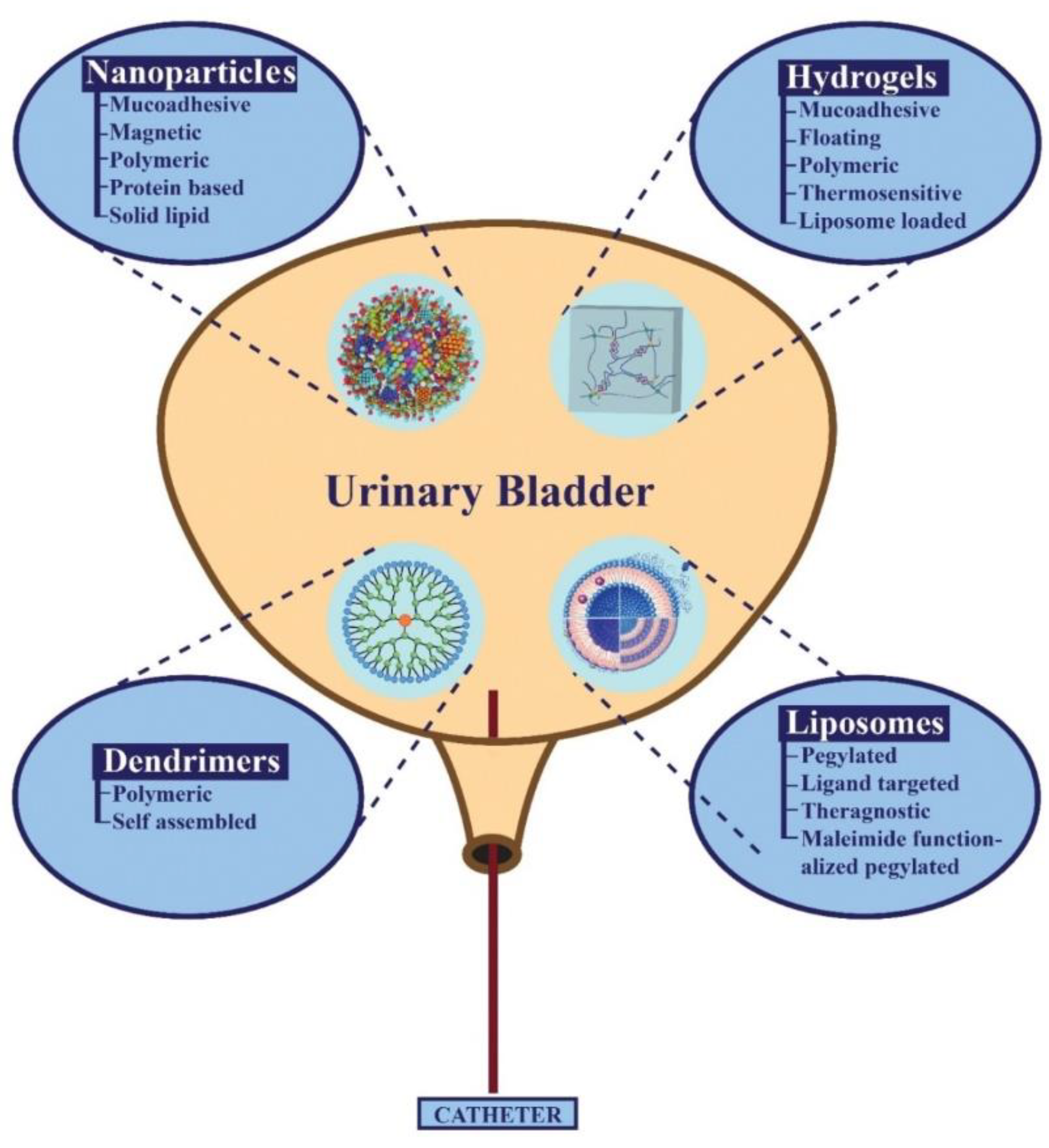
4. Types of Nano-Formulations Used in The Treatment of Bladder Diseases
4.1. Hydrogels as Reservoirs of The Drug
4.1.1. Mucoadhesive Hydrogels
4.1.2. Floating Platform Hydrogels
4.1.3. Polymeric Hydrogels
4.1.4. Thermo-Sensitive Hydrogels
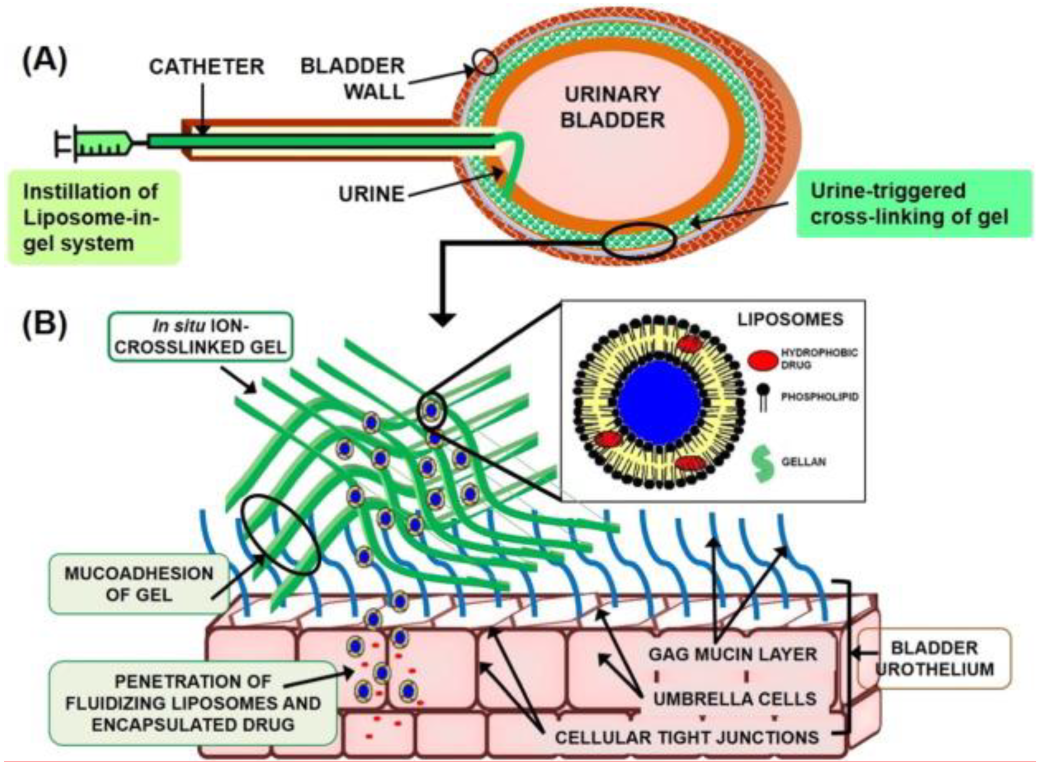
4.1.5. Liposome in Gel Systems (LP-Gel)
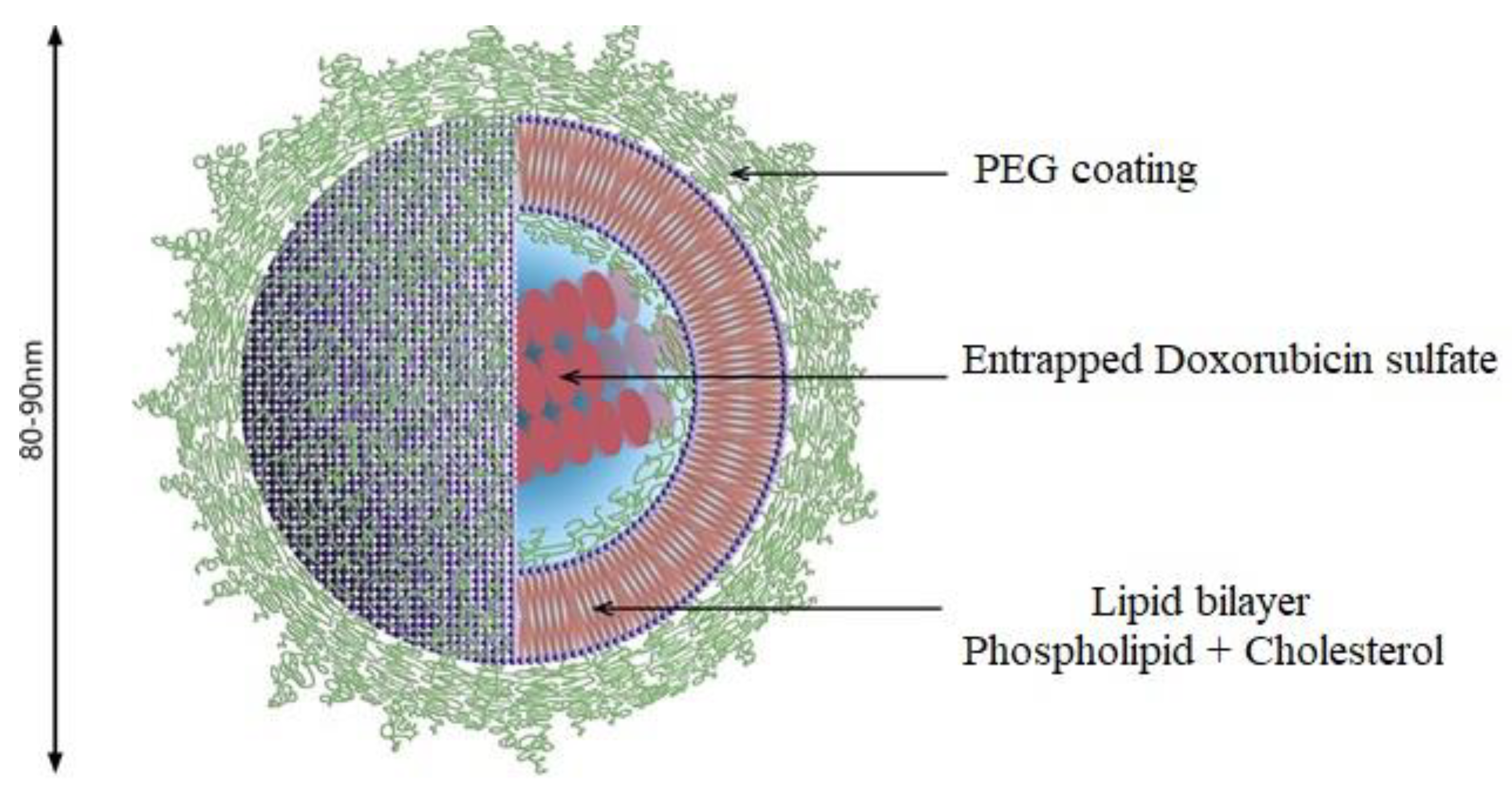
4.2. Liposomes as an Efficient IDDS
Maleimide-Functionalized PEGylated Liposomes (PEG-Mal)
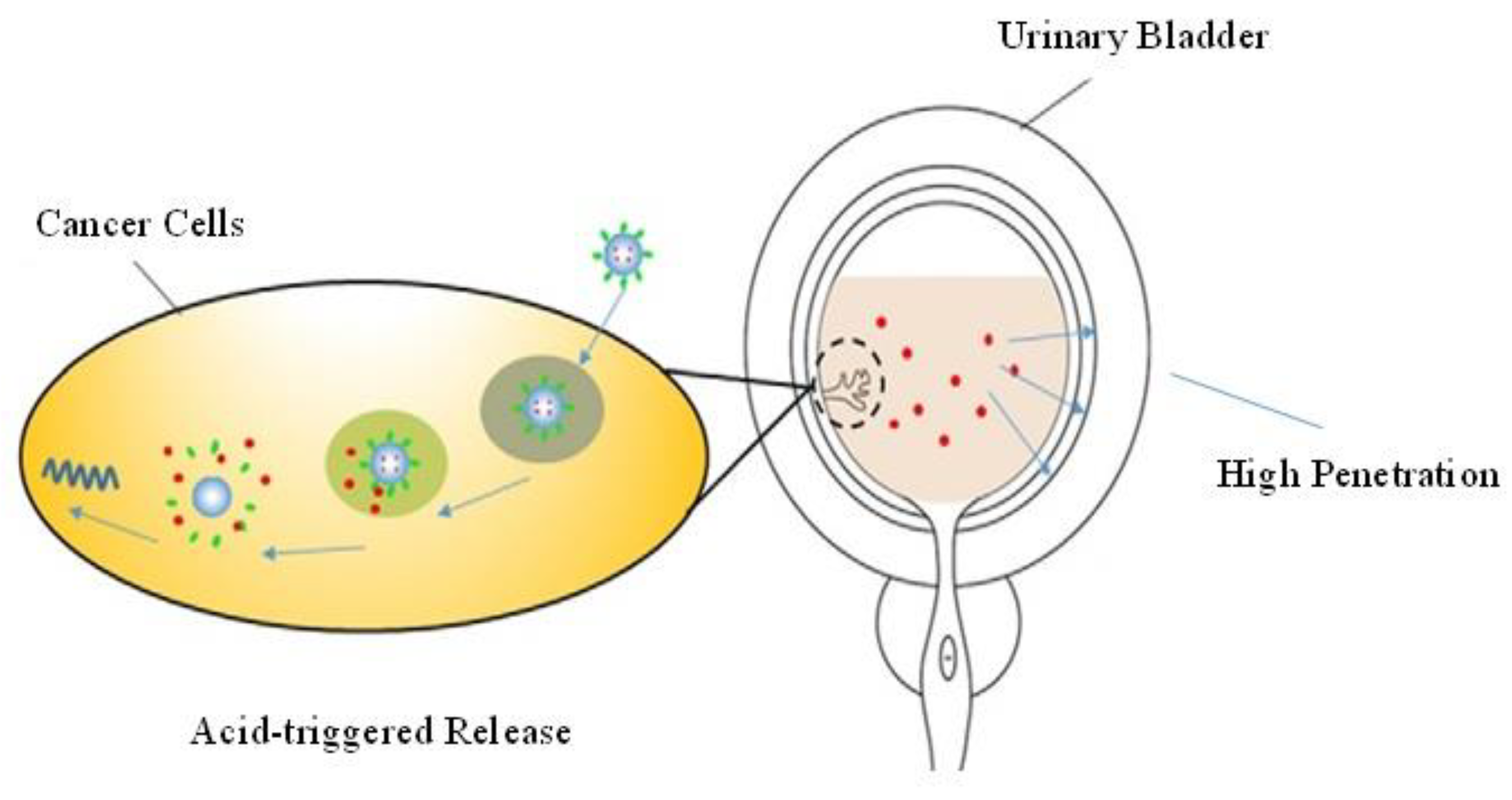
4.3. Nanoparticles as an Efficient IDDS
4.3.1. Polymeric Nanoparticles
4.3.2. Magnetic Nanoparticles
4.3.3. Mucoadhesive Nanoparticles
4.3.4. Solid Lipid Nanoparticles
4.3.5. Protein Nanoparticles
4.3.6. Dendrimers as Intravesical Drug Carriers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, Y.; Sato, Y.; Nakamura, T.; Harashima, H. Evolution of drug delivery system from viewpoint of controlled intracellular trafficking and selective tissue targeting toward future nanomedicine. J. Control. Release 2020, 327, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Wu, Y.-T.; Lin, K.-J.; Yu, T.-J.; Kuo, Y.-L.; Chang, L.-C. A hydrogel-based epirubicin delivery system for intravesical chemotherapy. Molecules 2016, 21, 712. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Kopp, Z.S.; Agatep, B.; Milsom, I.; Abrams, P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011, 108, 1132–1138. [Google Scholar] [CrossRef]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Colombel, M.; Soloway, M.; Akaza, H.; Böhle, A.; Palou, J.; Buckley, R.; Lamm, D.; Brausi, M.; Witjes, J.A.; Persad, R. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur. Urol. Suppl. 2008, 7, 618–626. [Google Scholar] [CrossRef]
- Hooton, T.M. Recurrent urinary tract infection in women. Int. J. Antimicrob. Agents 2001, 17, 259–268. [Google Scholar] [CrossRef]
- Giannantoni, A.; Di Stasi, S.M.; Chancellor, M.B.; Costantini, E.; Porena, M. New frontiers in intravesical therapies and drug delivery. Eur. Urol. 2006, 50, 1183–1193. [Google Scholar] [CrossRef]
- Ijaz, M.; Prantl, M.; Lupo, N.; Laffleur, F.; Asim, M.H.; Matuszczak, B.; Bernkop-Schnürch, A. Development of pre-activated α-cyclodextrin as a mucoadhesive excipient for intra-vesical drug delivery. Int. J. Pharm. 2017, 534, 339–347. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef]
- Tillmanns, H. A Text-Book of Surgery; Stimson, L., Ed.; D. Appleton and Company: New York, NY, USA, 1899. [Google Scholar]
- Yoon, H.Y.; Yang, H.M.; Kim, C.H.; Goo, Y.T.; Kang, M.J.; Lee, S.; Choi, Y. Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer. Expert Opin. Drug Deliv. 2020, 17, 1555–1572. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Xu, W.; Chen, J.; Hou, Y.; Wang, C.; Ding, J.; Chen, X. Mucoadhesive cationic polypeptide nanogel with enhanced penetration for efficient intravesical chemotherapy of bladder cancer. Adv. Sci. 2018, 5, 1800004. [Google Scholar] [CrossRef] [PubMed]
- Milcovich, G.; Antunes, F.E.; Farra, R.; Grassi, G.; Grassi, M.; Asaro, F. Modulating carbohydrate-based hydrogels as viscoelastic lubricant substitute for articular cartilages. Int. J. Biol. Macromol. 2017, 102, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Palugan, L.; Cerea, M.; Cirilli, M.; Moutaharrik, S.; Maroni, A.; Zema, L.; Melocchi, A.; Uboldi, M.; Filippin, I.; Foppoli, A. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X 2021, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Milcovich, G.; Antunes, F.E.; Grassi, M.; Asaro, F. Soft Nanoonions: A Dynamic Overview onto Catanionic Vesicles Temperature-Driven Transition. Int. J. Mol. Sci. 2020, 21, 6804. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Diamond, J.M. Na+ transport by rabbit urinary bladder, a tight epithelium. J. Membr. Biol. 1976, 28, 1–40. [Google Scholar] [CrossRef]
