Abstract
The oxidative stress resulting from the production of reactive oxygen species plays a vital role in inflammatory processes and is associated with neurodegenerative changes. In view of the ability of germinated brown rice (GBR) to improve learning and memory, this present study aimed to investigate the mechanistic basis of GBR’s neuroprotection in a high-fat diet (HFD)-induced oxidative changes in adult Sprague–Dawley rats. Ferulate-rich GBR ethyl acetate extract (GBR-EA; 100 mg/kg and 200 mg/kg body weight) was supplemented orally for the last 3 months of 6 months HFD feeding during the study. GBR-EA supplementation was found to improve lipid profile and serum antioxidant status, when compared to the HFD group. Elevated mRNA expressions of SOD1, SOD2, SOD3, Catalase, and GPX were demonstrated in the frontal cortex and hippocampus of GBR-EA treated animals. The pro-inflammatory changes induced by HFD in the hippocampus were attenuated by GBR-EA through the downregulation of CRP and TNF- α and upregulation of PPAR-γ. GBR also reduced the hippocampal mRNA expression and enzyme level of acetylcholinesterase. In conclusion, this study proposed the possible transcriptomic regulation of antioxidant and inflammation in neurodegenerative processes resulting from high cholesterol consumption, with an emphasis on GBR’s potential to ameliorate such changes.
1. Introduction
Reactive oxygen species (ROS) are continuously produced in the body via mitochondrial bioenergetics and oxidative metabolism, and they play a key role in the development of many diseases [1,2]. Increased dietary fat consumption contributes to obesity, which results in a chronic state of inflammation via the formation of white adipose tissue that secretes proinflammatory factors [3]. Furthermore, hypercholesterolemia is a metabolic disorder characterized by elevated total cholesterol levels in the blood, which can be caused by an unbalanced diet, obesity, inherited (genetic) diseases, or other diseases [4]. According to large clinical studies, hypercholesterolemia affects a significant population of adults in developed countries, as evidenced by this prevalence estimate. With a global population of 7.7 billion people in 2019, approximately 25 million people may have familial hypercholesterolemia [5].
Hypercholesterolemia is firmly linked to a pattern of chronic inflammation and has now been shown to have adverse effects on brain physiology and function, which is linked to neurodegenerative illnesses such as Alzheimer’s disease (AD) [6]. In afflicted regions of the brain from AD patients, aberrant pro-inflammatory cytokine production, activation of the inflammatory signaling cascade, acute-phase proteins, and other mediators have been identified [7,8]. Epidemiological research found that diets high in saturated fats were connected with an increased risk of developing endothelial dysfunction and AD [9,10]. Furthermore, patients with high cholesterol are found to be more likely to develop cognitive impairment, which is defined by a gradual deterioration in memory and other cognitive and executive capabilities [11]. Another study revealed that if the total cholesterol in the brain membrane rises, synapses do not function normally, affecting cognitive degradation in AD [12]. Moreover, an elevated low-density lipoprotein cholesterol (LDL-C) level was an independently associated risk factor for the development of AD. The pooled effect size revealed a substantial increase in the risk of AD for people with higher LDL-C levels [13]. According to previous research, elevated levels of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) cause the extracellular deposition of amyloid-β protein (Aβ), obstructing neuronal synaptic connections in the brain and increasing the risk of AD, as well as being associated with worse cognitive function [13,14]. Additionally, high cholesterol has been shown to affect the cholinergic system by decreasing ACh and choline acetyltransferase activity, while boosting AChE activity in the brain [15,16]. The usage of cholesterol-lowering medicines may have slowed the progression of AD [11], implying that they may provide protection against dementia [17].
Hypercholesterolemia is largely influenced by nutrition, hence many approaches to managing the condition center on changing one’s diet. It has been proven that food intake adjustment does not have to be substantial to have favorable long-term effects [11,18], and such changes are rather easy to implement. Dietary adjustments, such as increasing DHA intake or consuming foods rich in flavonoid, can help lower cholesterol and reduce the incidence of AD [18,19]. Oral DHA supplementation in animal tests has been shown to lessen the progression of Alzheimer’s-like brain degeneration [20]. Germinated brown rice (GBR) has numerous bioactives that contribute to its antioxidant effects [21]. Many of GBR’s functional properties can be attributed to its high antioxidant content, which includes ferulic acid, oryzanol, and gamma-aminobutyric acid (GABA), and which is increased during the germination process [22]. Antioxidant-rich foods have shown promise for the prevention of neurodegenerative illnesses such as AD [23]. GBR has also been shown to increase brain function, notably memory and learning [22,24]. Despite this, there is no evidence of the process at work. Thus, this study aimed to evaluate the effects of GBR extract on indicators of oxidative stress and inflammation in the brain of an in vivo hypercholesterolemia model of sporadic AD, as well as the mechanism of action.
2. Materials and Methods
2.1. Reagents
Brown rice of Malaysian mixed varieties was procured from PadiBeras Nasional (BERNAS) factory (Sri Tiram Jaya, Selangor, Malaysia). Hydrogen peroxide (H2O2) was purchased from Bendosen Laboratory Chemicals (Selangor, Malaysia) and sodium hypochlorite (NaOCl) was from Dexchem Industries Sdn. Bhd. (Penang, Malaysia. An AChE ELISA (enzyme-linked immunosorbent assay) kit was purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, Hubei, China). A Total RNA Extraction kit was purchased from RBC Bioscience Corp. (Taipei, Taiwan), and a GenomeLab™ GeXP Start Kit was from Beckman Coulter Inc. (Miami, FL, USA). MgCl2 and deoxyribonucleic acid (DNA) Taq polymerase were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA), Simvastatin was purchased from Pfizer (New York, NY, USA), and Donepezil hydrochloride was purchased from Cayman Chemical (Ann Arbor, MI, USA). Cholesterol and Bradford reagent were purchased from Amresco (Solon, OH, USA). Cholic acid was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Analytical grade ethanol was purchased from Merck (Darmstadt, Hesse, Germany). Palm oil and standard rat pellets were from Yee Lee Edible oils Sdn. Bhd. (Ipoh, Perak, Malaysia) and Specialty Feeds (Glen Forrest, WA, Australia), respectively. RCL2 Solution was purchased from Alphelys (Toulouse, Plaisir, France). Lipid profile kits were from Randox Laboratories Ltd. (Crumlin, County Antrim, UK).
2.2. Germination of Brown Rice and Extraction
Germination of brown rice was carried out according to a previous publication. The ground powder of germinated brown rice was subjected to ethyl acetate (1:4 w/v) extraction. The germinated brown rice ethyl acetate extract (GBR-EA) obtained comprised a considerable amount of γ-oryzanols (24-methylene cycloartanyl ferulate, campesteryl ferulate, cycloartenyl ferulate, as well as mixtures of β-sitosteryl ferulate and cycloartanyl ferulate), 2MHQ, cinnamic acid, rosmarinic acid, and guaiacol [25,26].
2.3. Diet Preparation
A high-fat diet (HFD) with 5% cholesterol was prepared by mixing a certain amount of normal rat pellet, oil, corn start, cholesterol, and cholic acid (Table 1), according to previous studies [27]. A sufficient amount of water was added to the mixture, to bind the components together. The HFD was cut into smaller pieces and dried in an incubator at 50 °C for 24 h, and fed to the rats according to their respective groups.

