Abstract
The way cells communicate is not fully understood. However, it is well-known that extracellular vesicles (EVs) are involved. Researchers initially thought that EVs were used by cells to remove cellular waste. It is now clear that EVs function as signaling molecules released by cells to communicate with one another, carrying a cargo representing the mother cell. Furthermore, these EVs can be found in all biological fluids, making them the perfect non-invasive diagnostic tool, as their cargo causes functional changes in the cells upon receiving, unlike synthetic drug carriers. EVs last longer in circulation and instigate minor immune responses, making them the perfect drug carrier. This review sheds light on the latest development in EVs isolation, characterization and, application as therapeutic cargo, novel drug loading techniques, and diagnostic tools. We also address the advancement in plant-derived EVs, their characteristics, and applications; since plant-derived EVs only recently gained focus, we listed the latest findings. Although there is much more to learn about, EV is a wide field of research; what scientists have discovered so far is fascinating. This paper is suitable for those new to the field seeking to understand EVs and those already familiar with it but wanting to review the latest findings.
1. Introduction
Extracellular vesicles (Evs) were first studied and described in 1946 [1] and once were regarded as a cellular waste [2]. These EVs gained considerable attention throughout the years [3] due to the active mechanism of their release from the cells [4]. These sacs were described as extracellular vesicles [5].
A recommended definition by the International Society for Extracellular Vesicles (ISEV) advised using the term extracellular vesicles as the characteristic label for particles released naturally from the cells, distinct by a lipid bilayer, and do not replicate. The short term is EVs [6]. In order to study EVs, the process should undergo characterization to identify the transmembrane luminal protein; the characterization process should include but not be limited to zeta potential, electron microscopy, and flow cytometry [7]. EVs have been described as the novel communication method between cells, either on a long or short-range signaling requirement [8]. EVs are heterogeneous sacs of membranous vesicles released from the cells into the surrounding environment [9].
The recognized role of EVs is to carry a molecular cargo including RNA and surface markers [10] originating from the mother cell and reflect their status [11] to be delivered to the receiving cells. Organisms that range from prokaryotic cells to higher eukaryotes can produce EVs. In mammals, all kinds of cells release EVs [12]. An increasing number of studies focused their interest in plant-derived EVs as therapeutic agents since plant source materials retain less toxic effects than chemotherapeutic-based therapy [13].
This review summarised the latest updates regarding mammalian and plant source EVs in terms of classification, cargo, uptake, and function. Furthermore, we reviewed the cutting-edge isolation and characterization techniques—their role as a diagnostic and therapeutic tool, safety assessment, and evolved results.
2. Classification and Biogenesis of EVs
ISEV has classified EVs based on their size into two prominent families; large EVs (200 nm) and small EVs (40–150 nm) [14]. These fluid-filled sacs can also be classified based on their biogenesis [5]. Moreover, it can be categorized and termed without complying with the standard definition [7]. The release mechanism (Figure 1) is a controlled process; lipid and lipid metabolite enzymes play the most significant role in this process [15]. There are three ways by which the cells release EVs [16].
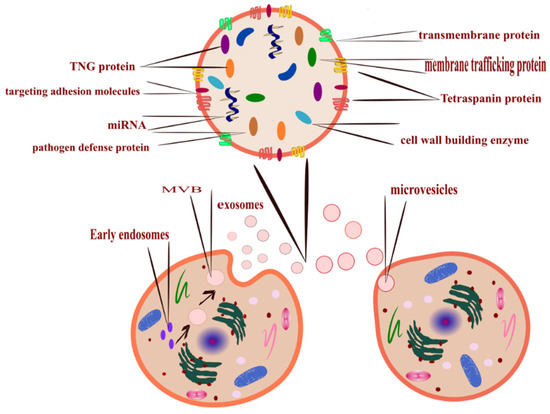
Figure 1.
All cells release EVs, the mechanism of EVs formation determines its type, microvesicles released after budding of the plasma membrane (right-bottom corner), and exosomes (left-bottom corner) released from MVBs that formed inside the cell from early endosomes. Its cargo is loaded upon maturation (top corner illustrates EVs cargo). EVs can also be classified according to size; exosomes are the smallest, and apoptotic bodies are the largest.
- Outward budding of the plasma membrane (shedding microvesicles, MVs) happens when the plasma membrane is reacting to external incitements such as inflammatory cytokines that provoke a response from the cell [17], leading to activation of the plasma membrane that will eventually release sacs 100–1000 nm in diameter that are released into the external surroundings including microparticles and microvesicles [18].
