A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy
Abstract
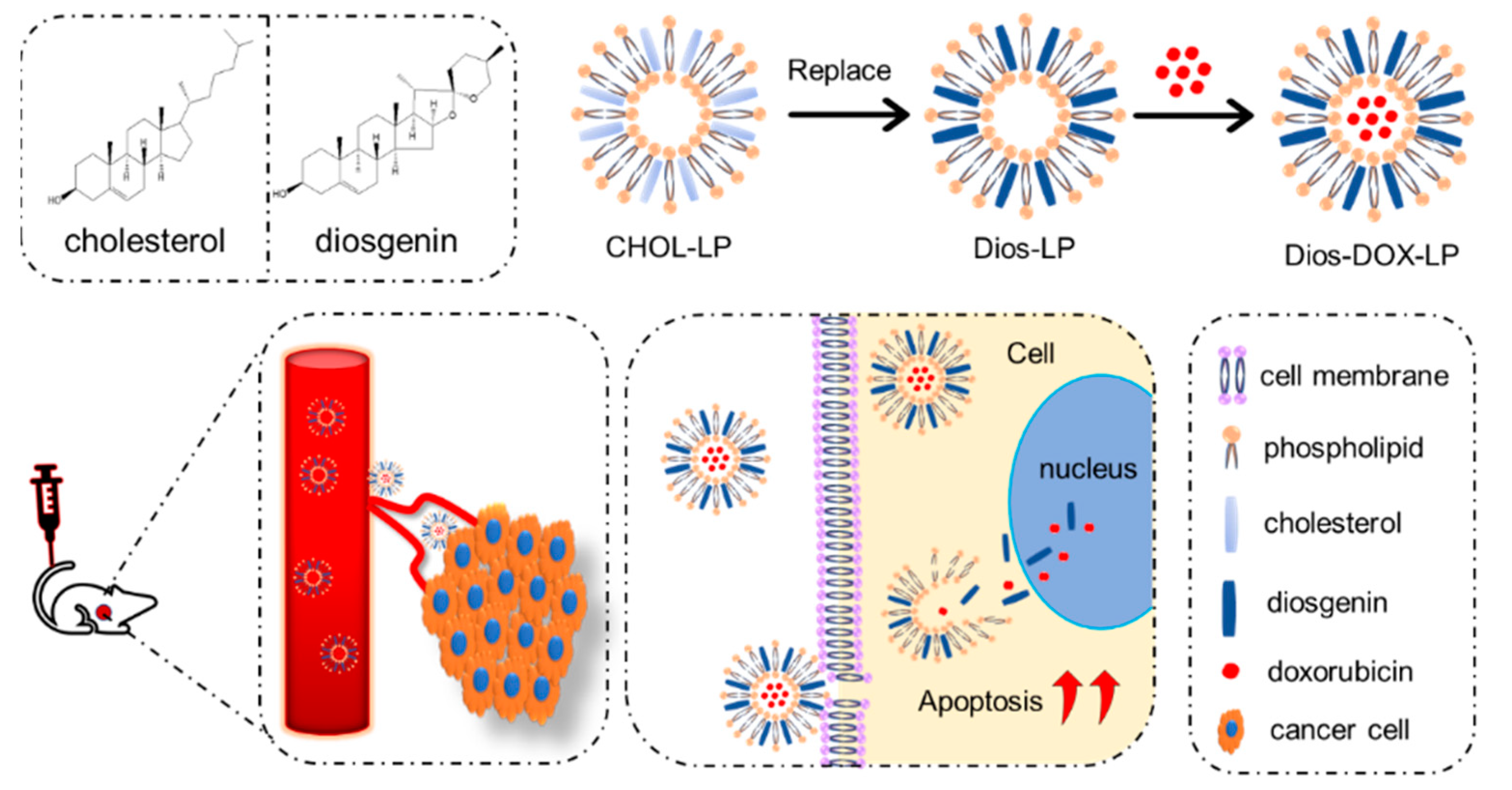
:1. Introduction
2. Materials and Methods
2.1. Materials
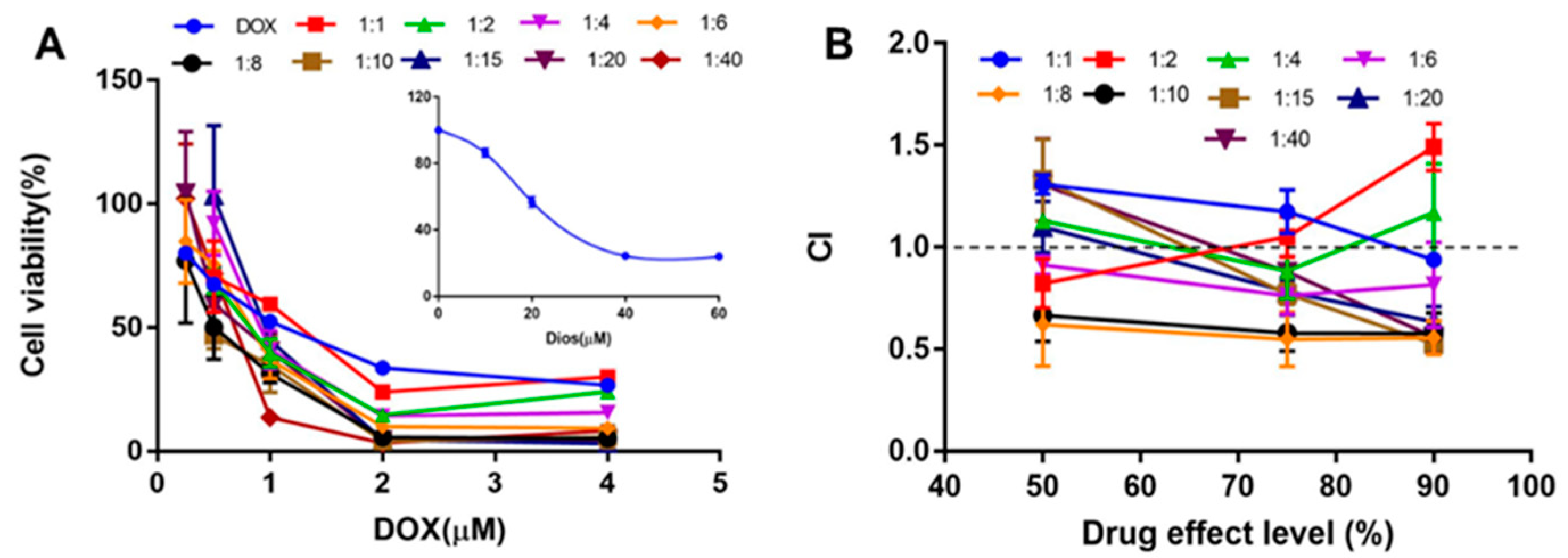
2.2. In Vitro Synergistic Effect of DOX and Dios on HepG2 Cell
2.3. Preparation of Liposomes
2.4. Characterization
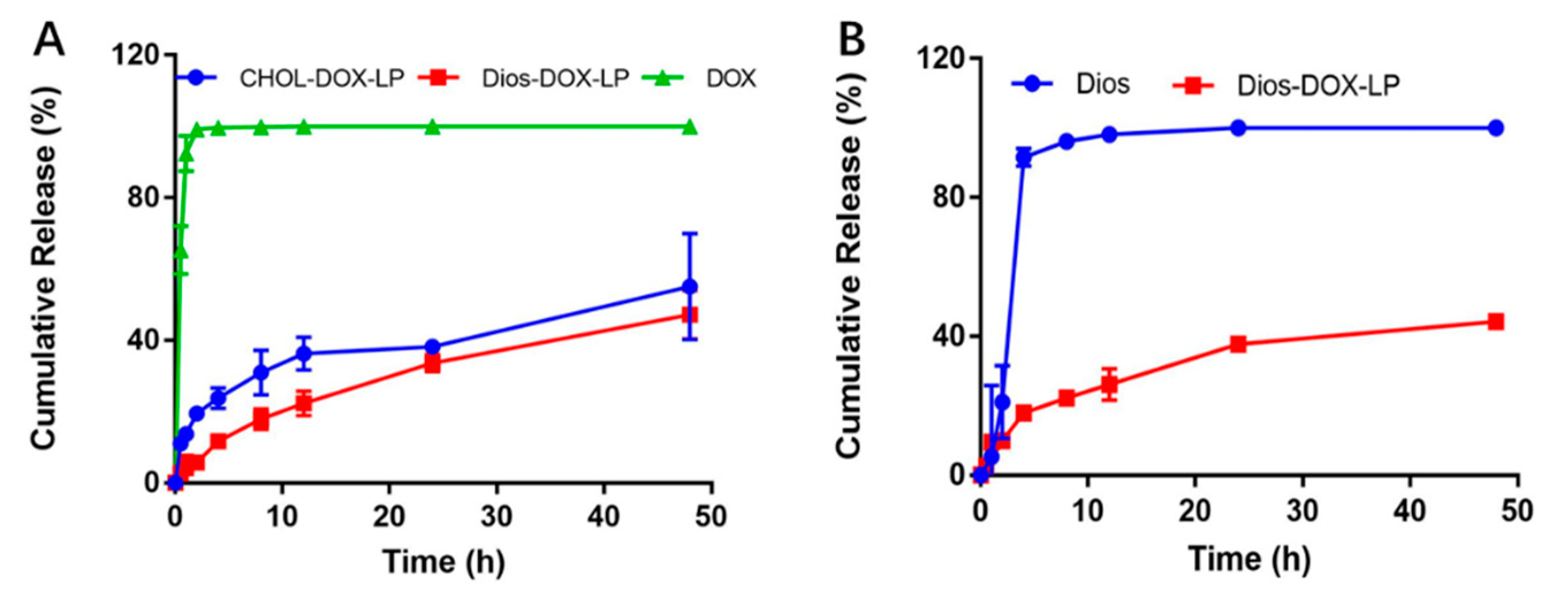
2.5. In Vitro Release of DOX and Dios from Liposomes
2.6. In Vitro Cell Cytotoxicity Assay
2.7. In Vitro Cell Apoptosis Assay
2.8. In Vivo Near-Infrared Imaging and Biodistribution
2.9. In Vivo Antitumor Activity
2.10. Statistical Analysis
3. Results
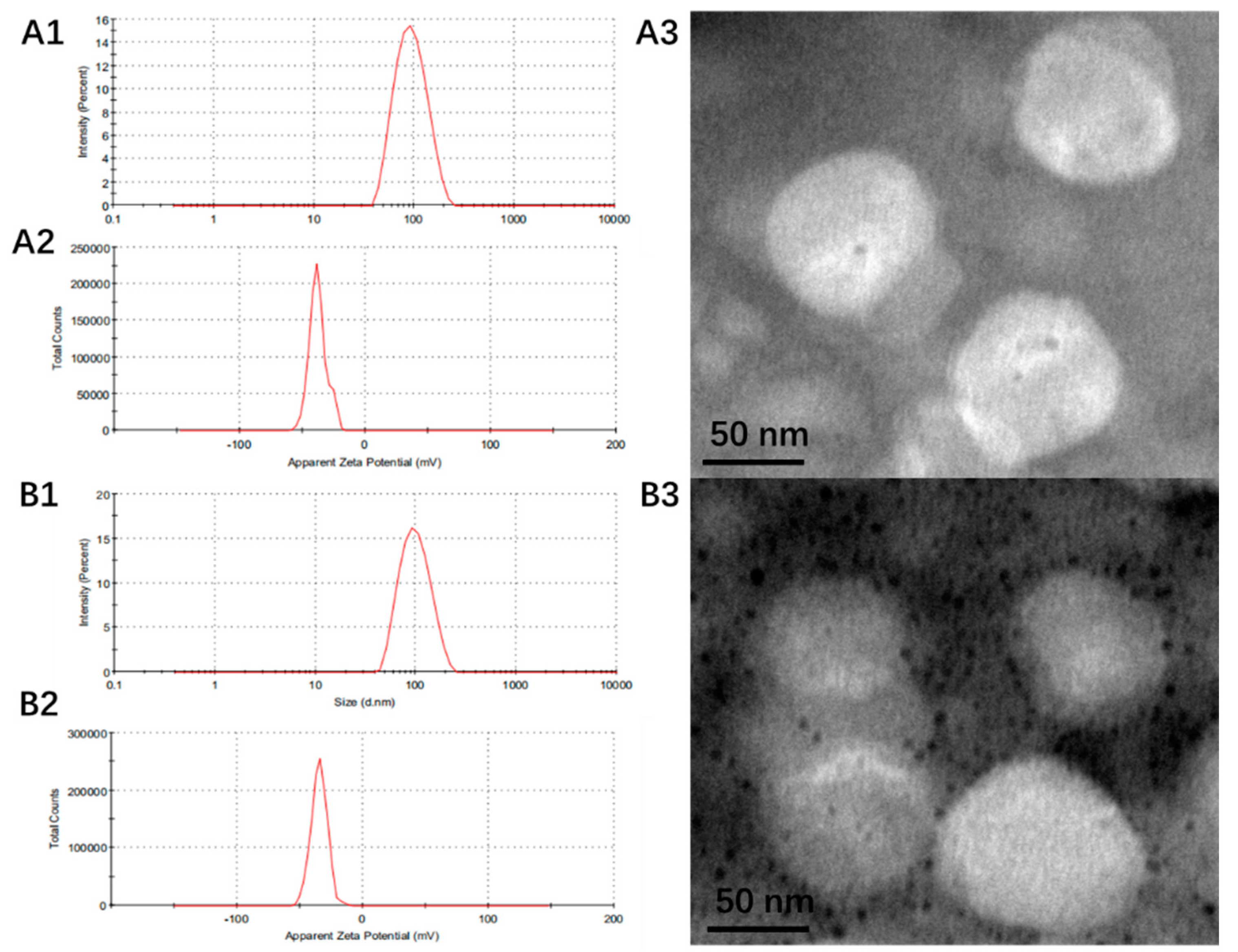
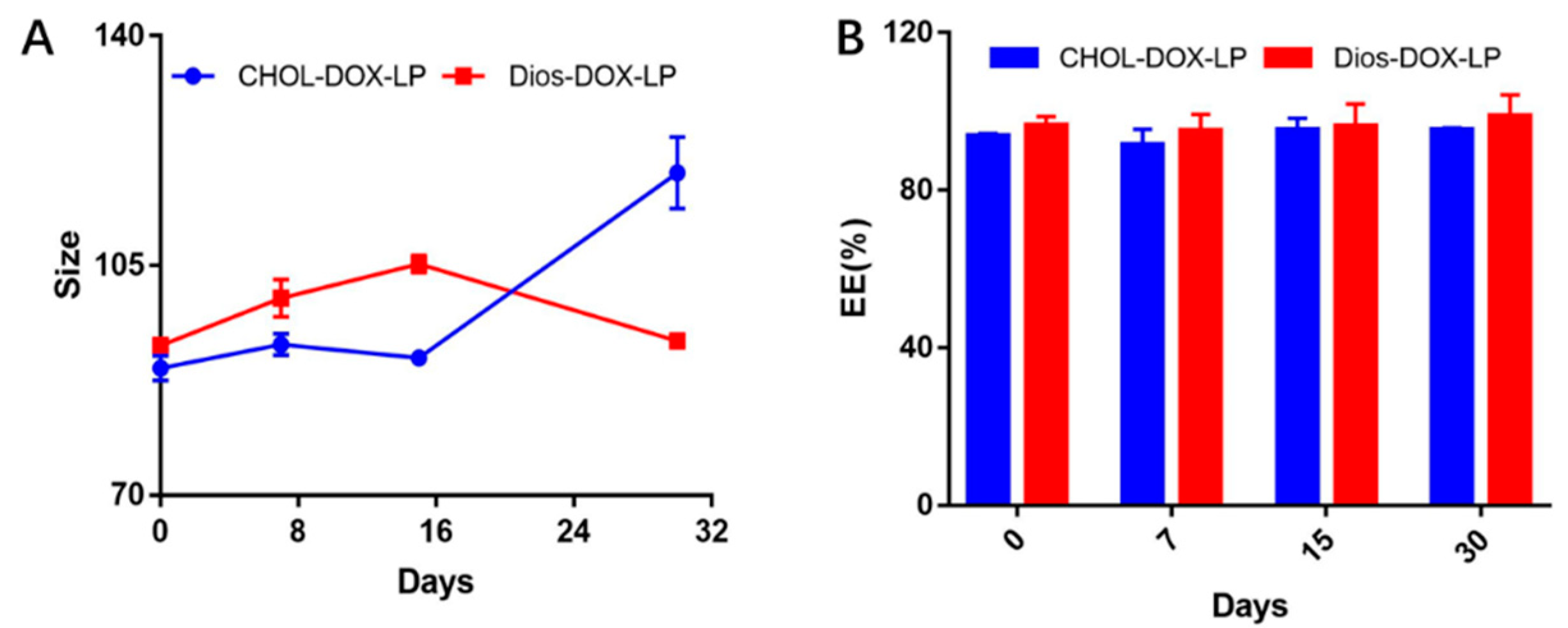
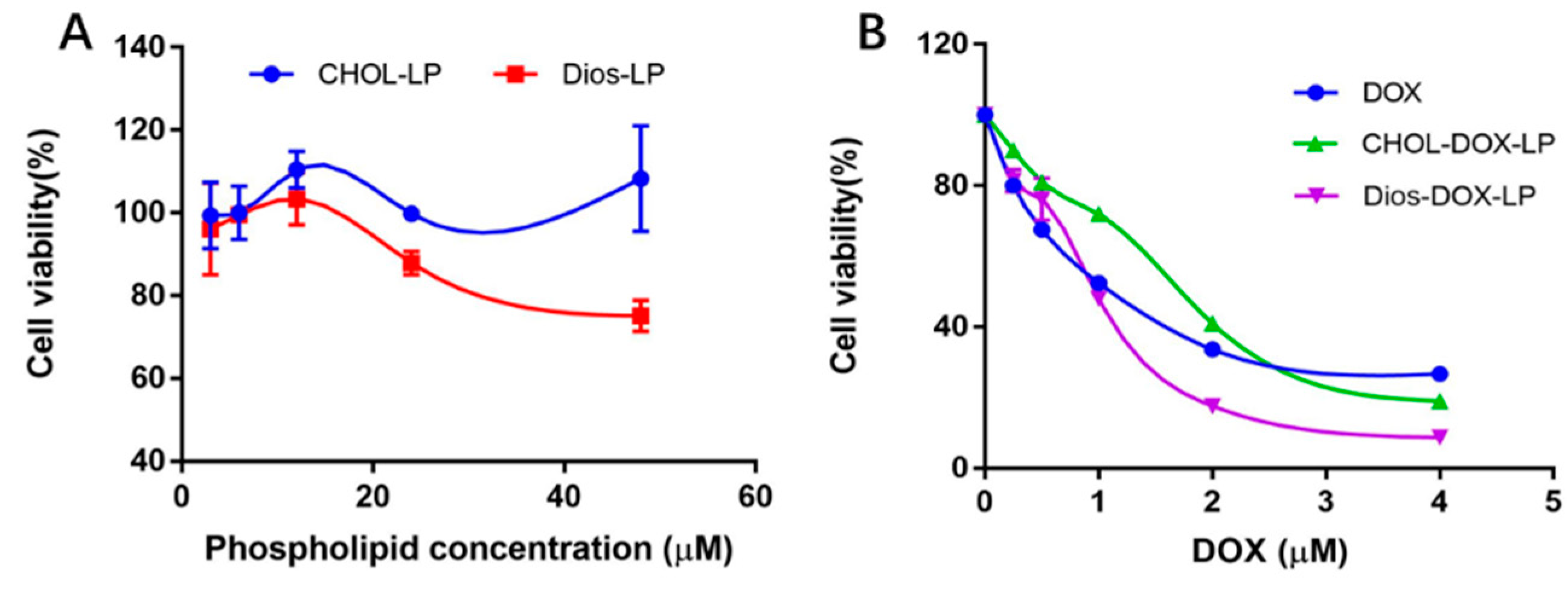
3.1. Preparation and Characterization of Liposomes
3.2. In Vitro Leakage and Release of DOX from Liposomes
3.3. In Vitro Cytotoxicity
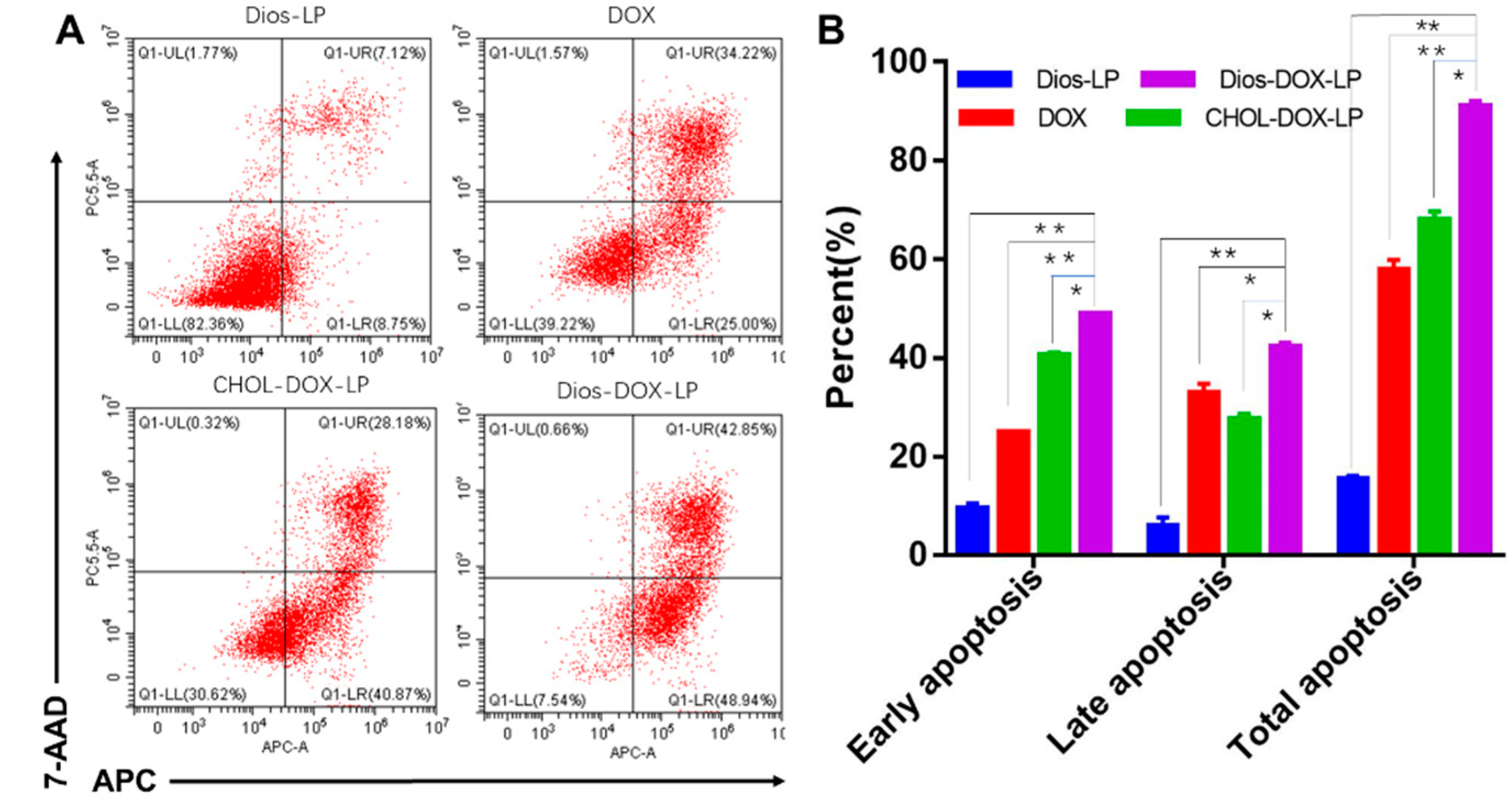
3.4. In Vitro Cell Apoptosis Assay
3.5. In Vivo Near-Infrared Imaging and Biodistribution
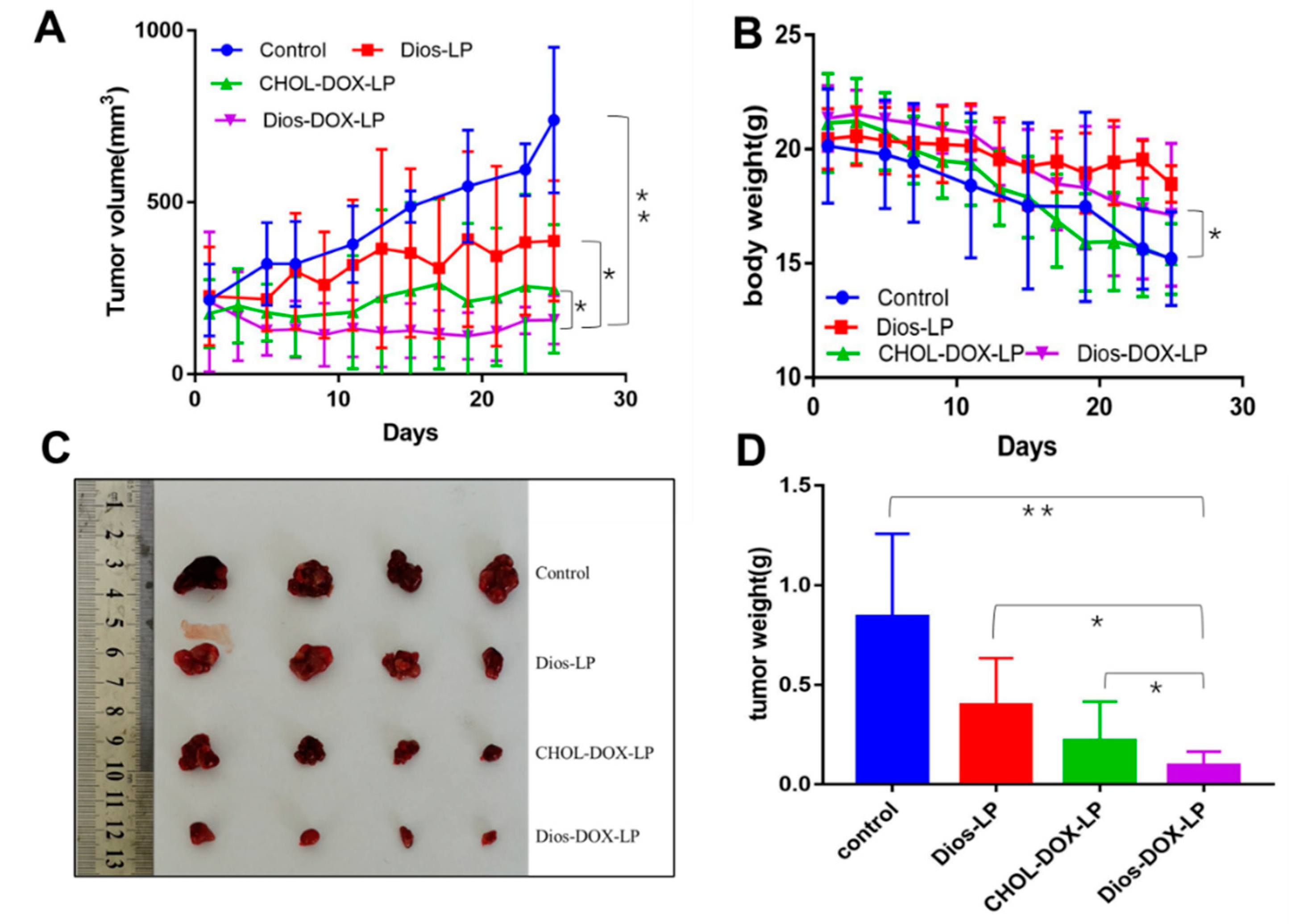
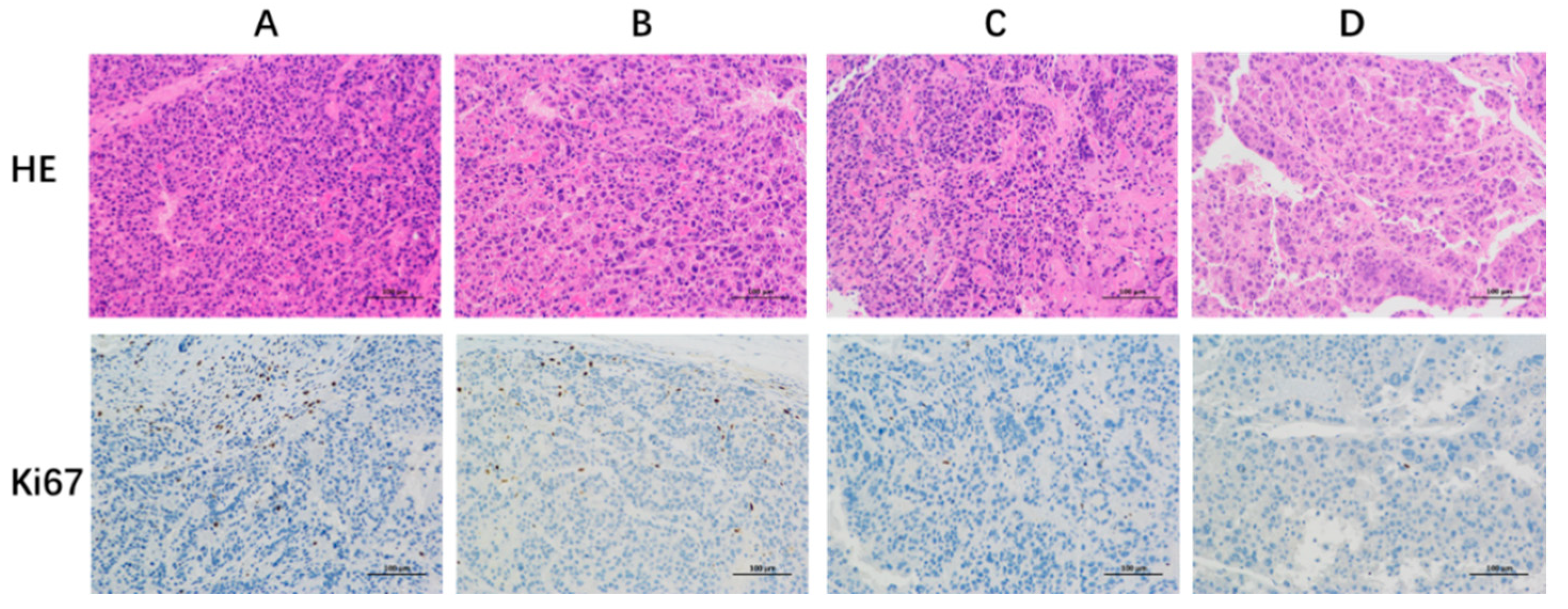
3.6. In Vivo Antitumor Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Josep, M.L.; Robin, K.K.; Augusto, V.; Amit, G.S.; Eli, P.; Sasan, R.; Riccardo, L.; Kazuhiko, K.; Jessica, Z.R.; Richard, S.F. