Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy
Abstract
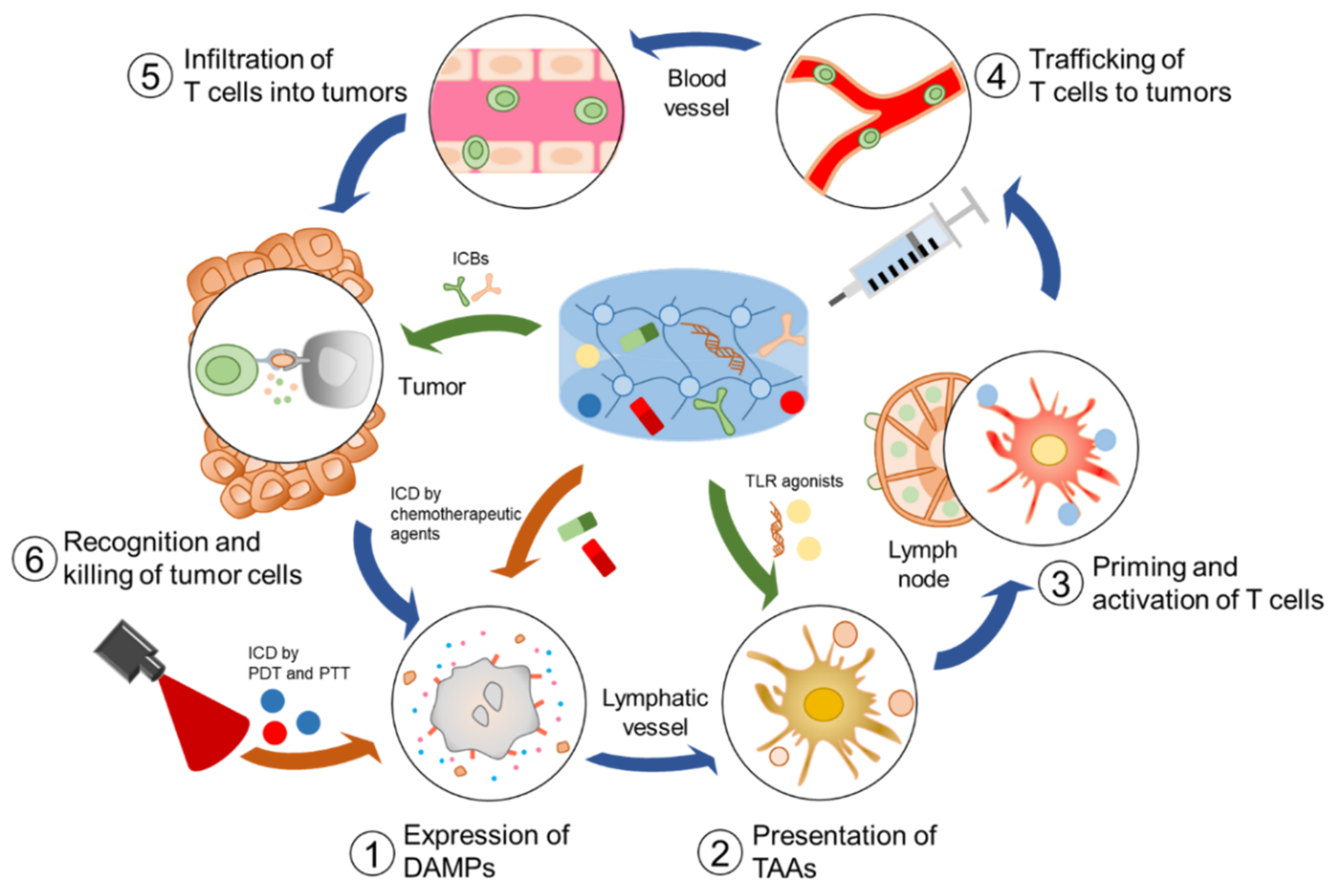
:1. Introduction
2. Injectable Hydrogels for Delivery of Immunotherapeutic Agents
3. Injectable Hydrogels for Combination Cancer Immunotherapy
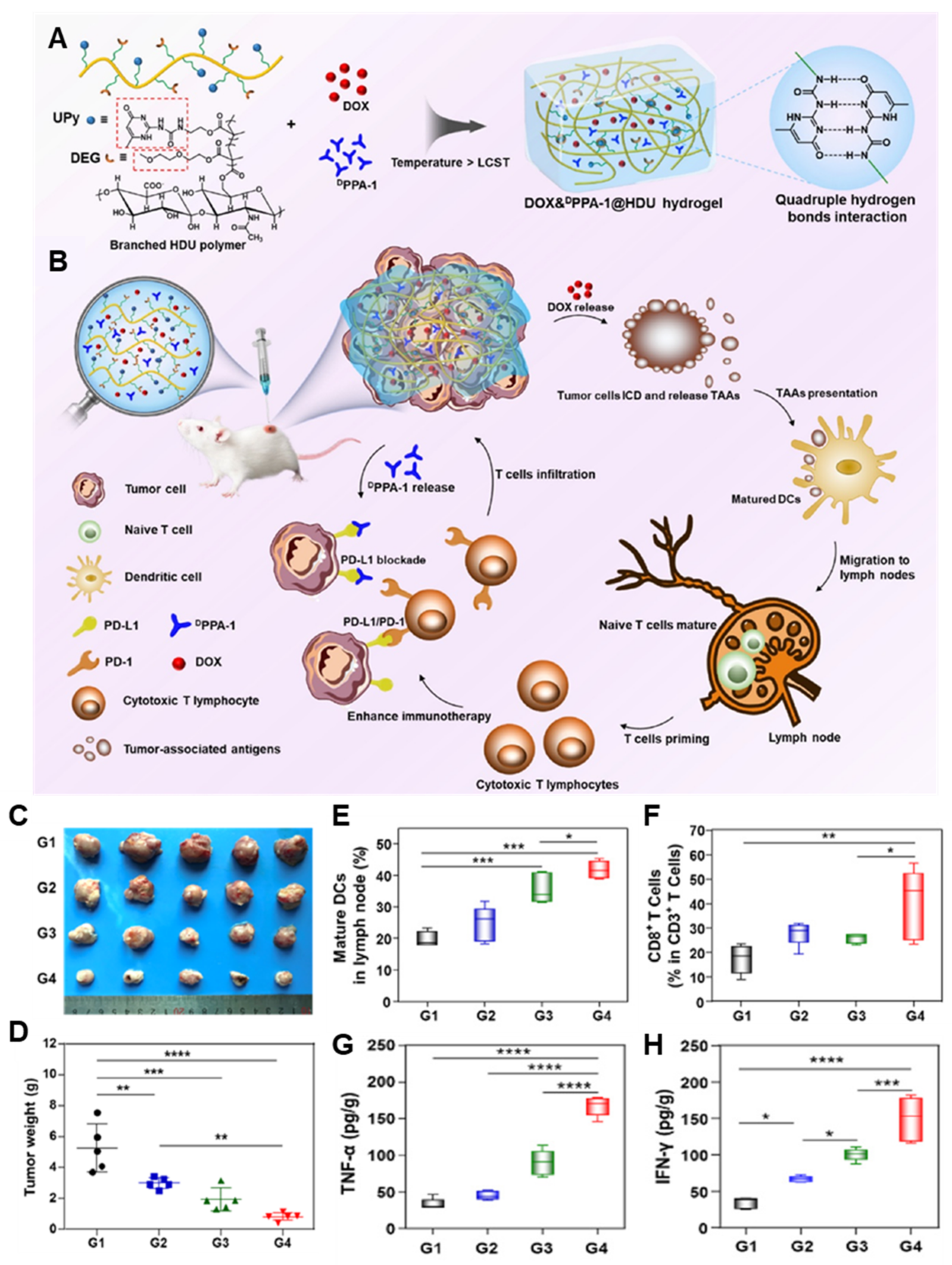
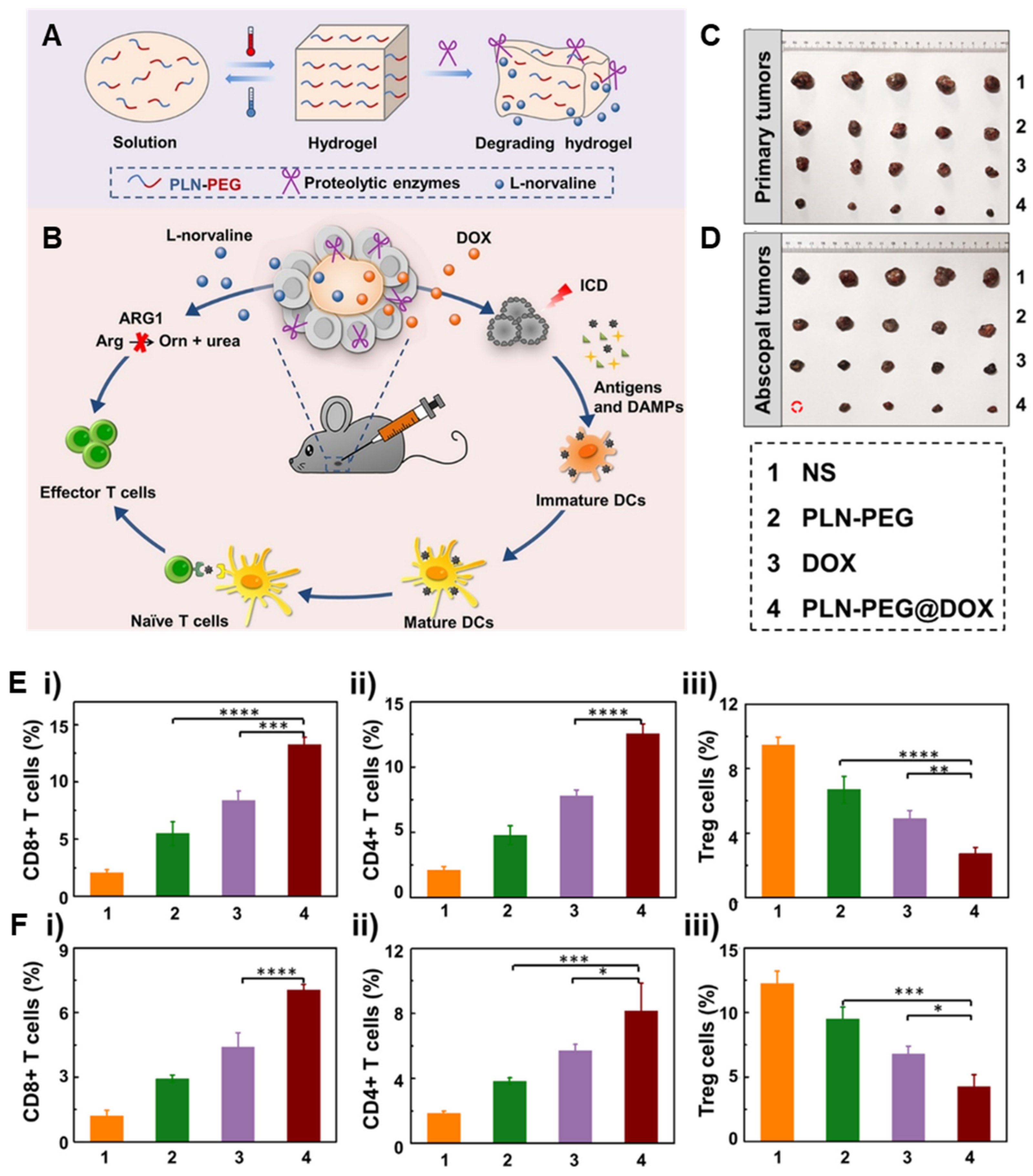
3.1. Combination Chemoimmunotherapy
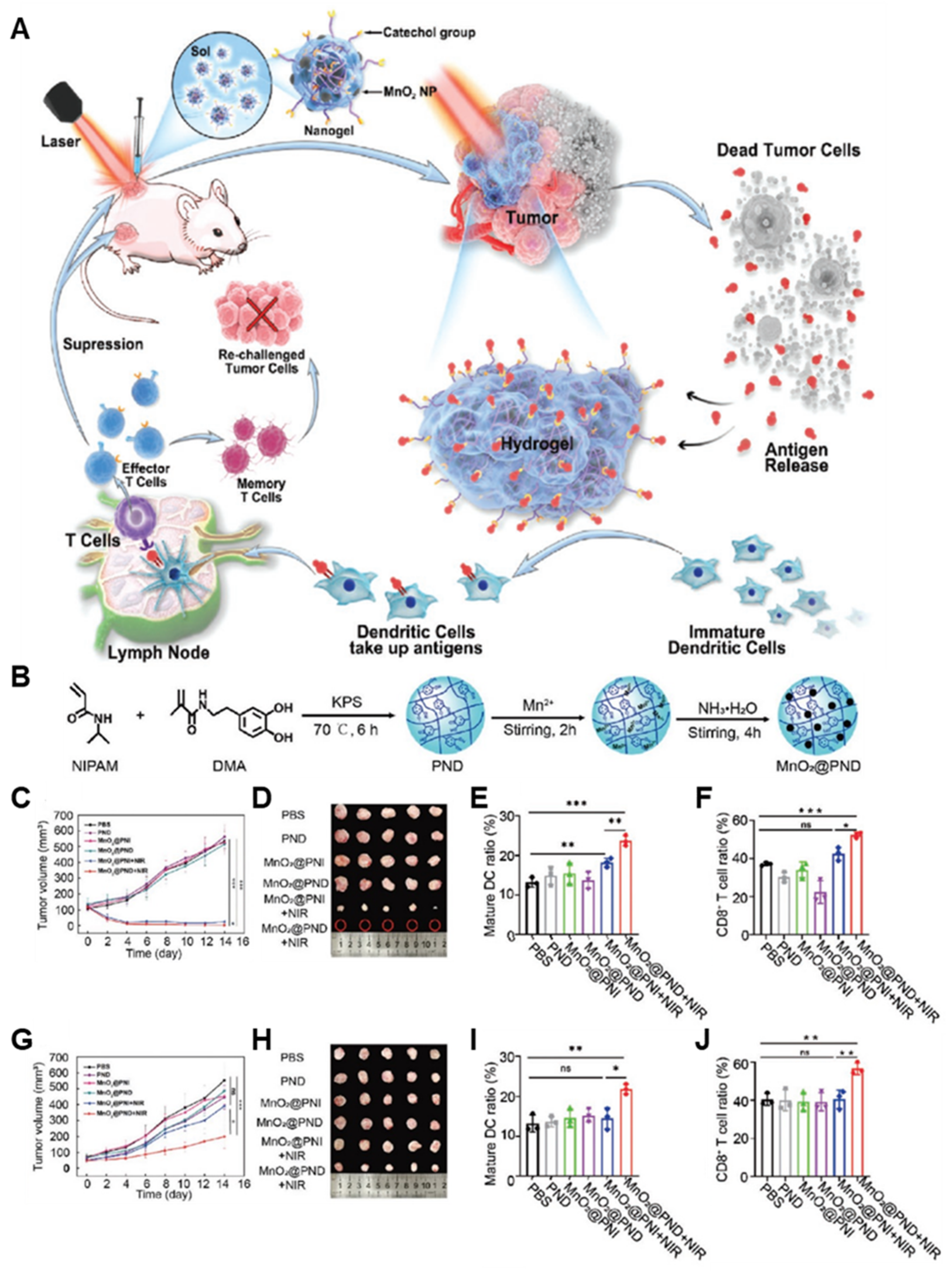
3.2. Combinational Photoimmunotherapy
3.3. Combinational Radioimmunotherapy
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tiwari, A.P.; Hwang, T.I.; Oh, J.-M.; Maharjan, B.; Chun, S.; Kim, B.S.; Joshi, M.K.; Park, C.H.; Kim, C.S. pH/NIR-responsive polypyrrole-functionalized fibrous localized drug-delivery platform for synergistic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 20256–20270. [Google Scholar] [CrossRef]
- Shin, D.H.; Kwon, G.S. Pre-clinical evaluation of a themosensitive gel containing epothilone B and mTOR/Hsp90 targeted agents in an ovarian tumor model. J. Control Release 2017, 268, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Elstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)—Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Xue, B.; Jia, Y.; Yuan, L.; Han, R.; Yang, F.; Peng, J.; Qian, Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019, 12, 1389–1399. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Vohidov, F.; Milling, L.E.; Chen, Q.; Zhang, W.; Bhagchandani, S.; Nguyen, H.V.-T.; Irvine, D.J.; Johnson, J.A. ABC triblock bottlebrush copolymer-based injectable hydrogels: Design, synthesis, and application to expanding the therapeutic index of cancer immunochemotherapy. Chem. Sci. 2020, 11, 5974–5986. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Li, W.; Yang, Q.; Tan, L.; Jia, Y.; Qu, Y.; Qian, Z. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Adv. Sci. 2018, 5, 1700891. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.-L.; Kheirolomoom, A.; Fite, B.Z.; Wu, B.; Lau, K.; Baikoghli, M.; Raie, M.N.; Tumbale, S.K.; Foiret, J. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release 2021, 330, 1080–1094. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.; Ding, L.; Dong, Y.; Wu, Z.; Wang, W.; Shen, M.; Duan, Y. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct. Target. Ther. 2019, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hang, Y.; Wang, Y.; Sleightholm, R.; Prajapati, D.R.; Bader, J.; Yu, A.; Tang, W.; Jaramillo, L.; Li, J. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano 2020, 14, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, H.Y.; Kim, J.; Yang, S.; Lee, J.; Choi, J.W.; Moon, Y.; Kim, J.; Lim, S.; Shim, M.K. Doxorubicin-Loaded PLGA nanoparticles for cancer therapy: Molecular weight effect of PLGA in doxorubicin release for controlling immunogenic cell death. Pharmaceutics 2020, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Watkins-Schulz, R.; Tiet, P.; Gallovic, M.D.; Junkins, R.D.; Batty, C.; Bachelder, E.M.; Ainslie, K.M.; Ting, J.P. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell-and CD8+ T cell-mediated anti-tumor immunity. Biomaterials 2019, 205, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Geisler, I.M.; Schneider, J.P. Evolution-based design of an injectable hydrogel. Adv. Funct. Mater. 2012, 22, 529–537. [Google Scholar] [CrossRef]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Chen, X. Injectable Hydrogels as Local Depots at Tumor Sites for Antitumor Immunotherapy and Immune-Based Combination Therapy. Macromol. Biosci. 2021, 21, 2100039. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.; Yang, S.; Lee, J.; Choi, J.; Moon, Y.; Kim, J.; Shim, N.; Cho, H.; Shim, M.K. Sustained and Long-Term Release of Doxorubicin from PLGA Nanoparticles for Eliciting Anti-Tumor Immune Responses. Pharmaceutics 2022, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Peppas, N.A. Hydrogels and scaffolds for immunomodulation. Adv. Mater. 2014, 26, 6530–6541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels 2021, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, C.; Chen, X. Injectable Hydrogels as Unique Platforms for Local Chemotherapeutics-Based Combination Antitumor Therapy. Macromol. Biosci. 2018, 18, 1800240. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A Checkpoint-Regulatable Immune Niche Created by Injectable Hydrogel for Tumor Therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Yang, A.; Bai, Y.; Dong, X.; Ma, T.; Zhu, D.; Mei, L.; Lv, F. Hydrogel/nanoadjuvant-mediated combined cell vaccines for cancer immunotherapy. Acta Biomater. 2021, 133, 257–267. [Google Scholar] [CrossRef]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Francis, D.M.; Thomas, S.N. In situ crosslinked hydrogel depot for sustained antibody release improves immune checkpoint blockade cancer immunotherapy. Nanomaterials 2021, 11, 471. [Google Scholar] [CrossRef]
- Xu, K.; Lee, F.; Gao, S.J.; Chung, J.E.; Yano, H.; Kurisawa, M. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy. J. Control Release 2013, 166, 203–210. [Google Scholar] [CrossRef]