Table 1.
Diet composition and treatments.
2.4. Animal Experiments
Seventy (70) 2-month-old, male Sprague–Dawley rats weighing between 250 and 280 g were used in this study. Rats were individually housed in stainless steel cages in a well-ventilated room with a 12/12-h light/dark cycle at an ambient temperature of 25–30 °C. Experiments were carried out according to the guidelines for the use of animals and approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicines, Universiti Putra Malaysia (Project approval number: UPM/IACUC/AUP-RO64/2013). The rats were divided into 7 groups, with 10 rats per group (n = 10).
After acclimatization, 1 mL of blood was withdrawn through retro orbital as baseline biochemical data. Next, all rats were fed with HFD for 3 months, except for the normal control group. After the induction period, the groups were subjected to respective interventions via oral gavage for another 3 months. Dosages of Probucol, Simvastatin, and Donepezil were chosen based on previous findings [28,29,30].
Simvastatin, Donepezil and GBR-EA extracts, at varying doses required for interventions (Table 1), were prepared in the form of a per ml water suspension/dissolved solution. The actual volume of water suspension/dissolved solution administered was subsequently adjusted based on the animal body weights in kg. The volumes used in the present interventions were in the range of 1.1–2.0 mL. As for Probucol, owing to its poor wettability, the drug was first dispersed in distilled water via sonication at a concentration of 25 mg/mL. Subsequently, 8 mL/kg of the prepared Probucol (in the range of 2.40–3.80 mL based on the animal body weight) was administered via the oral route [28].
Intervention lasted for 24 weeks in total. Body weights were measured weekly, while food intake was measured daily. At the end of the experiment, the animals were fasted overnight and sacrificed by decapitation under pentobarbital intraperitoneal anaesthesia (0.5 mg/g body weight). The blood was collected in serum separator tubes (BD Vacutainer, Plymouth, UK) via cardiac puncture. The brains, livers, kidneys, and hearts were immediately exteriorized, snap frozen in liquid nitrogen, and kept at −80 °C until analysis.
2.5. Determination of Serum Biochemical Profile
Serum total cholesterol, LDL, HDL, triglycerides, and glucose level were measured for all animals following blood collection using Randox analytical kits, according to the manufacturer’s instructions and using a Selectra XL chemistry analyzer instrument (Vita Scientific, Dieren, The Netherlands).
2.6. Determination of Serum Total Antioxidant Status
Serum total antioxidant status was determined by ABTS assay. An ABTS radical cation was generated by persulfate oxidation of ABTS. Serum antioxidant was measured by proper mixing 10 μL of a serum sample, 40 μL of ddH2O, and 950 μL of ABTS reagent. The mixtures were incubated in the dark at room temperature for 30 min. The absorbance was then read at 734 nm using a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). The radical scavenging activity of the serum was measured by the decrease in the absorbance, and % of radical scavenging activity was calculated using a standard curve (y = 0.736x − 0.132, R2 = 0.9978).
2.7. Analysis of Antioxidant and Inflammation-Related Gene Expressions
2.7.1. RNA Extraction
RNA was extracted from frontal cortex and hippocampal tissue using a Total RNA Isolation kit (RBC Bioscience Corp., Taiwan, China) as per the manufacturer’s instructions. The RNA concentration was determined using a NanoDrop spectrophotometer (Thermo Scientific Nanodrop, NanoDrop Technologies, Wilmington, DE, USA). The ratios of A260/230 and A260/280 were used to indicate the purity of the extracted total RNA.
2.7.2. Primer Design
Primers were designed on the GenomeLabeXpress Profiler software, using the Rattus norvegicus sequence adopted from the National Center for Biotechnology Information GenBank Database (http://www.ncbi.nlm.nih.gov/nucleotide/, accessed on 12 November 2013). Genes of interest, housekeeping genes, and the internal control are shown in Table 2. Specificity validation of the nucleotide sequences was performed using NCBI-nucleotide-BLAST. An additional 37 base pairs of universal tag sequences were attached to each forward and reverse primer. The primers were supplied by First Base Ltd. (Selangor, Malaysia), and diluted in 1 X TE Buffer to a concentration of 500 nM for reverse primers and 200 nM for forward primers.

Table 2.
Gene, accession number, and reverse and forward primer sequences used in GeXP Multiplex Gene Expression Analysis.
2.8. Reverse Transcription and Polymerase Chain Reaction
Reverse transcription (RT) and multiplex PCR of RNA samples (50 ng/μL) were carried out in an XP Thermal Cycler (BIOER Technology, Hangzhou, China), according to a previous publication [25].
2.9. GEXP Data Analysis
Data analysis was performed using eXpress Profiler software, as in a previous publication [25]. Normalization was performed with GAPDH, according to the manufacturer’s instructions.
2.10. Quantification of Acetylcholinesterase (AChE) Level
Brain AChE level was quantified in the hippocampal brain regions by ELISA using an Elabscience kit as per the manufacturer’s protocol. Briefly, the brain tissue (30 mg) was homogenized thoroughly in 180 μL of phosphate-buffered saline (PBS) and centrifuged at 100× g for 15 min at 4 °C. Prior to ELISA analysis, the protein concentrations of all samples were determined by standard Bradford assay [31]. AChE level was quantified using calorimetric sandwich ELISA kits and calculated based on the standard curve obtained.
2.11. Statistical Analysis
Data were analyzed using IBM SPSS Statistics (SPSS Inc., Chicago, IL, USA). The results were expressed as mean ± SD. One-way ANOVA and Tukey’s post hoc test were performed to determine the level of significance difference, where p < 0.05 was considered significant.
3. Result and Discussion
3.1. Effects of GBR-EA on Caloric Intake and Body Weight
Table 3 displays the food intake and weight of animals for each group. Food intake was not significantly different among all groups (p > 0.05), despite the different dietary interventions. Body weights at the beginning of the dietary feeding regimen were not significantly different among the groups (p > 0.05). However, at the end of intervention, increased body weight gain was observed in the HFD group compared with the normal control and other treatment groups. Treatment with Donepezil, Simvastatin, Probucol, and GBR-EA100 and GBR-EA200 showed a trend of lowered body weight changes, even though they were not significantly different compared to HFD group.

Table 3.
Food intake and body weight gain of experimental rats.
The fact that sporadic AD accounts for the majority (>90%) of AD cases, the choice of valid models is important to enable the evaluation of early pathological processes that are often not accessible in patients and that subsequently provide feasibility and aid in target discovery and drug development [32]. Oral cholesterol intake was selected on the basis of previous experiments by other researchers [26,33,34]. One confounding factor in studies utilizing high-fat and/or high-cholesterol diet is that such diets often results in substantial weight gain, which may stimulate Aβ accumulation in the brain region per se [34,35] and may have detrimental effects on cognition [34,36]. GBR’s effects in reducing weight gain were previously documented [37,38], and its anti-obesity effects were found to be through the regulation of pancreatic lipase, decrease in fat accumulation by inhibition of adipocyte differentiation and adipocytokine production, as well as the stimulation of lipolysis on adipocytes [39,40]. The reduction in weight gain for rats treated with GBR-EA in this study could be explained by its moderate effects on pancreatic lipase inhibition, reduced lipid accumulation in adipocytes, and stimulation of lipolysis [39,40].