- Inward budding of the endosomal membrane (early endosomes) results in the formation of multivesicular bodies (MVBs); these small size particles range in diameter 30–200 nm [18] and 30–150 nm [19]. The releasing mechanism of these MVBs starts by forming a sac inside the cell containing vesicles derived from the mother cell; eventually, these sacs will emerge with the plasma membrane and be released into the extracellular space, becoming exosomes [20].
- Apoptosis-derived EVs (ApoBDs). In this case, the vesicles are large in diameter (800–5000 nm); the mechanism of release occurred during programmed cell death [21]; eventually, these vesicles will be released into the extracellular space and become another type of EV [22].
Lázaro-Ibáñez et al. [23] suggested that there is more to EVs than a sole classification into the formerly mentioned classes, stating that EVs could be allocated into further subpopulations. For instance, when the vesicles were recently examined by TEM, the EVs were described as cup-shaped particles with no indication of their cargo. Furthermore, cryo-TEM technology was used to characterize EV subpopulations. The technique confirmed the presence of cup-shaped vesicles when negatively stained; some have a single membrane smaller than 100 nm [24].
Different EV types can be produced by a single cell exhibiting diversity within the subtypes. Various subcellular locations produce subtypes of EVs, either similar or distinct [25].
2.1. Exosomes
Exosomes are a type of extracellular vesicles, uniform in structure and composition [26]; the diameter of these exosomes are smaller than 150nm [27]. Some studies described their size as 40–150 nm [28]. We estimate the reason behind the variation in size is the absence of standard isolation and characterization methods.
Exosomes are found in all biological fluids [29], spherical membrane-bound vesicles [30], can transfer the biological cargo into a nearby cell that is adjacent to the mother cell, or can travel long distances in the circulation [31]. The meaning of the word exosome is from the Greek words ‘Exo’ outside, and ‘Soma’ means the body [6]. In terms of structure, the exosomes’ shell is lipophilic composed of lipid membrane ligands and receptors within the membrane, RNA, proteins, and other elements derived from the mother cell [32]. The core is aqueous; its amphiphilic properties enable them to compartmentalize, solubilize and introduce hydrophilic and hydrophobic materials [33]. Exosomes represent cell biopsy, reflecting the characteristics of the original cell that can be used for diagnostic purposes [3]. The exosomal protein plays a significant role in exosome-receptor cells interaction. The protein profile of the exosomes is different in each exosome, depending on their origin [34]. The protein cargo includes but not limited to the most common CD9 [35], CD36 [36], CD81 of the tetraspanin family [37], and TSG101 [38]. As for the lipid composition, exosomes contain cholesterol, sphingomyelin, hexosylceramides, phosphatidylserine, and saturated fatty acids; all are located in the plasma membrane [39]. The cargo can be transferred between cells by a molecular sorting mechanism [40].
Exosome biogenesis is a complex process that depends on several variables, including the mother cell’s stimulating signals. They are developed and maturated from multivesicular bodies (MVs) [18]; the procedure includes a particular sorting mechanism of intraluminal vesicles [41] and segregation of the cargoes on microdomains of the membrane of the MVs, inward pudding, and fusion afterward [42]. After the formation of exosomes are the steps of miRNA loading. RNA is transported by a protein that binds explicitly with RNA. It will be carried to the lipid raft-like region for the binding process then miRNAs with the highest affinity to the lipid-like area are secured and retained [43].
2.2. Microvesicles
Cytoskeletal and regulatory proteins control the biological shedding of MVBs, causing a redistribution of phospholipids and contraction of cytoskeletal protein. The activities of aminophospholipid translocases cause the translocation of phosphatidylserine to the leaflet of the outer membrane forming microvesicles. Microvesicle budding, in turn, is regulated by GTP-binding protein and ADP-ribosylation factor 6. The latter is involved in cell recycling and macrophages [44]. In the last stage of MVBs release, ESCRT and TSG101 interact with ALIX and ARRDC1 (arrestin domain-containing protein 1). The lipidomic profile of microvesicles is unique. Includes phospholipid phosphatidylserine (PS) that enhances their uptake by host cells. Additionally, sphingolipids, sphingomyelins, ceramide, phospholipid lysophosphatidylcholine, acylcarnitine, and fatty acyl esters participate in their biogenesis. Furthermore, other factors affect the formation of microvesicles. For example, the concentration of calcium in the extracellular environment plays a role in the structure of MVBs. As high calcium concentration will induce the scrambling of membrane phospholipids, improving MVBs formation [45].