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Varma, S.; Rajesh, S. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Grabarnick, P.E.; Andriyanov, A.V.; Han, H.; Sara, E.; Yechezkel, B. PEGylated Liposomes Remotely Loaded with the Combination of Doxorubicin, Quinine, and Indocyanine Green Enable Successful Treatment of Multidrug-Resistant Tumors. Pharmaceutics 2021, 13, 2181. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xiang, D.; Wang, T.; Yumei, Z.; Cuong, V.P.; Shufeng, Z.; Guoqin, J.; Yingchun, H.; Yimin, Z.; Yinglu, H.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Altayar, O.; O’Shea, R.; Raj, S.; Bassam, E.; Candice, W.; Shahnaz, S.; Yngve, F.Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Samuel, S. Diosgenin: Recent Highlights on Pharmacology and Analytical Methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Abdul, L.; Mir, T.; Muneeb, U.R.; Farrah, A.; Sarwat, S. Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: Probable role of p38MAPK and p53. Toxicol. Appl. Pharmacol. 2021, 258, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.F.; Moghadam, S.E.; Moridi Farimani, M.; Samad, N.E.; Marzieh, T.; Ehsan, J. A multi-targeting natural compound with growth inhibitory and anti-angiogenic properties re-sensitizes chemotherapy resistant