- Huang, W.C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A macroporous hydrogel dressing with enhanced antibacterial and anti-inflammatory capabilities for accelerated wound healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Gong, Y.; Zhu, W.; Zhu, J.; Pan, F.; Chao, Y.; Xiao, Z.; Liu, Y.; Wang, X. Vitamin C supramolecular hydrogel for enhanced cancer immunotherapy. Biomaterials 2022, 287, 121673. [Google Scholar] [CrossRef]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Wan, C.; Zou, Z.; Zhao, G.; Zhang, L.; Geng, Y.; Chen, T.; Huang, A.; Jiang, F.; Feng, J.-P.; et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano 2018, 12, 3295–3310. [Google Scholar] [CrossRef]
- Li, J.; Luo, G.; Zhang, C.; Long, S.; Guo, L.; Yang, G.; Wang, F.; Zhang, L.; Shi, L.; Fu, Y.; et al. In situ injectable hydrogel-loaded drugs induce anti-tumor immune responses in melanoma immunochemotherapy. Mater. Today Bio 2022, 14, 100238. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, Z.; Zhang, R.; Chen, Y.; Yang, X. Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 33874–33884. [Google Scholar] [CrossRef]
- Yang, A.; Dong, X.; Bai, Y.; Sheng, S.; Zhang, Y.; Liu, T.; Zhu, D.; Lv, F. Doxorubicin/CpG self-assembled nanoparticles prodrug and dendritic cells co-laden hydrogel for cancer chemo-assisted immunotherapy. Chem. Eng. J. 2021, 416, 129192. [Google Scholar] [CrossRef]
- Ren, X.; Wang, N.; Zhou, Y.; Song, A.; Jin, G.; Li, Z.; Luan, Y. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021, 124, 179–190. [Google Scholar] [CrossRef]
- Meng, Z.; Zhou, X.; Xu, J.; Han, X.; Dong, Z.; Wang, H.; Zhang, Y.; She, J.; Xu, L.; Wang, C.; et al. Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Adv. Mater. 2019, 31, e1900927. [Google Scholar] [CrossRef]
- Shu, G.; Zhu, W.; Jiang, Y.; Li, X.; Pan, J.; Zhang, X.; Zhang, X.; Sun, S.K. Persistent Luminescence Immune Hydrogel for Photodynamic-Immunotherapy of Tumors In Vivo. Adv. Funct. Mater. 2021, 31, 2104472. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.Y.; Lu, S.; Wu, Y.; Li, J.; Sun, X.; Yan, J. Injectable hydrogel platform with biodegradable Dawson-type polyoxometalate and R848 for combinational photothermal-immunotherapy of cancer. Biomater. Sci. 2022, 10, 1257–1266. [Google Scholar] [CrossRef]
- Fan, M.; Jia, L.; Pang, M.; Yang, X.; Yang, Y.; Kamel Elyzayati, S.; Liao, Y.; Wang, H.; Zhu, Y.; Wang, Q. Injectable Adhesive Hydrogel as Photothermal-Derived Antigen Reservoir for Enhanced Anti-Tumor Immunity. Adv. Funct. Mater. 2021, 31, 2010587. [Google Scholar] [CrossRef]