3.2. Effects of GBR and GBR-EA on Serum Biochemical Profile
Figure 1 shows the biochemical analysis of the serum at the end of the experiment. Total serum cholesterol (TC) differed among the groups, with the HFD group showing the highest elevation in comparison with the normal control groups (p < 0.05). Treatment with Probucol, a non-statin anti-hypercholesterolemic drug, showed a better reduction of cholesterol level in comparison with Simvastatin, but this was not statistically significant (p > 0.05). Interestingly, the Donepezil group showed a significantly reduced level of TC compared to HFD (p < 0.05). The reason behind the reduced serum cholesterol with Donepezil in this study is currently unknown. However, Donepezil was shown to reduce cholesterol accumulation in the brain cells both in vitro and in vivo via the inhibition of cholesterol synthesis [39], this may provide a clue regarding the modulation of cholesterol homeostasis by the drug in this study. LXRs act as a dominant regulator in cholesterol metabolism, including cholesterol synthesis, uptake, and trafficking, and ACh was shown to modulate cholesterol level through activating the LXR pathway [41]. Thus, the modulation of cholesterol homeostasis by Donepezil may be explained through its AChE inhibitory actions.
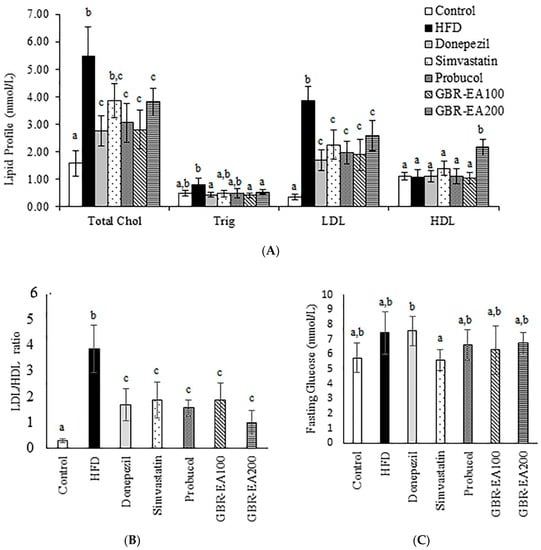
Figure 1.
Serum biochemical profile in rats fed with a high-fat diet (HFD) for 6 months, as determined using a chemistry analyzer. (A) Lipid profile, (B) LDL/HDL ratio, and (C) fasting glucose levels were measured in the serum. Values represent the mean ± SD. a–c Mean values with different letters were significantly different among the groups (p < 0.05).
The treatment with GBR-EA at 100 mg/kg BW and 200 mg/kg BW improved the condition when compared to HFD (p < 0.05). Meanwhile, the level of Triglycerides was shown to be elevated for the HFD group in comparison the with normal control group, with no statistically significant differences (p > 0.05). However, a significant reduction was shown for Donepezil, GBR-EA100, and GBR-EA200 when compared to the HFD group (p < 0.05). Other interventions showed no difference when compared to the HFD group. The LDL level in HFD group was found to be significantly higher when compared to the normal control group (p < 0.05). Treatment with Donepezil, Simvastatin, and Probucol showed significant reductions (p < 0.05). Similar effects were observed for GBR-EA at 100 mg/kg BW and 200 mg/kg BW.
It appears that the modulation of dietary lipids presents a potential treatment approach that is especially well suited for long-term chronic disease prevention. Unlike conventional pharmaceutical therapies, dietary interventions are essentially devoid of, or at least present fewer, unwanted side effects. The current findings are in agreement with previous reports in which treatment with GBR and its GABA- and ASG-rich extracts lowered the blood cholesterol level in HFD and STZ-induced diabetic rats [38,42]. Several other studies using GBR also reported similar results [43,44]. Previous findings indicated that the bioactives responsible for GBR’s hypocholesterolemic effects include GABA, phytosterol glycosides such as ASG, oryzanol, and phenolics compounds, which were found to be higher in GBR than in brown rice and white rice [42,44,45]. Therefore, it is likely that the hypocholesterolemic effects observed in the current study could be attributed to the presence of compounds such as guaiacol, 2MHQ, rosmaric acid, and γ-oryzanol components, as determined by a previous HPLC analysis [24]. The hypocholesterolemic effects of GBR were suggested to result from the cumulative effects of these reported compounds on the regulation of cholesterol metabolism markers, including ApoA1, LDL-R, lipoprotein lipase, and PPAR-γ [42,44,45].
On the other hand, the level of HDL among all groups was observed to have no significant difference, except for GBR-EA at 200 mg/kg BW, which showed a significant increment (p < 0.05). Fasting blood glucose level was not significantly different among the control and HFD groups (p > 0.05), even though the HFD group showed a slight elevation. Intervention with Simvastatin showed a reduced glucose level in comparison to HFD, even though this was not significantly different. Probucol, GBR-EA100, and GBR-EA200 did not show significant differences when compared to the control and HFD. Elevated fasting glucose levels and hyperlipidemia were attributed to neurological deficits [33,34,46], thus suggesting the role of glucose intolerance and/or insulin resistance with associated brain inflammation [46]. Even though it is difficult to separate the direct effect of impaired insulin homeostasis on the brain from the accompanying disruption in peripheral and central glucose homeostasis, it is clear that diet-induced alterations in lipid and glucose metabolism, as well as excessive caloric intake, activates certain mechanisms that are detrimental for neuronal plasticity and function.
3.3. Effects of GBR-EA on Serum Total Antioxidant Status
As shown in Figure 2, it was found that the level of ABTS radical scavenging activity in the serum was significantly reduced in HFD-fed rats when compared to the normal control group (p < 0.05). This finding revealed that the treatments with Donepezil, Probucol, and 100 mg/kg and 200 mg/kg of GBR-EA enhanced the scavenging activity significantly more than the HFD group (p < 0.05). Diet-induced oxidative stress secondary to high glycemic load and/or hypercholesterolemia may have a role in lowering antioxidant status in chronic diseases [47,48]. Chronic sustained hyperglycaemia was previously documented to cause low antioxidant status in a STZ-induced type 2 diabetes rat model fed a high-fat diet, and supplementation with GBR in their diet was found to increase the antioxidant level in the serum, in the same model [44]. The same study revealed that supplementation with white rice did not improve the antioxidant status. Even though a different model was used, the present study provided consistent findings, in which GBR-EA extract was able to improve serum antioxidant status. Maintenance of antioxidant status by GBR suggests that GBR and its extract (100 and 200 mg/kg BW) were able to replenish the supply of antioxidants that maintain serum antioxidant status and/or prevent the deterioration resulting from hypercholesterolemia. GBR’s effects on the improvement of biochemical profile and serum antioxidant status were consistent with the previous report on its antihypercholesterolemic effects in a diabetic rat model [44]. The effect of GBR on antioxidant status herein may have been a result of the higher antioxidant potentials of GBR, and likely due to the higher content of bioactive compounds in GBR [38,44].