- Born, M.; Pahner, I.; Ahnert-Hilger, G.; Jöns, T. The maintenance of the permeability barrier of bladder facet cells requires a continuous fusion of discoid vesicles with the apical plasma membrane. Eur. J. Cell Biol. 2003, 82, 343–350. [Google Scholar] [CrossRef]
- Lewis, S. The mammalian urinary bladder: It’s more than accommodating. Physiology 1986, 1, 61–65. [Google Scholar] [CrossRef]
- Lewis, S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol.-Ren. Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef]
- Tyagi, P.; Wu, P.-C.; Chancellor, M.; Yoshimura, N.; Huang, L. Recent advances in intravesical drug/gene delivery. Mol. Pharm. 2006, 3, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Poggi, M.M.; Johnstone, P.A.; Conner, R.J. Glycosaminoglycan content of human bladders: A method of analysis using cold-cup biopsies. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Hurst, R. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J. Urol. 1994, 12, 3–10. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chuang, Y.C.; Chancellor, M.B. Intravesical drug delivery for dysfunctional bladder. Int. J. Urol. 2013, 20, 552–562. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.; Griffith, G.J. Intravesical therapy: Does it affect the natural history of superficial bladder cancer. Semin. Urol. 1992, 10, 39–44. [Google Scholar] [PubMed]
- Lee, S.H.; Choy, Y.B. Implantable devices for sustained, intravesical drug delivery. Int. Neurourol. J. 2016, 20, 101. [Google Scholar] [CrossRef]
- Walker, M.; Masters, J.; Parris, C.E.; Hepburn, P.; English, P.J. Intravesical chemotherapy: In vitro studies on the relationship between dose and cytotoxicity. Urol. Res. 1986, 14, 137–140. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Crommelin, D.J.; Storm, G.; Jiskoot, W.; Stenekes, R.; Mastrobattista, E.; Hennink, W.E. Nanotechnological approaches for the delivery of macromolecules. J. Control. Release 2003, 87, 81–88. [Google Scholar] [CrossRef]
- Sansare, V.; Gupta, M.K.; Shrivastava, B. Chapter-3 Nanocarrier Mediated Urinary Bladder Targeted Drug Delivery. In Recent Research in Pharmaceutical; Weser Books: Zittau, Germany, 2021; Volume 25, p. 57. [Google Scholar]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2020; pp. 61–91. [Google Scholar]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Effect of Coumarate 3-zhydroxylase Down regulation on lignin structure. Nanomed. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Velluto, D.; Ricordi, C. Nanotechnology Advances in Drug Delivery. Nanoworld J. 2017, 3, S9–S17. [Google Scholar] [CrossRef]
- Silva, G.A. Introduction to nanotechnology and its applications to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Damgé, C.; Michel, C.; Aprahamian, M.; Couvreur, P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 1988, 37, 246–251. [Google Scholar] [CrossRef]
- Chatta, D.; Cottrell, L.; Burnett, B.; Laverty, G.; McConville, C. The use of water-soluble mucoadhesive gels for the intravesical delivery of epirubicin to the bladder for the treatment of non-muscle-invasive bladder cancer. J. Pharm. Pharmacol. 2015, 67, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Mostafid, H.; Khutoryanskiy, V.V. Advances in intravesical drug delivery systems to treat bladder cancer. Int. J. Pharm. 2017, 532, 105–117. [Google Scholar] [CrossRef]