- Chao, Y.; Xu, L.; Liang, C.; Feng, L.; Xu, J.; Dong, Z.; Tian, L.; Yi, X.; Yang, K.; Liu, Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018, 2, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-Responsive Smart Hydrogel Releasing Immune Adjuvant Synchronized with Repeated Chemotherapy or Radiotherapy to Boost Antitumor Immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Liu, J.; Li, H.; Su, Q.; Zhang, J.; Huang, P.; Wang, W.; Liu, J. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022, 16, 359–371. [Google Scholar] [CrossRef]
- Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2017, 14, 155–167. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Zhu, X.; Li, C.; Yan, M.; Pan, J.; Ma, G. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials 2020, 242, 119933. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Jiang, W.; Kumar, A.; Zhou, S.; Cao, Z.; Zhan, S.; Yang, W.; Liu, R.; Teng, Y. Nanoconjugates to enhance PDT-mediated cancer immunotherapy by targeting the indoleamine-2,3-dioxygenase pathway. J. Nanobiotechnol. 2021, 19, 182. [Google Scholar] [CrossRef]
- Ruan, H.; Bu, L.; Hu, Q.; Cheng, H.; Lu, W.; Gu, Z. Strategies of combination drug delivery for immune checkpoint blockades. Adv. Healthc. Mater. 2019, 8, 1801099. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022, in press. [CrossRef]
- Fan, D.-y.; Tian, Y.; Liu, Z.-j. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Vanmeerbeek, I.; Sprooten, J.; De Ruysscher, D.; Tejpar, S.; Vandenberghe, P.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; et al. Trial watch: Chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 2020, 9, 1703449. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Kroemer, G. Immunogenic cell death inducers as anticancer agents. Oncotarget 2014, 5, 5190–5191. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef]
- Veglia, F.; Gabrilovich, D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017, 45, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic cells and cancer: From biology to therapeutic intervention. Cancers 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, Y.; Takeuchi, O.; Akira, S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008, 60, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Yi, A.K.; Beaucage, S.L.; Conover, J.; Krieg, A.M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 1996, 93, 2879–2883. [Google Scholar] [CrossRef]