cancer. PLoS ONE 2019, 14, 218125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.Y.; Wang, H.P.; Chen, H.; Wu, Y.C.; Wang, L.; Pu, X.; Yue, G.; Zhang, L. Natural compound Tan-I enhances the efficacy of Paclitaxel chemotherapy in ovarian cancer. Ann. Transl. Med. 2020, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ha, J.; Park, O.J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005, 332, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, J.; Liu, X.; Li, X.; Zhang, W.; Dai, Z.; Lu, L.; Zhou, X.; Cai, J.; Zhang, X. The Origin and Evolution of the Diosgenin Biosynthetic Pathway in Yam. Plant Commun. 2020, 2, 100079. [Google Scholar] [CrossRef]
- Raju, J.; Mehta, R. Cancer Chemopreventive and Therapeutic Effects of Diosgenin, a Food Saponin. Nutr. Cancer 2009, 61, 27–35. [Google Scholar] [CrossRef]
- Khathayer, F.; Ray, S.K. Diosgenin as a Novel Alternative Therapy for Inhibition of Growth, Invasion, and Angiogenesis Abilities of Different Glioblastoma Cell Lines. Neurochem. Res. 2020, 45, 2336–2351. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jeon, B.K.; Lee, Y.E.; Woo, W.H.; Mun, Y.J. Diosgenin Induces Apoptosis in HepG2 Cells through Generation of Reactive Oxygen Species and Mitochondrial Pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 981675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fan, J.; Wang, Q.; Ju, D.; Feng, M.; Li, J.; Guan, Z.B.; An, D.; Wang, X.; Ye, L. Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells. Phytomedicine 2016, 23, 243–252. [Google Scholar] [CrossRef]
- Sikka, S.; Shanmugam, M.K.; Siveen, K.S.; Ong, T.H.; Yang, M.H.; Lee, J.H.; Rajendran, P.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; et al. Diosgenin attenuates tumor growth and metastasis in transgenic prostate cancer mouse model by negatively regulating both NF-κB/STAT3 signaling cascades. Eur. J. Pharmacol. 2021, 906, 174274. [Google Scholar] [CrossRef]
- Li, S.Y.; Shang, J.; Mao, X.M.; Fan, R.; Li, H.Q.; Li, R.H.; Shen, D.Y. Diosgenin exerts anti-tumor effects through inactivation of cAMP/PKA/CREB signaling pathway in colorectal cancer. Eur. J. Pharmacol. 2021, 908, 174370. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Z.H.; Hsu, C.C.; Lin, H.H.; Chen, J.H. In Vivo Protective Effects of Diosgenin against Doxorubicin-Induced Cardiotoxicity. Nutrients 2015, 7, 4938–4954. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Jhan, S.; Pethe, A.M. Double-loaded liposomes encapsulating lycopene β-cyclodextrin complexes: Preparation, optimization, and evaluation. J. Liposome Res. 2020, 30, 80–92. [Google Scholar] [CrossRef]