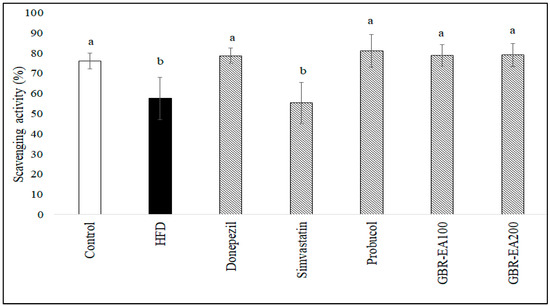
Figure 2.
Serum total antioxidant status in rats fed a high-fat diet (HFD) for 6 months, as determined by ABTS assay. Values represent the mean ± SD. a–b Mean values with different letters were significantly different among the groups (p < 0.05).
3.4. Effects of GBR-EA on Antioxidant and Inflammatory Gene Expressions
Figure 3 and Figure 4 show the expression of antioxidant genes, namely superoxide dismutases (SODs), catalase, and GPX, in both the frontal cortical and hippocampal regions of the rats’ brains. The current study showed that consumption of a high-cholesterol diet markedly suppressed SOD1, SOD2, and SOD3, as well as catalase and GPX in both the frontal cortex and hippocampus areas (p < 0.05). In contrast, treatment with GBR-EA (100 mg/kg and 200 mg/kg) significantly elevated the expression of all antioxidant genes, namely SOD1, SOD2, SOD3, catalase, and GPX in the frontal cortex, and SOD1, SOD2, catalase, and GPX in hippocampus, when compared to the HFD group (p < 0.05). However, GBR-EA at both doses did not show any significant difference compared to the HFD group for SOD3 expression in the hippocampus (p > 0.05). It was noted that the increment caused by GBR-EA was in a dose-dependent manner in the hippocampus for SOD1, while similar effects were observed for SOD2, catalase, and GPX in both the cortex and hippocampus. In the hippocampus, there was no significance difference for the expression of SOD2 and GPX among the GBR-EA100 and GBR-EA200 groups (p > 0.05). All GBR-EA groups showed no significant difference in terms of their frontal cortical catalase and hippocampal SOD3 expression (p > 0.05). Interestingly, treatment with Simvastatin did not show any significant differences when compared with HFD, in terms of all antioxidant genes (SOD1, SOD2, SOD3, catalase, and GPX), in both regions (p > 0.05).
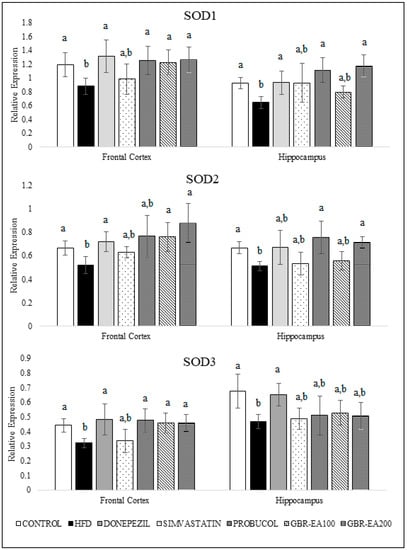
Figure 3.
Expression level of superoxide dismutases (SODs) in the frontal cortex and hippocampus of rats fed a high-fat diet (HFD) for 6 months, as determined using a multiplex GeXP analysis system. Values represent the mean ± SD. a–b Mean values with different letters were significantly different among the groups (p < 0.05).
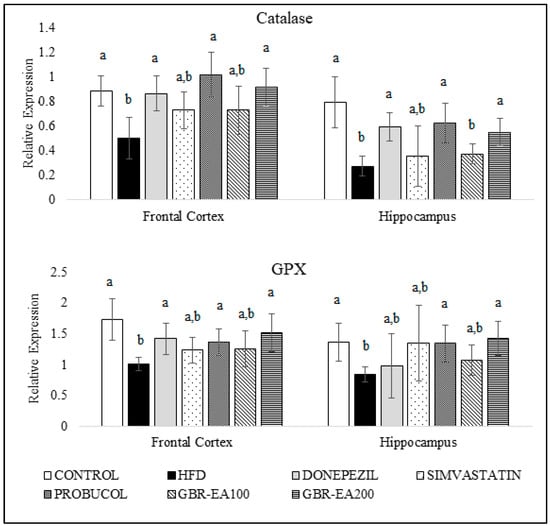
Figure 4.
Expression level of catalase and GPX in the frontal cortex and hippocampus of rats fed a high-fat diet (HFD) for 6 months, as determined using a multiplex GeXP analysis system. Values represent the mean ± SD. a–b Mean values with different letters were significantly different among the groups (p < 0.05).
It is recognized that oxidative stress is a major factor associated with the development and progression of AD and other forms of dementia. The brain is enriched with readily peroxidizable polyunsaturated fatty acids, and is preferentially susceptible to oxidative stress [49] due to its high oxygen consumption. Moreover, unlike other organs, the brain is not particularly well equipped with high antioxidant defenses. It has a low level of catalase and only moderate amounts of the endogenous antioxidant enzymes SOD and GPX. The hippocampus is the site of structural abnormalities associated with the early stages of AD and other cognitive dementias. The hippocampus, which is responsible for memory and cognition, is highly susceptible compared to other brain regions to a variety of insults including environmental toxicants, vascular risk factors, and metabolic perturbations [26,50], and was found to be susceptible to the oxidative stress caused by hypercholesterolemia [26,51].
The results from the current investigation, as well as those from other published studies, demonstrate that the consumption of a high-fat/cholesterol diet not only affects the peripheral vasculature, as implied by the serum antioxidant and biochemical profile, but also induces oxidative changes in these brain regions [26,34,51]. In this study, HFD consumption reduced SODs, catalase, and GPX in both the frontal cortical and hippocampal regions of the brain, implying a reduced defense against oxidative stress in both areas, which are highly prone to being affected in AD cases. In addition, it was revealed that SOD1 downregulation and deficiency promoted Aβ oligomerization and memory loss in a mouse model of AD [52,53]. Oxidative stress in the brain caused by excessive cholesterol intake was evidenced by the suppressed expression of antioxidant genes, as a result of overwhelming toxic stimuli that are able to surpass the cellular defense system. Therefore, the upregulation of antioxidant defenses is an attempt to boost endogenous antioxidants, to prevent oxidative damage on cells [54].
Interventions with antioxidants such as GBR exhibited neuroprotection, by which they are able to boost the cellular mechanism for clearing the free radicals and counteract the oxidative stress. The neuroprotective properties of GBR have been partly reported, and the mechanisms underlying its effects on the brain remain to be determined. The results of this current study indicate that the effect of GBR against oxidative stress may be mediated by the induction of endogenous antioxidant defenses through the upregulation of SOD1, SOD2, SOD3, catalase, and GPX, in both the frontal cortex and hippocampus. From this study, the upregulation of SODs by GBR and GBR-EA implies that both treatments efficiently regulate the conversion of O2− to the less reactive H2O2. The cumulative effects of GBR-EA are most likely due to the higher levels of bioactive contents in the extract, such as phenolics and oryzanol constituents, which have already been reported to possess high antioxidant properties [25,39].