- Nishikawa, M.; Mizuno, Y.; Mohri, K.; Matsuoka, N.; Rattanakiat, S.; Takahashi, Y.; Funabashi, H.; Luo, D.; Takakura, Y. Biodegradable CpG DNA hydrogels for sustained delivery of doxorubicin and immunostimulatory signals in tumor-bearing mice. Biomaterials 2011, 32, 488–494. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Xu, J.; Zhang, R.; Song, G.; Chao, Y.; Feng, L.; Han, F.; Dong, Z.; Li, B.; et al. Smart Nanoreactors for pH-Responsive Tumor Homing, Mitochondria-Targeting, and Enhanced Photodynamic-Immunotherapy of Cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Ding, W.; Xu, L.; Ma, Y.; Zhang, L. Recent advances of persistent luminescence nanoparticles in bioapplications. Nano-Micro Lett. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-K.; Wang, H.-F.; Yan, X.-P. Engineering persistent luminescence nanoparticles for biological applications: From biosensing/bioimaging to theranostics. Acc. Chem. Res. 2018, 51, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, F.; Tang, L.; Li, L. Synthesis of Cu-nanoparticle hydrogel with self-healing and photothermal properties. ACS Appl. Mater. Interfaces 2017, 9, 20895–20903. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Dong, K.; Luo, J.; Zhang, Q.; Cheng, Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials 2016, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Oh, J.-E.; Hong, J.; Chung, Y.; Lee, Y.; Park, K.D.; Kim, S.; Yun, C.-O. Optimized biodegradable polymeric reservoir-mediated local and sustained co-delivery of dendritic cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for cancer immunotherapy. J. Control Release 2017, 259, 115–127. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, X.; Jiang, M.; Zhu, L.; Zhang, Y.; Yang, W.; Xi, W.; Li, G.; Qian, J. Excretable IR-820 for in vivo NIR-II fluorescence cerebrovascular imaging and photothermal therapy of subcutaneous tumor. Theranostics 2019, 9, 5706–5719. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Im, H.-J.; Valdovinos, H.F.; Yu, B.; Goel, S.; Barnhart, T.E.; Huang, P.; Cai, W. Radiolabeled polyoxometalate clusters: Kidney dysfunction evaluation and tumor diagnosis by positron emission tomography imaging. Biomaterials 2018, 171, 144–152. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zuo, C.; Cheng, C.; Li, Q.; Zhang, L.; Wang, Z.; Shi, J. A polyoxometalate cluster paradigm with self-adaptive electronic structure for acidity/reducibility-specific photothermal conversion. J. Am. Chem. Soc. 2016, 138, 8156–8164. [Google Scholar] [CrossRef]
- Han, Y.; Qu, B.; Li, J.; Zhang, X.; Peng, X.; Li, W.; Zhang, R. A simple POM clusters for in vivo NIR-II photoacoustic imaging-guided NIR-II photothermal therapy. J. Inorg. Biochem. 2020, 209, 111121. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.H.; Gelfand, J.M.; Wysocka, M.; Troxel, A.B.; Benoit, B.; Surber, C.; Elenitsas, R.; Buchanan, M.A.; Leahy, D.S.; Watanabe, R. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood J. Am. Soc. Hematol. 2015, 126, 1452–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, T.; He, S.; Wang, Y. Toll-like receptor 7/8 agonist, R848, exhibits antitumoral effects in a breast cancer model. Mol. Med. Rep. 2015, 12, 3515–3520. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Angeles Ramos, D.M. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 4682. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. JNCI J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Golden, E.B.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012, 2, 88. [Google Scholar] [CrossRef]
- Cytlak, U.M.; Dyer, D.P.; Honeychurch, J.; Williams, K.J.; Travis, M.A.; Illidge, T.M. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat. Rev. Immunol. 2022, 22, 124–138. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Feng, Y.; Mu, R.; Wang, Z.; Xing, P.; Zhang, J.; Dong, L.; Wang, C. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat. Commun. 2019, 10, 2272. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Li, Q.; Feng, Z.; Huang, P.; Wang, W.; Liu, J. Development of injectable thermosensitive polypeptide hydrogel as facile radioisotope and radiosensitizer hotspot for synergistic brachytherapy. Acta Biomater. 2020, 114, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Dastidar, H.; Zhang, C.; Zemp, F.J.; Lau, K.; Ernst, M.; Rakic, A.; Sikdar, S.; Rajwani, J.; Naumenko, V. Smac mimetics and oncolytic viruses synergize in driving anticancer T-cell responses through complementary mechanisms. Nat. Commun. 2017, 8, 344. [Google Scholar] [CrossRef] [PubMed]

| Types | Hydrogels | Therapeutic Agents | Target Cancers | Strategies | Ref. |
|---|---|---|---|---|---|
| Chemoimmunotherapy | Melittin conjugated RADA32 | Melittin, DOX | B16F10 melanoma | - Chemoimmunotherapy using self-assembling peptide hydrogels that can induce cell membrane disruption in combination with DOX for eliciting antitumor immune responses. - Remodeling ITM by regulating innate immune cells and depleting M2-like TAMs. - Synergistic inhibition effect by disrupting tumor cell membrane associated with MDR. | [42] |
| PEGDA, PEGSH | DOX, R837 | B16F10 melanoma | - Chemoimmunotherapy using DOX- and immune adjuvant R837-loaded in situ crosslinkable hydrogel composed of four-arm PEGSH and PEGDA. - Synergistic therapeutic effect of DOX- and R837 on the metastatic progression of melanoma. | [43] | |