- Xu, S.Y.; Su, H.; Zhu, X.Y.; Li, X.Y.; Li, J.; Chen, X.; Wang, Q.; Hao, R.Y.; Yan, X.Y. Long-circulating Doxorubicin and Schizandrin A Liposome with Drug-resistant Liver Cancer Activity: Preparation, Characterization, and Pharmacokinetic. J. Liposome Res. 2021, 13, 107–118. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; AlSuhaymi, N.; Alsugoor, M.H.; et al. The Effect of Liposomal Diallyl Disulfide and Oxaliplatin on Proliferation of Colorectal Cancer Cells: In Vitro and In Silico Analysis. Pharmaceutics 2022, 14, 236. [Google Scholar] [CrossRef]
- Farzaneh, H.; Ebrahimi, N.M.; Mashreghi, M.; Saberi, Z.; Jaafari, M.; Teymouri, M. A study on the role of cholesterol and phosphatidylcholine in various features of liposomal doxorubicin: From liposomal preparation to therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef]
- Chaudhury, A.; Das, S.; Lee, R.F.; Tan, K.B.; Ng, W.K.; Tan, R.B.; Chiu, G.N. Lyophilization of cholesterol-free PEGylated liposomes and its impact on drug loading by passive equilibration. Int. J. Pharm. 2012, 430, 167–175. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019, 30, 143–156. [Google Scholar] [CrossRef]