- Quindós, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e172. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, Z.Y.; Duan, X.Y.; Jiang, L.J.; Cao, P.P.; Li, J.X.; Li, J.B. Design and evaluation of chitosan-β-cyclodextrin based thermosensitive hydrogel. Biochem. Eng. J. 2016, 111, 100–107. [Google Scholar] [CrossRef]
- Cai, G.; Hou, Z.; Sun, W.; Li, P.; Zhang, J.; Yang, L.; Chen, J. Recent developments in biomaterial-based hydrogel as the delivery system for repairing endometrial injury. Front. Bioeng. Biotechnol. 2022, 10, 894252. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.L.; Zhou, J.F.; Qi, X.; Shen, R. Thermo-sensitive hydrogel and their biomedical applications. IOP Conf. Ser. Earth Environ. Sci. 2021, 714, 032062. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, H.; Lu, X.; Yan, W.; Ji, Z. Enhanced photo/chemo combination efficiency against bladder tumor by encapsulation of DOX and ZnPC into in situ-formed thermosensitive polymer hydrogel. Int. J. Nanomed. 2018, 13, 7623. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, Y.H.; Zisman, A.; Jeshurun-Gutshtat, M.; Gerassi, T.; Hakim, G.; Vinshtok, Y.; Stav, K. Safety and feasibility of intravesical instillation of botulinum toxin—A in hydrogel-based slow-release delivery system in patients with interstitial cystitis-bladder pain syndrome: A pilot study. Urology 2018, 114, 60–65. [Google Scholar] [CrossRef]
- Arshad, S.; Asim, M.H.; Mahmood, A.; Ijaz, M.; Irfan, H.M.; Anwar, F.; Ali, M.Y. Calycosin-loaded nanostructured lipid carriers: In-vitro and in-vivo evaluation for enhanced anti-cancer potential. J. Drug Deliv. Sci. Technol. 2022, 67, 102957. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Pradhan, L.; Vasavada, S.; De, M.; Sarma, H.; Prakash, A.; Bahadur, D.; Dravid, V.P. Magneto-thermally responsive hydrogels for bladder cancer treatment: Therapeutic efficacy and in vivo biodistribution. Colloids Surf. B Biointerfaces 2015, 136, 625–633. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; More, P.; Banerjee, R. Urothelium-adherent, ion-triggered liposome-in-gel system as a platform for intravesical drug delivery. J. Control. Release 2017, 245, 147–156. [Google Scholar] [CrossRef]
- Sun, X.; Sun, P.; Li, B.; Liu, Y.; Wang, M.; Suo, N.; Yang, M.; Zhang, D.; Jin, X. A new drug delivery system for mitomycin C to improve intravesical instillation. Mater. Des. 2016, 110, 849–857. [Google Scholar] [CrossRef]
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical hydrogels as drug reservoirs. Trends Biotechnol. 2020, 38, 579–583. [Google Scholar] [CrossRef]
- Lin, T.; Wu, J.; Zhao, X.; Lian, H.; Yuan, A.; Tang, X.; Zhao, S.; Guo, H.; Hu, Y. In situ floating hydrogel for intravesical delivery of adriamycin without blocking urinary tract. J. Pharm. Sci. 2014, 103, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S. Re: A Floating Hydrogel System Capable of Generating CO2 Bubbles to Diminish Urinary Obstruction after Intravesical Instillation. J. Urol. 2016, 195, 525–526. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Y.; Wang, K.; Zhao, X.; Lian, H.; Wang, W.; Wang, H.; Wu, J.; Hu, Y.; Guo, H. Visualized intravesical floating hydrogel encapsulating vaporized perfluoropentane for controlled drug release. Drug Deliv. 2016, 23, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Yoon, H.Y.; Kim, C.H.; Goo, Y.T.; Choi, I.J.; Park, S.G.; Chang, I.H.; Choi, Y.W. Poloxamer 407-based Floating Hydrogels for Intravesical Instillation: Statistical Optimization Using Central Composite Design, Gel Erosion, and Drug Release. Bull. Korean Chem. Soc. 2021, 42, 72–79. [Google Scholar] [CrossRef]