2.3. Apoptotic Bodies
Significant morphological adjustments can occur to apoptotic cells, including the blebbing and protrusion of the membrane. The process will start by shortening the cell’s nucleus, followed by blebbing of the plasma membrane, and later the cell components will be disintegrated into distinct vesicles [25]. The apoptotic volume decrease (AVD) event starts within two hours after apoptosis. The process co-occurs with the blebbing of the plasma membrane, where cysteine proteases (caspases) can be activated due to internal enticements (disfunction of the mitochondria) or directly by external factors (activation of death receptors ligations) [45]. The apoptotic bodies will be released as a result. Besides, apoptotic bodies play a role in the phagocytosis of the apoptotic cell.
3. EV Cargo and Uptake
Both apoptotic and healthy cells release these sacs (vesicles) containing protein, including plasma membrane and endosomal protein [46], lipids [47] most commonly cholesterol, ceramide, sphingolipids, and phosphatidylserine [48], mRNA, miRNA [49], tRNA, Y RNA [2], DNA [23], ssDNA, mtDNA, dsDNA [50] and sugars obtained from the cell f origin [10].
3.1. EV RNA
RNA sorting into EVs is not fully understood, but several mechanisms could achieve the process. For example, the selective loading of miRNA into EVs is facilitated by RNA-binding protein, such as SUMO protein (hnRNBA1); this protein will identify GAGAG motifs of the miRNA and selectively load them into EVs. Furthermore, the production of neutral sphingomyelinase 2 (nSMase 2) by ceramide significantly impacts loading miRNA into EVs [25]. Another example, in liver functional cells hepatocyte, the synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP) is able to recognize GGCU motif in particular miRNA and augment their loading into EVs. Leading to the understanding that RNA binding proteins regulate the internalization of RNA inside EVs via miRNA-conserved motifs [51]. Once inside the recipient cell, EVs will release their cargo that will control gene expression through de novo translation and post-translational regulation of target mRNAs. Additionally, EVs can induce phenotypic changes due to their ability to change the recipient cell transcriptome and signaling activity [52].
Mammalian EVs are known to mediate the transportation of coding and non-coding RNAs. These significant components are protected inside the EVs and resume their function upon arrival to the host cell, imitating the mother cell. Still, EVs are enriched with mRNA and miRNA. Several non-coding RNAs have been detected, including transfer RNA (tRNAs), ribosomal RNA (rRNAs), and other types that are both a combination of coding and non-coding RNAs [53]. Extracellular RNA (exRNA) is usually enclosed by a membrane or found tightly associated with proteins [54].
The most abundant EV RNA is miRNA. The profile of the vesicle miRNA is different from their cell of origin. Indicating that miRNA is specific for EVs, and their sorting mechanism is selective. On the other hand, mRNA represents the minority of EV RNA. However, their concentration varies amongst EV types since mRNA is more abundant in MVBs than exosomes. EV mRNA can cause phenotypic changes in the receiving cells upon delivery. For example, the human telomerase reverse transcriptase gene (hTERT mRNA) can be localized into fibroblasts when transferred via EVs; upon entry, it enhances the cells’ multiplication life span, delays aging, and prevent the damage caused by DNA [55]. Another example is the presence of non-coding RNAs in glioblastoma-derived EVs; these EVs contain miR-21 that can trigger vascular endothelial growth factor signaling in human brain cells used as an angiogenic factor produced by the tumor cell. Furthermore, EVs derived from breast cancer cells enhance brain metastasis by changing the permeability of the brain [56]. EV mRNA can produce functional proteins; the active translation can occur within an hour after EV internalization [57].
3.2. Protein
The proteomic profile of EVs indicates that heat shock protein, cytoskeletal protein, transmembrane protein, and cytosolic proteins are the most abundant in EVs. Several proteins are considered markers for different types of EVs [28]. Common EV markers are listed in Table 1.

Table 1.
Common EV protein cargo is often used as EV markers.