- Chen, L.X.; Ding, Y.; Shen, Z.W.; Zhang, T.; Lan, J.S.; Yang, J.; Zhang, N.L.; Chen, G. Research progress on role of cholesterol in liposomes and replacement with sterols and saponins. Chin. Tradit. Herb. Drugs 2020, 51, 6396–6404. [Google Scholar] [CrossRef]
- Ondevilla, J.C.; Hanashima, S.; Mukogawa, A.; Umegawa, Y.; Murata, M. Diosgenin-induced physicochemical effects on phospholipid bilayers in comparison with cholesterol. Bioorg. Med. Chem. Lett. 2021, 36, 127816. [Google Scholar] [CrossRef]
- Schilt, Y.; Berman, T.; Wei, X.; Nativ, R.E.; Barenholz, Y.; Raviv, U. Effect of the ammonium salt anion on the structure of doxorubicin complex and PEGylated liposomal doxorubicin nanodrugs. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129849. [Google Scholar] [CrossRef]
- Wei, X.; Cohen, R.; Barenholz, Y. Insights into composition/structure/function relationships of Doxil(R) gained from “highsensitivity” differential scanning calorimetry. Eur. J. Pharm. Biopharm. 2016, 104, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®-The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944. [Google Scholar] [CrossRef]
- Lan, J.S.; Liu, L.; Zeng, R.F.; Qin, Y.H.; Hou, J.W.; Xie, S.S.; Yue, S.; Yang, J.; Ho, R.J.; Ding, Y.; et al. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021, 407, 127212. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, G.; Zheng, H.; Mu, D.; Chen, H.; Lu, Z.; He, P.; Zhang, Y.; Liu, C.; Lin, Z.; et al. Biomimetic nanoparticles blocking autophagy for enhanced chemotherapy and metastasis inhibition via reversing focal adhesion disassembly. J. Nanobiotechnol. 2021, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Liu, L.; Wang, X.R.; Shuai, W.P.; Hu, Y.; Han, M.; Gao, J.Q. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int. J. Nanomed. 2016, 11, 1395–1412. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Xing, Y.; Wang, X.; Zhang, H.; Xia, M.; Wang, D. Dual-pH Sensitive Charge-Reversal Drug Delivery System for Highly Precise and Penetrative Chemotherapy. Pharm. Res. 2020, 37, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]


| DOX/Lipid Ratio (w/w) | Size (nm) | PDI | EE (%) |
|---|---|---|---|
| 1:30 | 99.4 ± 6.2 | 0.12 ± 0.03 | 98.77 ± 2.04 |
| 2:30 | 101.5 ± 4.6 | 0.17 ± 0.04 | 100.17 ± 6.86 |
| 4:30 | 98.6 ± 4.6 | 0.15 ± 0.03 | 86.37 ± 2.35 |
| Liposome | Size (nm) | PDI | Zeta (mV) | EE (%) | DL (%) | ||
|---|---|---|---|---|---|---|---|
| DOX | Dios | DOX | Dios | ||||
| CHOL-LP | 88.8 ± 5.5 | 0.24 ± 0.03 | −33.8 ± 5.2 | / | / | / | / |
| CHOL-DOX-LP | 90.3 ± 4.8 | 0.16 ± 0.02 | −38.4 ± 0.8 | 93.81 ± 0.53 | / | 2.54 ± 0.01 | / |
| Dios-LP | 98.2 ± 3.4 | 0.10 ± 0.02 | −33.8 ± 0.8 | / | 91.47 ± 4.27 | / | 14.83 ± 0.69 |