| HAMA, DEGMA, UPyMA | DOX, PD-L1 antagonist D peptide | CT26 colorectal carcinoma | - DEGMA and UPyMA-conjugated HAMA supramolecular hydrogel encapsulating DOX and PD-L1 antagonist D peptide. - PD-L1 antagonist D peptide-based locoregional block of the PD-1/PD-L1 pathway, resulting in potentiating T cell-mediated immune responses and minimizing side effects. | [44] | |
| PEG-4000, α-CD | PEI-CA-DOX, CpG, DCs | B16 melanoma | - pH-sensitive adjuvant DOX prodrug- and DCs co-laden hydrogel for alleviating immunosuppressive TME by increasing tumor antigen generation and antigen presentation. - Promoting antigen presentation by the adjuvant and the implanted exogenous DCs. | [45] | |
| PLN-PEG | DOX, L-norvaline | B16F10 melanoma | - DOX-loaded L-norvaline-based polypeptide-b-PEG (PLN-PEG) hydrogel for combination chemoimmunotherapy by inducing ICD and blocking the arginase 1 (ARG1) pathway. - High drug loading efficiency and negligible side effects by using ARG1 inhibitor L-norvaline. | [46] | |
| Photoimmunotherapy | PEGDA | Ce6-Cat, R837-loaded PLGA NPs | 4T1 mammary carcinoma and CT26 colorectal carcinoma | - Ce6-CAT- and R837-loaded PLGA NPs-co-laden light-triggered hydrogel for reversing immunosuppressive TME in photodynamic immunotherapy of cancers. - Effective multi-round PDT and immunotherapy based on persistent tumor hypoxia relief. | [47] |
| Alginate-Ca2+ | Ce6, R837 | 4T1 mammary carcinoma | - “Turning solid into gel” strategy using PLM- and R837-co-laden alginate-Ca2+ hydrogel for rechargeable photodynamic immunotherapy of cancers. - Sustained and rechargeable efficient PDT with the PLM-based continuous internal light source. | [48] | |
| PEG, IR-820-α-CD | IR820, CpG | B16 melanoma | - Genipin-crosslinked CpG NPs-loaded PEG-IR-820-α-CD hydrogel for combinational photothermal immunotherapy. The hydrogel induces a hyperthermal effect, generating tumor antigens. - High loading efficiency and long-term release of CpG by self-crosslinked structures. - Image-guided therapy based on the PEG-IR-820-α-CD hydrogel for precise tumor treatment. | [49] | |
| GG | POM, R848 | 4T1 mammary carcinoma | - Dawson-type (P2Mo18) POM- and R848-co-laden gellan gum (GG) hydrogel for combinational photothermal immunotherapy. - High photothermal conversion effect and photostability during repeated treatment cycles. | [50] | |
| PND | MnO2 | 4T1 mammary carcinoma | - MnO2 nanoparticle-based in situ-formable thermosensitive nanogels that can capture tumor antigen for enhancing anticancer immune response and immune memory effect after photothermal immunotherapy. - Tumor antigen adsorption by using the adhesive antigen reservoir system, resulting in prolonging immune stimulation and enhancing DC recruitment. | [51] | |
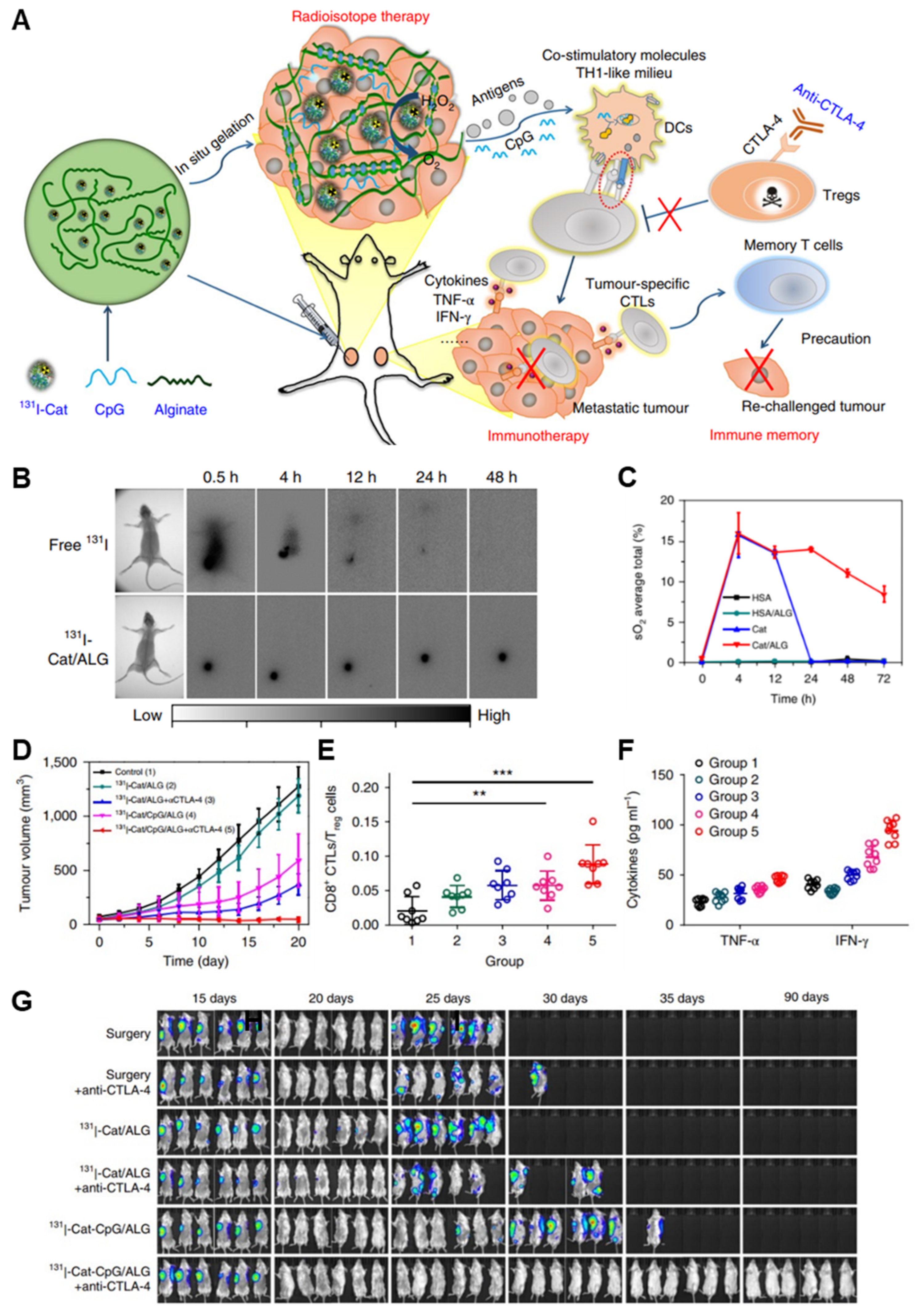