Protein is essential when EVs are being formed and released. During EV release, specific proteins are needed for membrane formation and to finish the curving and pudding of the plasma membrane; after this, protein participates in the separation process and pinching of the formed membrane. Furthermore, protein is responsible for fusing MVBs with the plasma membrane. In turn, these processes are done sequentially; they mostly require the same protein [58]. Lipids, tetraspanin, and ESCRT regulate the sorting of proteins into EVs. Other proteins packed into EVs are the results of their biogenesis process. Post-translation modifications (PTMs) similarly control the selective process of protein sorting into EVs since they regulate the protein’s function, structure, and subcellular localization [25].
3.3. EV Uptake
EV uptake can be divided into three primary levels: cellular, intracellular, or tissue. On a cellular level, EVs appear to interact with the receptors on the plasma membrane. The intracellular level uptake typically occurs via endocytosis, each cell with a specific endocytic pathway. The ability of EVs to transfer their RNA into receiving cells indicates that these vesicles prompt endogenous mechanisms for cargo delivery. Finally, an example of tissue-level uptake is EVs’ ability to cross the blood-brain barrier; these EVs facilitate the intracellular communication between neuronal cells [51].
The type of recipient cells appears to control how EVs are taken up. Most commonly, phagocytosis is the mechanism behind the cellular uptake of EVs. Moreover, the magnitude of the process depends on the recipient cell’s phagocytic capacities. On the other hand, the direct fusion of EVs with the plasma membrane can only occur in acidic conditions, such as the case in tumor cells [59]. When The endosomal/lysosomal system takes up EVs, Membrane-associated proteins also appear to be involved in EV uptake into cellular compartments [60]. On the other hand, endocytosis is the most recognized EV uptake mechanism; it is an active engulfment process that incorporates clathrin-mediated endocytosis, macropinocytosis, or phagocytosis. Still, it is not fully understood if the mechanism relies on specific EV-surface proteins or receptors [61].
Additionally, cell-specific factors can control EV uptake; this could be via direct contact, uptake, fusion, degradation, or a combination. For instance, protein interaction between EVs and host cells is a regulating factor in direct contact interaction. Furthermore, the lipids on the EVs membrane could be recognized by cells permitting non-specific uptake or fusion [60].
Some reports confirmed that a saturable transport mechanism mediates the uptake of EVs by the Caco-2 cell line. Furthermore, the protein contents of both EVs and the Caco-2 cell line affect EV uptake [62]. Additionally, these plant EVs have a therapeutic impact on cancer cells, such as the case in a study conducted by Zhang et al. [63] used the density gradient ultracentrifugation method to isolate ginger-derived EVs. A colon cancer cell line was treated with these vesicles to evaluate their uptake and therapeutic effect. The vesicles were taken up by cancer cells, and traces of plant-derived lipids were detected in the treated cell line, confirming these vesicles’ ability to transfer their cargo. Further investigations confirmed that these EVs have no toxic effect on human cells [63].
The last few years offered several EVs isolation methods that provided an excellent yield and promising quality isolates. Nevertheless, further analytical and comparative studies are needed to aid the scientists in determining their experimental approach [64]. Furthermore, the need for standard isolation, characterization, and storage guidelines is most urgent since the comparability between different studies cannot be achieved since the accuracy of the reported data are unknown [65].
The International Society for Extracellular Vesicles (ISEV) conducted a survey that invited the leaders in the field to answer questions related to EV biogenesis, cargo loading, release, and uptake by other cells. The answers were collected and vetted regarding the uptake mechanism of EVs.
It remains largely unclear how EVs interact with cells, and what dictates the next step (signaling, uptake, or fusion) of the bound EVs. The molecular drivers (e.g., proteins, lipids, sugars, nucleic acids) of EV-cell interactions are largely unknown. including how these vary among cell types, with cell state, or among EV subtypes [60].
4. Plant-Derived EVs
Human and animal EVs were the subject of research, but plant-derived EVs and their cargo are also the focus of research (Table 2). Plant extracellular vesicles demonstrated therapeutic functions via the oral route in animals. Additionally, plants are readily available, been consumed daily without any toxic effect or immune response in humans, and their assessment is the subject of many studies [70]. Plant-derived EVs were evaluated for their therapeutic activities towards tumor cells and their abilities to be used as drug carriers. These EVs were extracted from edible plants [71]. Only recently did plant-derived EVs enter the recruitment processes for clinical trials.

Table 2.
Assortment of isolated plant-derived EVs and their characterization compared to mammal-derived EVs.