Figure 5 shows the expression of genes involved in the inflammatory pathway, namely CRP, NOS1, PPAR-γ, and TNFα. It was found that the expression of CRP and NOS1 in the frontal cortex showed no changes between groups (p > 0.05), while the expression of both genes was significantly elevated in the hippocampal region for the HFD group as compared to the control (p < 0.05). Treatment with Simvastatin and Probucol significantly attenuated the HFD-induced CRP elevation in the hippocampal area (p < 0.05), while the Donepezil group exhibited a similar expression to the HFD group. Treatment with GBR-EA200 showed a significant decrease in the expression of CRP (p < 0.05), when compared to HFD. Treatment with Probucol significantly lowered the expression of NOS1 compared with HFD (p < 0.05). However, no significant difference was noted in the expression of NOS1 for Donepezil and Simvastatin compared to HFD. The consumption of HFD was found to reduce the expression of PPAR-γ in both the frontal cortex and hippocampus, when compared to the normal control (p < 0.05). However, in the frontal cortex, no significant differences were found for all intervention groups compared to the HFD group (p > 0.05). On the other hand, in the hippocampal area, the expression of PPAR-γ was significantly elevated in the groups treated with GBR-EA at 100 and 200 mg/kg BW, when compared with HFD (p < 0.05). However, the expression was not significantly different when comparing among all the treated groups (p > 0.05). Meanwhile, no significant difference was found for the treatments with Donepezil, Simvastatin, and Probucol when compared with HFD (p > 0.05).
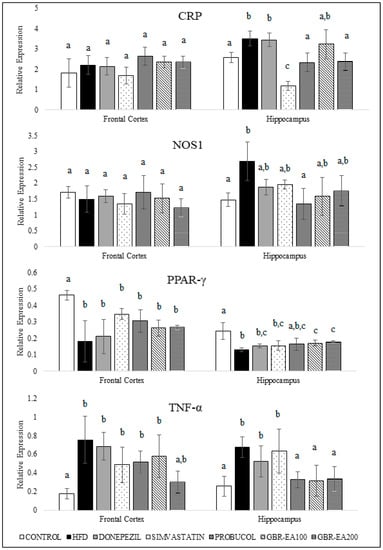
Figure 5.
Expression level of CRP, NOS1, PPAR-γ, and TNF-α in the frontal cortex and hippocampus of rats fed a high-fat diet (HFD) for 6 months, as determined using a multiplex GeXP analysis system. Values represent the mean ± SD. a–c Mean values with different letters were significantly different among the groups (p < 0.05).
On the other hand, the HFD group showed a significant increase in the expression of TNF-α for both the frontal cortex and hippocampus compared to the control (p < 0.05). No changes were observed for Donepezil and Simvastatin in the frontal cortical and hippocampal region. Treatment with Probucol tended towards lowering the TNF-α expression. Even though the expression was not significantly different from the HFD group in the frontal cortex (p > 0.05), Probucol was found to reduce the expression of TNF-α significantly in the hippocampus (p < 0.05). In addition, this study revealed that the TNF-α expression was decreased significantly in both regions for groups treated with GBR-EA100 and GBR-EA200, when compared to HFD (p < 0.05), thus implying their anti-inflammatory actions. It has already been suggested that PPAR-γ and its agonists will increase SOD, catalase, and IDE expressions, which are protective to the brain [51,55].
Interestingly, the transcriptional changes of inflammatory biomarker expressions in the frontal cortex and hippocampus of the HFD group in this study were consistent with an increased risk of neurodegenerative diseases. The findings from this study showed that hypercholesterolemia resulted in inflammatory processes in the frontal cortex and hippocampus, in which the induction with cholesterol reduced the expression of PPAR-γ in both regions. Both experimental and clinical studies have shown that brain function is sensitive to inflammatory mediators, which may involve various mechanisms, including an acute phase response to damaged tissue, as well as a response to accumulated Aβ [26,56]. High levels of serum cholesterol not only induce vascular changes similar to the early inflammatory lesions of atherosclerosis but, in addition, induced the blood–brain barrier permeability and localized neuroinflammatory changes observed [57]. Brain microvessels isolated from AD patients presented high levels of cytokines and chemokines, thus supporting the contribution of cerebrovascular inflammation in AD pathogenesis [58]. In addition, the highest levels of cytokine binding were demonstrated in certain areas associated with learning and memory, including regions of the cortex and hippocampus [59,60].
In the current study, Donepezil exhibited potential antioxidant properties in an HFD-induced animal model. It was found that Donepezil’s antioxidant regulation involves the regulation of SODs, catalase, and GPX. The antioxidative effects of Donepezil were previously documented [52,61], thus supporting the findings from the present study. Indeed, Donepezil is known to inhibit AChE enzymes, thus causing an increased level of ACh in the synaptic cleft. ACh has been reported to regulate NO levels in the brain and confer neuroprotective properties [62,63]. However, Donepezil did not alter the regulation of inflammatory-related biomarkers such as CRP, TNFα, NOS1, and PPARγ.
Previous findings showed that increased biomarkers such as CRP, NOS1, and TNF-α in the brain resulted in a high susceptibility to neurodegeneration [64,65,66]. Consistent with this notion, and based on the relevance of CRP and Aβ in transgenic mice and in vitro models, it was demonstrated that CRP is a potential trigger of Aβ production, thus it is speculated that CRP may be elevated with Aβ pathogenesis in the early stages of AD [67,68]. CRP inhibitor effectively inhibited the CRP-induced upregulations of Aβ-related production, thus providing important evidence of their associations; therefore, CRP may be a novel target for early AD intervention [67,68]. Furthermore, not only TNF-α inhibits the transport of Aβ from the brain to the periphery, elevated TNF-α levels may increase the brain accumulation of Aβ [69,70]. Moreover, the secretion of Aβ may also be further enhanced by TNF-α, thus resulting in a vicious cycle between Aβ and TNF-α in the AD brain [71]. As exhibited by previous findings, this present study supported the antioxidant effects of Probucol in the brain. In this animal model, Probucol was found to upregulate the expression of all antioxidant genes, namely SODs, catalase, and GPX. The neuroprotective effects of Probucol in the brain might also be explained by its anti-inflammatory effects through the regulation of NOS1, CRP, and TNFα, particularly in the hippocampus.
3.5. Effects of GBR-EA on Hippocampal Acetylcholinesterase Gene and Enzyme Level
As shown in Figure 6, the expression of AChE mRNA did not differ among all groups in the frontal cortex. However, in the hippocampus, the expression was significantly increased with the consumption of HFD compared to the control (p < 0.05). With treatment with AChE inhibitor, Donepezil was found to downregulate the expression of AChE compared to HFD (p < 0.05). Simvastatin, Probucol, and GBR-EA (particularly at 200 mg/kg) also showed significantly reduced expressions of the gene (p < 0.05). Furthermore, the level of AChE in the hippocampus was measured, and it was found that the protein level was significantly increased in HFD group when compared to the normal control group (Figure 7). In concordant with the mRNA expressions, treatment with Donepezil, an AChE inhibitor, as well as Simvastatin and GBR-EA at both doses, markedly reduced AChE levels (p < 0.05). However, even though the mRNA expression was elevated for Probucol, the groups showed a significantly reduced level of AChE enzyme compared to the HFD group (p < 0.05). There was no significance difference between the GBR-EA groups, when compared among the two groups (p > 0.05).