- Goo, Y.T.; Yang, H.M.; Kim, C.H.; Kim, M.S.; Kim, H.K.; Chang, I.H.; Choi, Y.W. Optimization of a floating poloxamer 407-based hydrogel using the Box-Behnken design: In vitro characterization and in vivo buoyancy evaluation for intravesical instillation. Eur. J. Pharm. Sci. 2021, 163, 105885. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-glycerophosphate in situ gelling mucoadhesive systems for intravesical delivery of mitomycin-C. Int. J. Pharm. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Mahrous, G.M.; Alanazi, F.K. Novel in-situ gel for intravesical administration of ketorolac. Saudi Pharm. J. 2018, 26, 845–851. [Google Scholar] [CrossRef]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Tyagi, P.; Li, Z.; Chancellor, M.; De Groat, W.C.; Yoshimura, N.; Huang, L. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm. Res. 2004, 21, 832–837. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Soler, R.; Bruschini, H.; Martins, J.R.; Dreyfuss, J.L.; Camara, N.O.; Alves, M.T.; Leite, K.R.; Truzzi, J.C.; Nader, H.B.; Srougi, M. Urinary glycosaminoglycans as biomarker for urothelial injury: Is it possible to discriminate damage from recovery? Urology 2008, 72, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cheang, T.; Tang, B.; Xia, H.; Xing, Z.; Chen, Z.; Fang, Y.; Chen, W.; Xu, A.; Wang, S. The inhibition of human bladder cancer growth by calcium carbonate/CaIP6 nanocomposite particles delivering AIB1 siRNA. Biomaterials 2013, 34, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Leakakos, T.; Ji, C.; Lawson, G.; Peterson, C.; Goodwin, S. Intravesical administration of doxorubicin to swine bladder using magnetically targeted carriers. Cancer Chemother. Pharmacol. 2003, 51, 445–450. [Google Scholar] [CrossRef]
- Li, T.-J.; Huang, C.-C.; Ruan, P.-W.; Chuang, K.-Y.; Huang, K.-J.; Shieh, D.-B.; Yeh, C.-S. In vivo anti-cancer efficacy of magnetite nanocrystal-based system using locoregional hyperthermia combined with 5-fluorouracil chemotherapy. Biomaterials 2013, 34, 7873–7883. [Google Scholar] [CrossRef]
- Yang, H.-W.; Hua, M.-Y.; Liu, H.-L.; Tsai, R.-Y.; Pang, S.-T.; Hsu, P.-H.; Tang, H.-J.; Yen, T.-C.; Chuang, C.-K. An epirubicin–conjugated nanocarrier with MRI function to overcome lethal multidrug-resistant bladder cancer. Biomaterials 2012, 33, 3919–3930. [Google Scholar] [CrossRef]
- Hsieh, D.-S.; Wang, H.; Tan, S.-W.; Huang, Y.-H.; Tsai, C.-Y.; Yeh, M.-K.; Wu, C.-J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef]
- Bangham, A. A correlation between surface charge and coagulant action of phospholipids. Nature 1961, 192, 1197–1198. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef]
- Johnson, J.W.; Nayar, R.; Killion, J.J.; Von Eschenbach, A.C.; Fidler, I.J. Binding of liposomes to human bladder tumor epithelial cell lines: Implications for an intravesical drug delivery system for the treatment of bladder cancer. Sel. Cancer Ther. 1989, 5, 147–155. [Google Scholar] [CrossRef]
- Reimer, K.; Fleischer, W.; Brögmann, B.; Schreier, H.; Burkhard, P.; Lanzendörfer, A.; Gümbel, H.; Hoekstra, H.; Behrens-Baumann, W. Povidone-iodine liposomes—An overview. Dermatology 1997, 195 (Suppl. S2), 93–99. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Korting, H.C.; Braun-Falco, O. Liposome preparations: A step forward in topical drug therapy for skin disease? A review. J. Am. Acad. Dermatol. 1989, 21, 1271–1275. [Google Scholar] [CrossRef]
- Tsuruta, T.; Muraishi, O.; Katsuyama, Y.; Ichino, M.; Ogawa, A. Liposome encapsulated doxorubicin transfer to the pelvic lymph nodes by endoscopic administration into the bladder wall: A preliminary report. J. Urol. 1997, 157, 1652–1654. [Google Scholar] [CrossRef]