For example, the studied edible plants included corn, tomatoes, tobacco, sunflower, rice, and grapefruit; the latter showed an immune modulation effect in the intestine [72].
Additionally, lemons and grapes-derived EVs were isolated, characterized, and thoroughly studied to establish their beneficial effect [73]. Gut bacteria can take up plant exosomes like nanoparticles, contain RNA and siRNA in vitro, and modulate their communication with the host cells by altering their genetic makeup. These EVs, particularly their RNA, instigate tissue repair and antimicrobial immunity [74].
Moreover, Wang et al. [75] engineered grape fruit-derived nanoparticles to be delivered to tumor mice model intravenously. The nanoparticles transported chemotherapeutic agents to target cells showing enhanced efficiency and specificity. Furthermore, the safety of these nanoparticles was tested in vivo; the results showed no toxicity. Furthermore, these vesicles originated from a natural source; they provided efficient drug delivery with no toxic effect on humans [76].
EVs derived from plant sources have a structure and cargo similar to EVs isolated from mammal cells. Furthermore, the cargo of plant EVs contains proteins, bioactive lipids, and both mRNA and microRNA, and can transfer this significant cargo to other cells, just like mammal EVs [13]. One of the research subjects was corn, tomatoes, tobacco, sunflower, rice, and grapefruit; the latter showed an immune modulation effect in the intestine. There are also clinical trials using plant EVs to prevent cancer [72]. Gut bacteria can take up plant exosomes like nanoparticles, contain RNA and siRNA in vitro, and modulate their communication with the host cells by altering their genetic makeup. These EVs, particularly their RNA, instigate tissue repair and antimicrobial immunity [74].
5. EV Function
EVs can travel long distances through the blood circulation and reach various tissues affecting the signaling process [82]. EVs can modulate several pathological and physiological functions [83]; both actions can be used as disease biomarkers, giving them a significant advantage in the biomedical field [84], including immune function and metastasis [85]; this is achieved by transporting their cargo between cells [86] (Table 3).

Table 3.
Mammal-derived EVs were thoroughly investigated; their involvement in several pathological and physiological functions was recorded in various studies.
The cargo within the EVs can interact with the receiving cell, causing them to function as mediators [87]. These vesicles cannot self-replicate [7]; their significance is presented in the RNA cargo [54].
On the other hand, EVs play a significant role in anti-inflammatory and antiapoptotic actions [88]. It could either work as protected vesicles that expel invading pathogen from the infected cell or as vector-transmitted virulence factors that will facilitate the infection [33]; for example, CNS-derived EVs illustrate defensive functions by releasing anti-inflammatory reactions [86]. In addition to its therapeutic functions, EVs released from infected cells are capable of acting similar to the mother cell, such as viruses [89], transferring viral RNA and other pathogenic proteins [90] that behave as barriers to prevent the immune mechanism from recognizing the virus. Thus, EVs have a vital role in the process of a viral infection, such as the case in malaria, and are involved in the communication between infected cells, viruses, and healthy cells [91]. Cancer cells can direct EVs as a messenger to transfer aggressive traits to the neighbor cells [84]; also, EVs can help the mobility of cancer cells, which is accomplished by forming lumps that will ensure persistence migration of the tumor [92], several studies concluded that tumor-derived EVs are part of tumor angiogenesis [93].
In the receptor cells, EVs can bind directly to the plasma membrane by activating the surface receptors of these cells or the endocytic membrane [50]; this action will instigate the release of intraluminal content in the cytoplasm of the recipient cells; this step is a significant activity that leads to the release of miRNA and mRNA [42].
Not only do mammals release EVs, a parasite, for example, Heligmosomoides polygyrus, releases EVs upon entry to the host cells that block the pro-inflammatory TNF-α and IL-6 to deliver the virulence cargo to manipulate the immune response [94]. Bacteria also release EVs essential for their survival and development; these bacterial EVs are referred to as outer membrane vesicles OMVs because they protrude from the cell’s outer membrane, 20–250 nm in size [95].
6. EVs as a Disease Biomarker and Diagnostic Tool
There is an increased interest in using EVs as disease biomarkers and liquid biopsy due to their availability in all biological fluids and the ability to transfer its cargo intact to the receptor cell. EVs as a disease biomarker have been the subject of several studies, especially cancer research [10]. EVs gained significant interest due to their relationship to pathological processes and gene delivery in short or long distances [109]. When EVs indicate dynamic changes in their content, they are directly related to the mother cell and their pathological condition, reflecting reliable disease biomarkers [110].