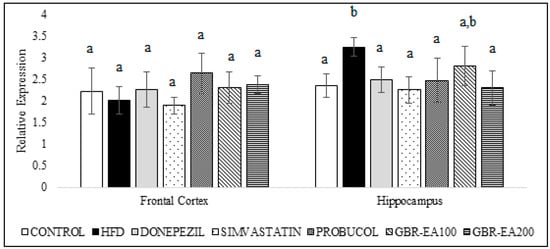
Figure 6.
Expression level of acetylcholinesterase in the frontal cortex and hippocampus of rats fed a high-fat diet (HFD) for 6 months, as determined using a multiplex GeXP analysis system. Values represent the mean ± SD. a–b Mean values with different letters were significantly different among the groups (p < 0.05).
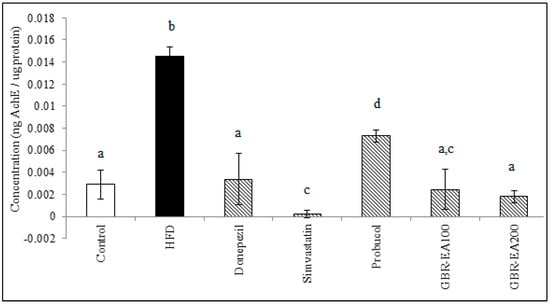
Figure 7.
Acetylcholinesterase level in the hippocampus of rats fed with a high-fat diet (HFD) for 6 months, as determined by ELISA. Values represent the mean ± SD. a–d Mean values with different letters were significantly different among the groups (p < 0.05).
Elevated AChE is proposed as an early event associated with hypercholesterolemia-induced cognitive impairments [16,72]. In a previous study, the AChE level in the hippocampus suggested that a high level of fat in the diet for a period of 12 weeks did not disrupt the cholinergic system [73]. It should be noted that lowered hippocampal AChE levels have been observed after long-term consumption of a high-fat diet [74], whilst a cholesterol-enriched diet showed decreased ChAT-positive neurons in the basal nucleus [15], thereby suggesting longer term manipulation may be necessary. Thus, in this present study, HFD was induced for 3 months before starting the intervention, together with HFD for another 3 months. A possible interaction was suggested between cholesterol and membrane-bound cholinergic receptors, and thus increased cholesterol levels may modulate their cholinergic function.
HFD consumption was reported to increase AChE enzyme activity in the brain, and this study showed similar results in the expression of the AChE gene and the increased AChE level in hippocampus, as measured by ELISA. The anticholinesterase effect of Simvastatin in the present study also corroborated that of previous findings [75,76]. A similar result was obtained in that study for the use of Donepezil, a cholinesterase inhibitor, thus validating the current findings. GBR-EA extract has shown promising effects on the inhibition of AChE gene expression, as well as in its activity in the rat hippocampus, suggested to be due to the presence of certain compounds, particularly ferulic acid and oryzanol components [24], that have been reported to inhibit AChE competitively [77,78] and are likely to possess a binding ability with the AChE structure.
Previous studies have shown that hypercholesterolemia is commonly accompanied by antioxidant deregulation, oxidative damage, inflammation, and altered cholinergic signaling in the brain. These led to cognitive alteration, due to the impairment of areas associated with learning and memory processes, particularly the prefrontal cortex and hippocampus [16,33,51]. The present study demonstrated that the neuroprotective effects of GBR may partly be regulated through modifications of antioxidants (SODs, catalase, and GPX) and attenuation of the inflammatory process in the hippocampus. Nevertheless, the underlying mechanism of AD pathogenesis remains elusive, and other than neuroinflammation, AD patients are also characterized by amyloid plaques, neurofibrillary tangles of tau protein, loss of neuronal connections, and cell death, as well as mitochondrial dysfunction and proteasome inhibition [79,80]. While the accumulated knowledge demonstrates that modulation of the cholinergic system might be beneficial for attenuating the development of Aβ formation, and restoring cholinergic neurotransmission and consequently improving cognition in AD, future studies could be directed at investigating the effect of GBR and its extracts in modulating other AD pathogeneses.
4. Conclusions
In conclusion, the results from this study reinforce the link between diet-induced hypercholesterolemia with molecular perturbation in the brain, highlighting the role of oxidative stress and inflammation. More importantly, this study also demonstrated that GBR-EA is neuroprotective against cholesterol-induced damage in the brain, as evidenced by the improved biochemical profile, increased serum antioxidant, and the regulatory effects on antioxidant (SODs, catalase, and GPX) gene expression. The neuroinflammatory alterations in the hippocampus were improved by GBR-EA through a reduction of AChE levels, as well as the downregulation of CRP and TNF- α and upregulation of PPAR-γ mRNA expressions. The findings from the current study also substantiated the use of adult hypercholesterolemic rats as a model to study neurodegenerative changes in the brain, especially at the molecular level.
Author Contributions
Conceptualization, N.H.A., N.I. and M.U.I.; Experimental parts, data analysis, and interpretation, N.H.A., N.I. and D.J.O. Original draft preparation, N.H.A., N.I. and S.N.H.O.; Manuscript review and editing, N.H.A., N.I., M.U.I., D.J.O. and S.N.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Padiberas Nasional Berhad (BERNAS), Malaysia (Project Number: 63536).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicines, Universiti Putra Malaysia (Project approval number: UPM/IACUC/AUP-RO64/2013).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Authors acknowledge the support from the Universiti Putra Malaysia, in technical assistance during the completion of this work. The authors would like to express their gratitude for the financial support received from the Universiti Malaysia Sabah, for the funding of the publication fee.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Costa, M. Nutritionally mediated oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2013, 2013, 610950. [Google Scholar] [CrossRef] [PubMed]
- Csaba, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of hypercholesterolemia-induced oxidative/nitrative stress in the heart. Oxid. Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Kucharska, E.; Garcez, M.L.; Scarpatto Rodrigues, M.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease—A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Lambert, E.A.; Phillips, S.; Belski, R.; Tursunalieva, A.; Eikelis, N.; Sari, C.I.; Dixon, J.B.; Straznicky, N.; Grima, M.; Head, G.A.; et al. Endothelial Function in Healthy Young Individuals Is Associated with Dietary Consumption of Saturated Fat. Front. Physiol. 2017, 8, 876. [Google Scholar] [CrossRef]
- Graves, S.I.; Baker, D.J. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin. Pharmacol. Toxicol. 2020, 127, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sáiz-Vazquez, O.; Puente-Martínez, A.; Ubillos-Landa, S.; Pacheco-Bonrostro, J.; Santabárbara, J. Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sci. 2020, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Alzheimer’s disease: The amyloid cascade hypothesis: An update and reappraisal. J. Alzheimer’s Dis. 2006, 9, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zou, L.; Zhou, R.; Zhang, M.; Gu, S.; Zheng, J.; Hukportie, D.N.; Wu, K.; Huang, Z.; Yuan, Z.; et al. Long-Term Increase in Cholesterol Is Associated with Better Cognitive Function: Evidence from a Longitudinal Study. Front. Aging Neurosci. 2021, 13, 691423. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Tang, M.-X.; Luchsinger, J.; Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004, 61, 705–714. [Google Scholar] [CrossRef]