- Hikosaka, S.; Hara, I.; Miyake, H.; Hara, S.; Kamidono, S. Antitumor effect of simultaneous transfer of interleukin-12 and interleukin-18 genes and its mechanism in a mouse bladder cancer model. Int. J. Urol. 2004, 11, 647–652. [Google Scholar] [CrossRef]
- Nogawa, M.; Yuasa, T.; Kimura, S.; Tanaka, M.; Kuroda, J.; Sato, K.; Yokota, A.; Segawa, H.; Toda, Y.; Kageyama, S.; et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J. Clin. Investig. 2005, 115, 978–985. [Google Scholar] [CrossRef]
- Zang, Z.; Mahendran, R.; Wu, Q.; Yong, T.; Esuvaranathan, K. Non-viral tumor necrosis factor-alpha gene transfer decreases the incidence of orthotopic bladder tumors. Int. J. Mol. Med. 2004, 14, 713–720. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef]
- Cortesi, R.; Nastruzzi, C. Liposomes, micelles and microemulsions as new delivery systems for cytotoxic alkaloids. Pharm. Sci. Technol. Today 1999, 2, 288–298. [Google Scholar] [CrossRef]
- Rajaganapathy, B.R.; Chancellor, M.B.; Nirmal, J.; Dang, L.; Tyagi, P. Bladder uptake of liposomes after intravesical administration occurs by endocytosis. PLoS ONE 2015, 10, e0122766. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.; Wasan, K.M.; Lopez-Berestein, G. Liposomal polyene antibiotics. Methods Enzymol. 2005, 391, 304–313. [Google Scholar]
- Connor, R.; Engler, H.; Machemer, T.; Philopena, J.; Horn, M.; Sutjipto, S.; Maneval, D.; Youngster, S.; Chan, T.; Bausch, J. Identification of polyamides that enhance adenovirus-mediated gene expression in the urothelium. Gene Ther. 2001, 8, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratliff, T.L. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. J. Urol. 2005, 173, 2198–2199. [Google Scholar] [CrossRef]
- Kuball, J.; Wen, S.F.; Leissner, J.; Atkins, D.; Meinhardt, P.; Quijano, E.; Engler, H.; Hutchins, B.; Maneval, D.C.; Grace, M.J. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J. Clin. Oncol. 2002, 20, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.O.; Chuang, Y.-C.; Tyagi, P.; Yokoyama, T.; Yoshimura, N.; Huang, L.; De Groat, W.C.; Chancellor, M.B. Intravesical liposome administration—A novel treatment for hyperactive bladder in the rat. Urology 2003, 61, 656–663. [Google Scholar] [CrossRef]
- Chancellor, M.B.; Yoshimura, N. Treatment of interstitial cystitis. Urology 2004, 63, 85–92. [Google Scholar] [CrossRef]
- Tyagi, P.; Chancellor, M.B.; Li, Z.; De Groat, W.C.; Yoshimura, N.; Fraser, M.O.; Huang, L. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. J. Urol. 2004, 171, 483–489. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lee, W.-C.; Lee, W.-C.; Chiang, P.-H. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J. Urol. 2009, 182, 1393–1400. [Google Scholar] [CrossRef]
- Frangos, D.N.; Killion, J.J.; Fan, D.; Fishbeck, R.; Fidler, I.J. The development of liposomes containing interferon alpha for the intravesical therapy of human superficial bladder cancer. J. Urol. 1990, 143, 1252–1256. [Google Scholar] [CrossRef]
- Parsons, C.L.; Benson, G.; Childs, S.J.; Hanno, P.; Sant, G.R.; Webster, G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J. Urol. 1993, 150, 845–848. [Google Scholar] [CrossRef]
- Parsons, C. A model for the function of glycosaminoglycans in the urinary tract. World J. Urol. 1994, 12, 38–42. [Google Scholar] [CrossRef]