Furthermore, their non-invasive traits as disease biomarkers because of their miscellaneous content and their variety of biologically active components [15]. They consist of overlapping content, and their communication and functional mechanisms are not entirely different amongst all EV types. Providing a comprehensive investigation tool for some diseases, including cancer, cardiovascular diseases, neurological diseases, and infection [5]. Indicating that the detection of mutated EV-RNA is an effective tool to diagnose cancer [111].
Since cancer cells also produce EVs rich in a mitochondrial membrane protein that can be isolated from plasma that increases in melanoma, ovarian, and breast cancer [112]. There are specific features explicit for tumor-derived EVs that were the focal point of the focus of several studies as non-invasive diagnostic tools. These markers include epithelial cell adhesion molecules, epidermal growth factor receptors, and mucin [113]. EV-enriched proteins play a role in cancer development and can be used to diagnose metastatic cancer with a weak prognosis [114]. Tumor EVs are detected in almost all bodily fluids, including blood, urine, and cerebrospinal fluid. Confirms that EVs are circulating cancer biomarkers, as they represent in liquid biopsy in the breast, prostate, ovarian cancer, and melanoma [39].
The detection of EVs and their subset can predict diseases and anticipate the risk factors or development of a pathological process. Hence, EVs have also been used in a Framingham risk score (FRS) tool, a risk assessment approach [115].
The appropriate approach to use EVs in diagnosis is to study the nature of these vesicles and their origin. For example, analysis of plasma EVs confirmed their involvement with a specific type of cancer. Furthermore, some biomarkers are released directly from the cancer cell; others can be identified by their specific protein. All these indicators and tools can be incorporated with traditional tools to study cancer prognosis, development and, possible prediction [116].
For example, EVs were tested as early non-invasive diagnoses of colorectal cancer (CRC); the isolated EVs expressed tumor-specific protein circulating extracellularly, which can be utilized as early stages diagnostic tools [117]. Other than employing EVs in cancer diagnosis, some research focused on using EVs to diagnose rapidly progressed diseases that instigate a challenge for early diagnosis. Such is the case in Pleural effusion; EVs can be a promising source to detect this disease. After metabolic and lipidomic characterization of Pleural effusion-derived EVs, the results indicated that these EVs are enriched in specific metabolic cargo with a noticeable variation from tuberculosis-derived EVs and malignant tissue, indicating a promising future to use these particular characteristics as an early diagnostic tool [118].
In a study conducted by Saenz-Pipaon et al. [10] aiming to evaluate the potential use of EVs as a diagnostic tool for Peripheral Arterial Diseases PAD associated with cardiovascular conditions, the study concluded that EVs are likely reliable liquid biopsy to predict the PAD molecular component signature. Additionally, EVs play a significant role in the lungs by controlling the airways’ homeostasis and providing a significant circulating disease biomarker in chronic obstructive pulmonary disease and potential therapeutic due to its cellular communication abilities [38].
Cheng et al. [119] conducted detailed profiling of EVs collected from the frontal lope of Alzheimer’s patients. The study investigated the RNA makeup of these EVs and the changes associated with disease prognosis. The results indicated that EVs are involved in Transcriptomic deregulation of miRNA expression, achieved by horizontal transfer of the RNA via EVs. The study suggested that these early changes can be used to indicate and diagnose Alzheimer’s [119]. EVs also helps in providing long-term disease monitoring and predicting possible relapse. Moreover. A study by Melo et al. [120] acknowledged that the number of exosomes was significantly elevated in vivo in pancreatic cancer before the disease was detected by screening techniques [120].
7. Application of EVs as a Therapeutic or Drug Delivery Agent
Evidence showed that EVs function systematically as a natural response and drug delivery vehicle and can be employed for target delivery (Figure 2). EVs are detectable in most biological fluids in disease and immune response [121]. EVs can be used as original therapeutic agents or as delivery agents. Most studies focused on the possibility of using them as drug delivery cargo to a specific target due to its membrane protein [67]. Once the drug is encapsulated inside EVs, the advantage is that the drug works with the elements naturally present in them. In other cases, EVs only act as a drug carrier protecting the drug, passing it safely to the target location [73].