- Ullrich, C.; Pirchl, M.; Humpel, C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol. Cell. Neurosci. 2010, 45, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.L.G.; de Oliveira, J.; Engel, D.F.; Walz, R.; de Bem, A.F.; Farina, M.; Prediger, R.D.S. Hypercholesterolemia induces short-term spatial memory impairments in mice: Up-regulation of acetylcholinesterase activity as an early and causal event? J. Neur. Transm. 2014, 121, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.S.; Lin, C.L.; Lin, M.C.; Sung, F.C.; Kao, C.H. Decreased prevalence of dementia associated with statins: A national population-based study. Eur. J. Neurol. 2015, 22, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N. The Potential of Dietary Bioactive Compounds against SARS-CoV-2 and COVID-19-Induced Endothelial Dysfunction. Molecules 2022, 27, 1623. [Google Scholar] [CrossRef] [PubMed]
- Oksman, M.; Iivonen, H.; Hogyes, E.; Amtul, Z.; Penke, B.; Leenders, I.; Broersen, L.; Lütjohann, D.; Hartmann, T.; Tanila, H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis. 2006, 23, 563–572. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef] [PubMed]
- Md Zamri, N.D.; Imam, M.U.; Abd Ghafar, S.A.; Ismail, M. Antioxidative Effects of Germinated Brown Rice-Derived Extracts on H2O2-Induced Oxidative Stress in HepG2 Cells. Evid Based Complement Alternat. Med. 2014, 2014, 371907. [Google Scholar] [CrossRef] [PubMed]
- Demeekul, K.; Suthammarak, W.; Petchdee, S. Bioactive Compounds from Germinated Brown Rice Protect Cardiomyocytes Against Simulated Ischemic/Reperfusion Injury by Ameliorating Mitochondrial Dysfunction. Drug Des. Dev. Ther. 2021, 15, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Mulè, F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, H.; Tian, S.; Yin, J.; Chen, Q.; Ma, L.; Cui, S.; Niu, Y. Protective effects of pre-germinated brown rice diet on low levels of Pbinduced learning and memory deficits in developing rat. Chem. Biol. Interact. 2010, 184, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.H.; Ismail, M.; Ismail, N.; Imam, M.U.; Alitheen, N.B.M.; Abdullah, M.A. Germinated brown rice alters Aβ (1-42) aggregation and modulates Alzheimer’s disease-related genes in differentiated human SH-SY5Y cells. Evid. Based Complement. Altern. Med. 2015, 2015, 153684. [Google Scholar] [CrossRef]
- Bilyaminu, A.; Yakasai, H.M.; Zawawi, N.; Ismail, M. Compositional analyses of white, brown and germinated forms of popular Malaysian rice to offer insight into the growing diet-related diseases. J. Food Drug Anal. 2018, 26, 706–715. [Google Scholar]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed. Pharmacother. 2017, 95, 780–788. [Google Scholar] [CrossRef]
- Shudo, J.; Pongpeerapat, A.; Wanawongthai, C.; Moribe, K.; Yamamoto, K. In vivo assessment of oral administration of probucol nanoparticles in rats. Biol. Pharm. Bull. 2008, 31, 321–325. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest attenuates high fat diet-induced oxidative stress and inflammation via regulation of hepatic antioxidant and inflammatory genes. BMC Complement. Alt. Med. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Julia, H.; Helene, L.; Jochen, K. Effects of Ginkgo biloba extract EGb 761, donepezil and their combination on central cholinergic function in aged rats. J. Pharm. Pharm. Sci. 2015, 18, 634–646. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Van Dam, D.; De Deyn, P.P. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br. J. Pharmacol. 2011, 164, 1285–1300. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Abi, I.; Adeniyi, S.O.; Imam, M.U. An assessment of the neurobehavioural effect of cannabidiol and omega-3 in high-fat–diet-induced dementia in albino mice. Alzheimer’s Dement. 2021, 17, e057466. [Google Scholar] [CrossRef]
- Banks, W.A.; Abrass, C.K.; Hansen, K.M. Differentiating the influences of aging and adiposity on brain weights, levels of serum and brain cytokines, gastrointestinal hormones, and amyloid precursor protein. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 21–29. [Google Scholar] [CrossRef]
- Moy, G.A.; McNay, E.C. Caffeine prevents weight gain and cognitive impairment caused by a high-fat diet while elevating hippocampal BDNF. Physiol. Behav. 2013, 109, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kim, Y.S.; Choi, I.W.; Seog, H.M.; Park, Y.D. Anti-obesity and cholesterol-lowering effects of germinated brown rice in rats fed with high fat and cholesterol diets. Korean J. Food Sci. Technol. 2006, 38, 674–678. [Google Scholar]
- Hao, C.-L.; Lin, H.-L.; Ke, L.-Y.; Yen, H.-W.; Shen, K.-P. Pre-germinated brown rice extract ameliorates high-fat diet-induced metabolic syndrome. J. Food Biochem. 2019, 43, e12769. [Google Scholar] [CrossRef]
- Lim, S.M.; Goh, Y.M.; Kuan, W.B.; Loh, S.P. Effect of germinated brown rice extracts on pancreatic lipase, adipogenesis and lipolysis in 3T3-L1 adipocytes. Lipids Health Dis. 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Bulanawichit, W.; Kirdin, T.; Boonsong, T. Effects of brown rice and germinated brown rice extracts from Thai rice cultivars (PL2 and KDML105) on adipogenic, adipocytokine, and antioxidant genes in 3T3-L1 adipocytes. J. Nat. Sci 2018, 17, 79–96. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, Y.; Kim, H.S.; Kang, I.; Hong, I.S.; Choi, S.W.; Yu, K.R.; Kang, K.S. Donepezil enhances Purkinje cell survival and alleviates motor dysfunction by inhibiting cholesterol synthesis in a murine model of Niemann pick disease type C. J. Neuropathol. Exp. Neurol. 2014, 73, 234–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imam, M.U.; Ismail, M.; Omar, A.R.; Ithnin, H. The hypocholesterolemic effect of germinated brown rice involves the upregulation of the apolipoprotein A1 and low-density lipoprotein receptor genes. J. Diabetes Res. 2013, 2013, 134694. [Google Scholar] [CrossRef] [PubMed]
- Mohd. Esa, N.; Abdul Kadir, K.K.; Amom, Z.; Azlan, A. Improving the lipid profile in hypercholesterolemia-induced rabbit by supplementation of germinated brown rice. J. Agric. Food Chem. 2011, 59, 7985–7991. [Google Scholar] [CrossRef]
- Imam, M.U.; Musa, S.N.A.; Azmi, N.H.; Ismail, M. Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic rats. Int. J. Mol. Sci. 2012, 13, 12952–12969. [Google Scholar] [CrossRef]