| Dios-DOX-LP | 99.4 ± 6.2 | 0.12 ± 0.03 | −33.3 ± 2.5 | 98.77 ± 2.04 | 87.75 ± 2.93 | 2.67 ± 0.55 | 14.23 ± 0.47 |
| IC50 | DOX | Dios-LP | CHOL-DOX-LP | Dios-DOX-LP |
|---|---|---|---|---|
| DOX | 1.03 ± 0.12 | / | 1.42 ± 0.02 | 0.87 ± 0.08 * |
| Dios | / | 33.93 ± 2.47 | / | 5.20 ± 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Lan, J.; Li, Z.; Zeng, R.; Wang, Y.; Zhen, L.; Jin, H.; Ding, Y.; Zhang, T. A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy. Pharmaceutics 2022, 14, 1685. https://doi.org/10.3390/pharmaceutics14081685
Chen L, Lan J, Li Z, Zeng R, Wang Y, Zhen L, Jin H, Ding Y, Zhang T. A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy. Pharmaceutics. 2022; 14(8):1685. https://doi.org/10.3390/pharmaceutics14081685
Chicago/Turabian StyleChen, Lixia, Jinshuai Lan, Zhe Li, Ruifeng Zeng, Yu Wang, Lu Zhen, Haojieyin Jin, Yue Ding, and Tong Zhang. 2022. "A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy" Pharmaceutics 14, no. 8: 1685. https://doi.org/10.3390/pharmaceutics14081685
APA StyleChen, L., Lan, J., Li, Z., Zeng, R., Wang, Y., Zhen, L., Jin, H., Ding, Y., & Zhang, T. (2022). A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy. Pharmaceutics, 14(8), 1685. https://doi.org/10.3390/pharmaceutics14081685