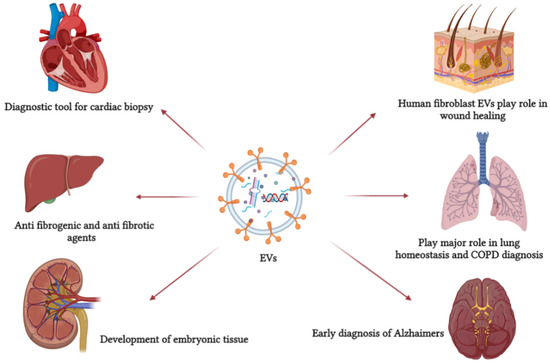
Figure 2.
EVs are involved in several pathological and physiological processes. It can be used as a primary non-invasive diagnostic tool and, because of its role in cell communication, it can be used as a drug carrier. This figure refers to some EV functions in defense mechanisms, cellular communication, and normal physiological processes.
Several studies indicated that EVs derived from stem cells could be used as a non-invasive treatment for a brain injury or brain infection, as these EVs retain the same therapeutic functions as stem cells. Furthermore, the secretomes of stem cells, including EVs, are the active compounds that attribute to their therapeutic roles; in addition, stem cell-derived
EVs can transfer the required genetic information and protein to accomplish the therapeutic process. Several studies applied stem cell-derived EVs on injured brain cells; the EVs acted as therapeutic agents on these cells [122]. Additionally, immune cells also release EVs that act similarly to their origin and can be used in therapeutic applications. For example, dendritic cells (DC) release EVs that activate CD4+ T cells [67] via the endocrine pathway, causing enhanced cardiac performance and reducing the time needed for wound healing in myocardial infarction. EVs derived from macrophages are used in plasmin encoding therapeutic protein and vaccination [123]. Salivary EVs prevent the Zika virus attachment to the host cell, explaining why the virus is rarely transferred via saliva. However, the same study stated that the salivary EVs are not effective against SARS-CoV-2 that majorly communicate via salivary droplets [124].
There are a variety of challenges facing scientists when developing a therapeutic approach. These included toxicity, safety, target specificity, and large-scale production. Furthermore, a synthesized nanoparticle can solve the target delivery issue. However, it must undergo in vivo safety and toxicity assessment, and there is the issue with costly large-scale production. Using extracellular derived nanoparticles as drug carriers provides a possible solution since they contain lipid, RNA, and protein [63]. As EVs transfer their content from the mother cell, it is the most critical attribute in drug delivery and cell communication [36]. Their cargo, specifically RNA, was of particular interest due to the diverse population of miRNA with target genes that can influence and regulate the biological process in mammalian cells [125]. Additionally, a growing body of facts indicates that cell apoptosis proteins are involved in EV formation. Unveiling the interaction pathway between EVs and autophagy that promotes cancer cell motility, providing significant advantages in early diagnosis and cancer treatment using EVs [126]. The protein profile of EVs derived from mesenchymal cells retains a therapeutic effect observed in vivo. These proteins include osteoprotegerin and angiogenin [127].
Drug loading into EVs can be divided into two strategies. The first is directly loading the drug into the exosomes; the second is targeting the mother cell by loading drugs during the biogenesis of exosomes [34]. Furthermore, in the case of loading lipophilic drugs, the process is relatively easy due to the interaction between EVs lipid bilayer and the drug, which occurs via hydrophobic interaction [128]. Several methods were utilized to achieve exogenous loading of EVs with drugs, including electroporation, incubation, sonication, and thawing. The level of success differs from one method to another; some cause degradation to either EVs or their cargo [129].
The first time EVs were used as a drug cargo was when San et al. [130] used EV-encapsulated curcumin fused with the plasma membrane. It was reported that EVs isolated from the plasma of humans and rats showed a protective mechanism against Acute myocardial ischemia/reperfusion [131]. Successfully, EVs were loaded with anti-cancer and RNA-based drugs and cancer vaccination. These applications are based on cancer immunotherapy, indicating that the success of this approach is that EVs can be taken up efficiently by macrophages and tumor cells [132]. Other drugs loaded into EVs include a lipophilic drug with a small molecular weight and RNA-based drugs, for example, small interfering RNA (siRNA) [128]. Furthermore, the blood-brain barrier is the most selective in the human body. There are three primary methods to deliver drugs and therapeutic components into the brain, including invasive, pharmacological, and physiological approaches—the first approach is based on delivering drugs by breaching the blood-brain barrier. The pharmacological method includes modifying the active compound to enter the brain via a passive crossing. The last approach is the most efficient; it depends on the naturally present receptors found in abundance on the surface of the blood–brain barrier. These receptors provide easy and equal distribution of the loaded drugs into the brain. EVs possess two significant advantages as the usage will be non-invasive and were proven to cross the blood-brain barrier easily [133].