- Imam, M.U.; Ishaka, A.; Ooi, D.J.; Zamri, N.D.M.; Sarega, N.; Ismail, M.; Mohd. Esa, N. Germinated brown rice regulates hepatic cholesterol metabolism and cardiovascular disease risk in hypercholesterolaemic rats. J. Funct. Foods 2014, 8, 193–203. [Google Scholar] [CrossRef]
- Mehta, B.K.; Singh, K.K.; Banerjee, S. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int. J. Neurosci. 2019, 129, 252–263. [Google Scholar] [CrossRef]
- Jayaraman, R.; Subramani, S.; Sheik Abdullah, S.H.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef]
- Ashfaq, F.; Butt, M.S.; Bilal, A.; Suleria, H.A.R. Hepatoprotective effects of red cabbage in hypercholesterolemic diet-induced oxidative stressed rabbits. Curr. Bioact. Compd. 2020, 16, 469–480. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen radicals and the nervous system. Trends Neurosci. 1985, 8, 22–26. [Google Scholar] [CrossRef]
- Walsh, T.J.; Emerich, D.F. The hippocampus as a common target of neurotoxic agents. Toxicology 1988, 49, 137–140. [Google Scholar] [CrossRef]
- Norsharina, I.; Ismail, M.; Azmi, N.H.; Firdaus, M.; Bakar, A.; Yida, Z.; Stanslas, J.; Sani, D.; Basri, H.; Abdullah, M.A. Beneficial effects of TQRF and TQ nano-and conventional emulsions on memory deficit, lipid peroxidation, total antioxidant status, antioxidants genes expression and soluble Aβ levels in high fat-cholesterol diet-induced rats. Chem. Biol. Interact. 2017, 275, 61–73. [Google Scholar]
- Murakami, K.; Murata, N.; Noda, Y.; Tahara, S.; Kaneko, T.; Kinoshita, N.; Hatsuta, H.; Murayama, S.; Barnham, K.J.; Irie, K.; et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011, 286, 44557–44568. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Wang, X.; Xiang, X.T.; Wu, Y.M.; Hu, J.; Li, Y.Y.; Dong, Y.L.; Tan, Y.Q.; Wu, X. Inhibition of GPR17 with cangrelor improves cognitive impairment and synaptic deficits induced by Aβ1–42 through Nrf2/HO-1 and NF-κB signaling pathway in mice. Int. Immunopharmacol. 2021, 101, 108335. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.H.; Tsai, H.D.; Chen, Y.C.; Wu, J.S.; Lin, T.N. Anti-apoptotic actions of ppar-γ against ischemic stroke. Mol. Neurobiol. 2010, 41, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflam. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thirumangalakudi, L.; Prakasam, A.; Zhang, R.; Bimonte-Nelson, H.; Sambamurti, K.; Kindy, M.S.; Bhat, N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008, 106, 475–485. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.A.; Lamar, M.; Zhang, A.; Ren, X.; Ajilore, O.; Pandey, G.N.; Kumar, A. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. Int. J. Geriatr. Psyc. 2018, 33, 104–112. [Google Scholar] [CrossRef]
- Bourgognon, J.-M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef]
- Munishamappa, V.; Seethalakshmi; Vijayakumar, A.E.; Rajathilagam, T. Evaluation of the antioxidant activity of donepezil-in vitro study. Nat. J. Physiol. Pharm. Pharmacol. 2019, 9, 108. [Google Scholar]
- Liu, J.; Lee, T.J.F. Mechanism of prejunctional muscarinic receptormediated inhibition of neurogenic vasodilation in cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, 194–204. [Google Scholar] [CrossRef]
- Priyanka, H.P.; Singh, R.V.; Mishra, M.; ThyagaRajan, S. Diverse agerelated effects of Bacopa monnieri and donepezil in vitro on cytokine production, antioxidant enzyme activities, and intracellular targets in splenocytes of F344 male rats. Int. Immunopharmacol. 2013, 15, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shen, Y.; Li, R. Targeting TNF: A therapeutic strategy for Alzheimer’s disease. Drug Discov. Today 2014, 19, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.-Y.; Yao, Y.M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Kim, M.; Jung, J.; Jeong, N.Y. Inhibition of neuronal nitric oxide synthase by ethyl pyruvate in schwann cells protects against peripheral nerve degeneration. Neurochem. Res. 2019, 44, 1964–1976. [Google Scholar] [CrossRef]
- Bi, B.-T.; Lin, H.-B.; Cheng, Y.-F.; Zhou, H.; Lin, T.; Zhang, M.-Z.; Li, T.-J.; Xu, J.-P. Promotion of β-amyloid production by C-reactive protein and its implications in the early pathogenesis of Alzheimer’s disease. Neurochem. Int. 2012, 60, 257–266. [Google Scholar] [CrossRef]
- Hilal, S.; Ikram, M.A.; Verbeek, M.M.; Franco, O.H.; Stoops, E.; Vanderstichele, H.; Niessen, W.J.; Vernooij, M.W. C-reactive protein, plasma amyloid-β levels, and their interaction with magnetic resonance imaging markers. Stroke 2018, 49, 2692–2698. [Google Scholar] [CrossRef]
- Evi, P.; Tzara, O.; Zenelak, S.; Georgopoulos, S. Genetic deletion of tumor necrosis factor-α attenuates amyloid-β production and decreases amyloid plaque formation and glial response in the 5xfad model of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 165–181. [Google Scholar]
- Clark, I.A.; Vissel, B. Therapeutic implications of how TNF links apolipoprotein E, phosphorylated tau, α-synuclein, amyloid-β and insulin resistance in neurodegenerative diseases. Br. J. Pharmacol. 2018, 175, 3859–3875. [Google Scholar] [CrossRef]
- Blasko, I.; Marx, F.; Steiner, E.; Hartmann, T.; Grubeck-Loebenstein, B. TNFα plus IFNγ induce the production of Alzheimer’s β-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Akhtar, M.; Abdin, M.Z.; Islamuddin, M.; Shaharyar, M.; Najmi, A.K. Rosuvastatin ameliorates cognitive impairment in rats fed with high-salt and cholesterol diet via inhibiting acetylcholinesterase activity and amyloid beta peptide aggregation. Hum. Exp. Toxicol. 2018, 37, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Kosari, S.; Badoer, E.; Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav. Brain Res. 2012, 235, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, R.R.; Da Silva, A.C.; Morsch, V.M.; Corrêa, M.C.; Schetinger, M.R. Diet-induced changes in AChE activity after long-term exposure. Neurochem. Res. 2004, 29, 2251–2255. [Google Scholar] [CrossRef]
- Cibicková, L.; Palicka, V.; Cibicek, N.; Cermakova, E.; Micuda, S.; Bartosova, L.; Jun, D. Differential effects of statins and alendronate on cholinesterases in serum and brain of rats. Physiol. Res. 2007, 56, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, Anant, and Rishabh Singh. Pharmacological evaluation of combined neuroprotective effect of Melatonin and Simvastatin against LPS and STZ induced memory impairment in rodents. Asian J. Pharm. Pharmacol. 2019, 5, 316–325. [Google Scholar] [CrossRef]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine–ferulic acid hybrids as multi-potent antiAlzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, V.K.; Singh, D.K. Kinetics of enzyme inhibition by active molluscicidal agents ferulic acid, umbelliferone, eugenol and limonene in the nervous tissue of snail Lymnaea acuminata. Phytother. Res. 2009, 23, 172–177. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Hyman, B.T.; Spires-Jones, T.L. Beyond the neuron–cellular interactions early in Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).