- Lee, W.C.; Chuang, Y.C.; Lee, W.C.; Chiang, P.H. Safety and dose flexibility clinical evaluation of intravesical liposome in patients with interstitial cystitis or painful bladder syndrome. Kaohsiung J. Med. Sci. 2011, 27, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonglairoum, P.; Brannigan, R.P.; Opanasopit, P.; Khutoryanskiy, V. Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery. J. Mater. Chem. B 2016, 4, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Gabizon, A. Liposomes designed to avoid the reticuloendothelial system. Prog. Clin. Biol. Res. 1990, 343, 85–93. [Google Scholar] [PubMed]
- Lin, H.-Y.; Thomas, J.L. Peg−Lipids and Oligo (ethylene Glycol) surfactants enhance the ultrasonic permeabilizability of liposomes. Langmuir 2003, 19, 1098–1105. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhao, Y.; Zheng, X. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia: A literature review of pharmaceutical and clinical aspects. Eur. J. Hosp. Pharm. 2021, 28, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Wu, S.-C.; Tsai, J.-W.; Yu, T.-J.; Tsai, T.-R. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int. J. Pharm. 2009, 376, 195–203. [Google Scholar] [CrossRef]
- Stern, J.M.; Cadeddu, J.A. Emerging use of nanoparticles for the therapeutic ablation of urologic malignancies. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Alexis, F.; Rhee, J.-W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Taleghani, A.S.; Nakhjiri, A.T.; Khakzad, M.J.; Rezayat, S.M.; Ebrahimnejad, P.; Heydarinasab, A.; Akbarzadeh, A.; Marjani, A. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: A review. J. Mol. Liq. 2021, 328, 115417. [Google Scholar] [CrossRef]
- Matsumoto, A.; Cabral, H.; Sato, N.; Kataoka, K.; Miyahara, Y. Assessment of tumor metastasis by the direct determination of cell-membrane sialic acid expression. Angew. Chem. 2010, 122, 5626–5629. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Synthesis and evaluation of boronated chitosan as a mucoadhesive polymer for intravesical drug delivery. J. Pharm. Sci. 2019, 108, 3046–3053. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Taleghani, A.S.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2021, 27, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Gommersall, L.; Shergill, I.S.; Ahmed, H.U.; Hayne, D.; Arya, M.; Patel, H.R.; Hashizume, M.; Gill, I.S. Nanotechnology and its relevance to the urologist. Eur. Urol. 2007, 52, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette–Guérin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Biju, V.; Mundayoor, S.; Omkumar, R.V.; Anas, A.; Ishikawa, M. Bioconjugated quantum dots for cancer research: Present status, prospects and remaining issues. Biotechnol. Adv. 2010, 28, 199–213. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Jordan, J.L.; Henderson, S.; Elson, C.M.; Zhou, J.; Kydonieus, A.; Downie, J.; Lee, T.D. Use of a Sulfated Chitosan Derivative to Reduce Bladder Inflammation in the Rat. Urology 2007, 70, 1014–1018. [Google Scholar] [CrossRef]
- Barthelmes, J.; Perera, G.; Hombach, J.; Dünnhaupt, S.; Bernkop-Schnürch, A. Development of a mucoadhesive nanoparticulate drug delivery system for a targeted drug release in the bladder. Int. J. Pharm. 2011, 416, 339–345. [Google Scholar] [CrossRef]
- Xu, X.; Liu, K.; Jiao, B.; Luo, K.; Ren, J.; Zhang, G.; Yu, Q.; Gan, Z. Mucoadhesive nanoparticles based on ROS activated gambogic acid prodrug for safe and efficient intravesical instillation chemotherapy of bladder cancer. J. Control. Release 2020, 324, 493–504. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89. [Google Scholar]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- M Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 42, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Woods, S.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef]