| Radioimmunotherapy | Alginate-Ca2+ | 131I-Cat, CpG | 4T1 mammary carcinoma and CT26 colorectal carcinoma, prostate cancer PDX model, rabbit VX2 liver cancer | - 131I-Cat- and CpG-co-laden injectable hydrogel that causes oxygenation by Cat-triggered decomposition of endogenous H2O2, enhancing radioimmunotherapy efficacy with a low dose of radioactivity. Based on the “vaccine-like” function of hydrogels, distant metastatic cancers were effectively eliminated when combining treatment with anti-CTLA-4 antibody. - Less invasive and easy application compared to the implantable 125I beads. - Long-term relief of tumor hypoxia and effective primary tumor elimination under low doses of radiation. | [52] |
| Aapt-conjugated alginate | CpG-cAptamer, oxaliplatin | 4T1 mammary carcinoma and CT26 colorectal carcinoma | - Aapt-conjugated alginate-based injectable hydrogel that can hybridize with CpG-cAptamer, resulting in ATP-responsive release of CpG by low doses of oxaliplatin or X-rays for the synergistic immune responses. - Immune adjuvant release synchronized with low-dose repeated RT, resulting in synergistic tumor elimination and long-term immune-memory effects. | [53] | |
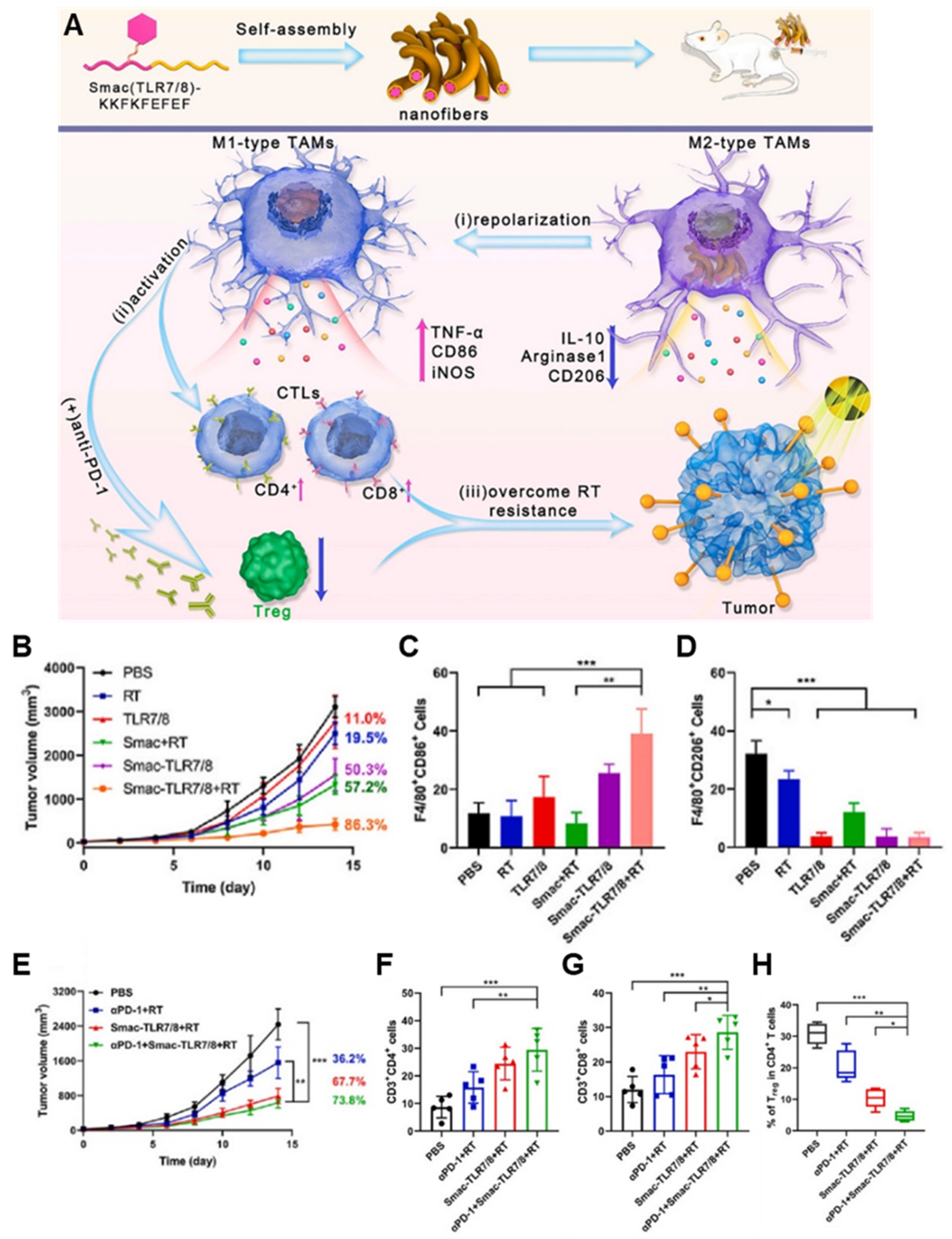
| Smac-conjugated TLR7/8 receptor agonist peptide | Smac-conjugated TLR7/8 receptor agonist peptide | B16 melanoma | - Self-assembled Smac-TLR7/8 peptide hydrogel that can repolarize tumor-associated macrophages into M1 type for improving anticancer immune responses by relieving radio-resistant TME. - Modulating the ITM and overcoming the radioresistance by reprogramming TAMs polarization toward the M1 phenotype. - Improving radio sensitivity and reducing the radiation dose due to the Smac mimetic peptide. | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, Y.; Kim, D.-H.; Yoon, H.Y.; Kim, K. Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy. Pharmaceutics 2022, 14, 1908. https://doi.org/10.3390/pharmaceutics14091908
Kim J, Choi Y, Kim D-H, Yoon HY, Kim K. Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy. Pharmaceutics. 2022; 14(9):1908. https://doi.org/10.3390/pharmaceutics14091908
Chicago/Turabian StyleKim, Jeongrae, Yongwhan Choi, Dong-Hwee Kim, Hong Yeol Yoon, and Kwangmeyung Kim. 2022. "Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy" Pharmaceutics 14, no. 9: 1908. https://doi.org/10.3390/pharmaceutics14091908
APA StyleKim, J., Choi, Y., Kim, D.-H., Yoon, H. Y., & Kim, K. (2022). Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy. Pharmaceutics, 14(9), 1908. https://doi.org/10.3390/pharmaceutics14091908