- Shin, S.-C.; Kim, J.-Y.; Oh, I.-J. Mucoadhesive and Physicochemical Characterization of Carbopol-Poloxamer Gels Containing Triamcinolone Acetonide. Drug Dev. Ind. Pharm. 2000, 26, 307–312. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255. [Google Scholar] [CrossRef]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Tonglairoum, P. 6-Maleimidohexanoic acid-grafted chitosan: A new generation mucoadhesive polymer. Carbohydr. Polym. 2018, 202, 258–264. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Singpanna, K.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-bearing hyaluronic acid coated polyvinyl pyrrolidone/hydroxyl propyl-β-cyclodextrin/clotrimazole nanofibers for oral candidiasis treatment. In Key Engineering Material; Trans Tech Publications Ltd.: Bäch, Switzerland,, 2019. [Google Scholar]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Catechol-functionalized succinyl chitosan for novel mucoadhesive drug delivery. Key Eng. Mater. 2019, 819, 21–26. [Google Scholar] [CrossRef]
- Fontaine, S.D.; Reid, R.; Robinson, L.; Ashley, G.W.; Santi, D.V. Long-term stabilization of maleimide–thiol conjugates. Bioconjug. Chem. 2015, 26, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Doxorubicin-loaded chitosan-alginate nanoparticles with dual mucoadhesive functionalities for intravesical chemotherapy. J. Drug Deliv. Sci. Technol. 2021, 63, 102481. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Sharma, R.; Chuttani, K.; Mishra, A.; Murthy, R. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J. Control. Release 2005, 105, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, L.; Gu, W.; Gao, Y.; Lin, L.; Zhang, Z.; Xi, Y.; Li, Y. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 2009, 30, 226–232. [Google Scholar] [CrossRef]
- Subedi, R.K.; Kang, K.W.; Choi, H.-K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur. J. Pharm. Sci. 2009, 37, 508–513. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation II. Biomedical applications and current status of peptide and protein nanoparticulate delivery systems. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 53–65. [Google Scholar] [CrossRef]
- Kratz, F.; Beyer, U.; Roth, T.; Tarasova, N.; Collery, P.; Lechenault, F.; Cazabat, A.; Schumacher, P.; Unger, C.; Falken, U. Transferrin conjugates of doxorubicin: Synthesis, characterization, cellular uptake, and in vitro efficacy. J. Pharm. Sci. 1998, 87, 338–346. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Lu, Z.; Yeh, T.-K.; Tsai, M.; Au, J.L.-S.; Wientjes, M.G. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin. Cancer Res. 2004, 10, 7677–7684. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly (amidoamine)(PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Tang, M.X.; Redemann, C.T.; Szoka, F.C. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem. 1996, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Myc, A.; Beals, J.; Thomas, T.P.; Bander, N.H.; Baker, J.R. Synthesis and in vitro testing of J591 antibody− dendrimer conjugates for targeted prostate cancer therapy. Bioconjug. Chem. 2004, 15, 1174–1181. [Google Scholar] [CrossRef]
- Yuan, A.; Yang, B.; Wu, J.; Hu, Y.; Ming, X. Dendritic nanoconjugates of photosensitizer for targeted photodynamic therapy. Acta Biomater. 2015, 21, 63–73. [Google Scholar] [CrossRef]
- Wang, Z.; Itoh, Y.; Hosaka, Y.; Kobayashi, I.; Nakano, Y.; Maeda, I.; Umeda, F.; Yamakawa, J.; Kawase, M.; Yag, K. Novel transdermal drug delivery system with polyhydroxyalkanoate and starburst polyamidoamine dendrimer. J. Biosci. Bioeng. 2003, 95, 541–543. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, K.; Lin, T.; Chen, W.; Yuan, A.; Wu, J.; Hu, Y.; Guo, H. Drug delivery system based on dendritic nanoparticles for enhancement of intravesical instillation. Int. J. Nanomed. 2017, 12, 7365. [Google Scholar] [CrossRef] [Green Version]


| Category | Hydrogel | Bonding | Diseases | Limitations | Reference |
|---|---|---|---|---|---|
| Mucoadhesive | Cellulose-based | Hydrogen bonding | Bladder cancer | Sudden drug release | [40] |
| PCL-PTSUO-PEG | Hydrophobic | Bladder cancer | Irregular release, less stability | [48] | |
| TC-3 | Hydrophobic + hydrogen bonding | Bladder cancer; interstitial cystitis | Shorter retention period | [49] | |
| PNIPAM | Covalent | Bladder cancer | Less stability | [51] | |
| Gelatine and glutaraldehyde | Covalent | Bladder cancer | Toxicity | ||
| Floating | perfluoropentane | Hydrophobic | Bladder cancer | Irregular pH balance | [55] |
| Poloxamer-407/NaHCO3 | Hydrophilic | Bladder cancer | pH dependency | [59] | |
| Thermo- sensitive | Chitosan/β-glycerophosphate | Covalent | Bladder cancer; interstitial cystitis | [60] | |
| Poloxamer and chitosan | Covalent | Bladder cancer | [61] |
| Composition | Disease | Properties | Reference |
|---|---|---|---|
| Vesicles of the lipid bilayer | Can be used alone; used as an agent in gene therapy of bladder cancer patients; neurotoxin-loaded liposomes used in interstitial cystitis | Suitable for both hydrophobic and hydrophilic drugs; surface functionalization; improved cellular uptake | [88] |
| Lipid bilayer of positively charged multilamellar lipid vesicles | Detrusor hyper-reflexia | Suitable for the intravesical delivery of capsaicin with less tissue damage | [80] |
| Phospholipid bilayer vesicles | Hypersensitive bladder | This liposome can efficiently deliver botulinum toxin A intravesically without injection | [82] |
| Multiamellar phospholipid bilayer | Superficial bladder cancer | Augmented the antiproliferative activity of IFN-alpha after encapsulation within multilamellar liposome | [83,84] |
| Nanoparticle | Size (nm) | Composition | Disease | Properties | Reference. |
|---|---|---|---|---|---|
| Polymeric nanoparticles | 90–300 | PLGA; PECA | Used with epirubicin in the treatment of bladder cancer | Controlled drug delivery; biodegradable | [100] |
| Magnetic nanoparticle | 5000 | Iron oxide | Used with doxorubicin, guided by the external magnetic field | Hypothermia; aids in imaging | [68] |
| Mucoadhesive nanoparticles | 10–110 | Chitosan based | Hypothermia effect induced by SPR | NIR radiation activation | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Qamar, S.; Rehman, M.U.; Tahir, M.A.; Ijaz, M.; Ahsan, A.; Asim, M.H.; Nazir, I. Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases. Pharmaceutics 2022, 14, 1909. https://doi.org/10.3390/pharmaceutics14091909
Sarfraz M, Qamar S, Rehman MU, Tahir MA, Ijaz M, Ahsan A, Asim MH, Nazir I. Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases. Pharmaceutics. 2022; 14(9):1909. https://doi.org/10.3390/pharmaceutics14091909
Chicago/Turabian StyleSarfraz, Muhammad, Shaista Qamar, Masood Ur Rehman, Muhammad Azam Tahir, Muhammad Ijaz, Anam Ahsan, Mulazim Hussain Asim, and Imran Nazir. 2022. "Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases" Pharmaceutics 14, no. 9: 1909. https://doi.org/10.3390/pharmaceutics14091909
APA StyleSarfraz, M., Qamar, S., Rehman, M. U., Tahir, M. A., Ijaz, M., Ahsan, A., Asim, M. H., & Nazir, I. (2022). Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases. Pharmaceutics, 14(9), 1909. https://doi.org/10.3390/pharmaceutics14091909