Employing EVs as a drug carrier has several advantages compared to platelets. To mention a few, (1) EVs are smaller in size, which is an advantage when delivering drugs to cancer cells. For instance, EVs were more efficient in delivering the membrane protein SIRPa than ferritin. (2) EVs are originated from different sources. They are smaller and less toxic than lysosomes, with enhanced tissue biocompatibility and tolerance. (3) The cargo within EVs is protected by the double lipid layer that reduces the degradation and improves their biological stability. (4) Target cells can exclusively distinguish the specific receptors present on the EV membrane, reducing systemic toxicity and lowering drug outflow compared to conventional drug administration methods. (5) It is established that EVs could pass the BBB, giving an advantage in treating brain tumors. (6) Due to EVs’ ability to circulate the blood, the drug’s effect can last longer in the system [71].
8. Drug Loading into EVs
Loading EVs with foreign substances for therapy continues to grow and develop [134]., enhancing the therapeutic potentials of these particles. The loading approach can be categorized into EV alteration or cellular modification [44]. Furthermore, it can be achieved post EV isolation (exogenous). Or during the biogenesis of EVs within the mother cell (endogenous) [51].
EVs were tested to be used as a vehicle for drug delivery systems. One of these applications was developed by delivering systemically injected exosomes to target epidermal growth factor receptors in breast cancer; the study stated that these exosomes successfully conveyed miRNA and anti-tumor agents into epidermal growth factor receptors [135]. Conjugating gold nanoparticles developed another system into the doxorubicin anti-cancer drugs that were physically loaded into the exosomes. The study stated that the target cell successfully took up these modified exosomes [136]. Another modified EV consists of exosomes-liposomes fusion by repeated freeze-thawing mechanism; this method successfully altered the treated exosomes surface, resulting in enhanced colloidal stability and reduced immunogenicity [135]. The significant advantage in using EVs as a drug delivery agent or as a therapeutic agent on their own is the ability of these EVs to control their RNA, proteins, and lipids and employ it as a tissue-specific response [121]. Furthermore, EVs are being engineered and modified to enhance the encapsulation of the drug and the targeting accuracy [137].
Although EVs proved to have significant targeting abilities, some studies indicated that EVs were accumulated in the spleen and liver and later removed by macrophages. Two techniques were developed to correct the off-target internalization of EVs. The first is based on modifying the surface of EVs using polymers, increasing the duration of EV blood circulation and possibilities of target accumulation. The second approach is based on modifying the surface of EVs by adding a part of a molecule that has an affinity to the target cell or tissue [138].
Advantages of EV-Drug Delivery Technique
The internal cargo of EVs is protected by their membrane, likely by encapsulation, primarily originating from the cytosol of the mother cell, which will be transferred to the recipient cell upon fusion. Since the intraluminal vesicles are originated in the acidic condition of the multivesicular body, EVs and their protein should be resistant to pH. Additionally, upon entering the endosomal pathway, EVs will also undergo progressive acidification [139].
In addition, since EVs are encapsulated phages, some demonstrate higher tolerance for harsh conditions, providing higher efficacy when orally administered. The pH conditions in tumor cells may range from 6.0 to 6.8. Initial data concluded that cancer cells cultured in a physiological pH 7.4 released a significantly lower concentration of EVs than those cultured in 6.4 pH. Concluding that, cells release EVs at low pH to avoid toxin accumulation [140].
Ogawa et al. [139] studied the stability of EVs under different pH conditions. The study stated that EVs are stable in functionality and shape under acidic conditions for at least 3 h. In case of EVs rupture, the action can be due to membrane penetration by protease. The process is facilitated by fatty acid cleavage mediated by phospholipase A2, leading to EV surface protein degradation [139]. The surface protein profile on the EVs’ membrane is important due to proteases damaging the extracellular environment. For example, the IgA present on the surface of EVs protects these vesicles from breaking down [141].
9. EV Isolation Techniques
Several methods to isolate EVs are available. Such methods are ultracentrifugation isolation and microfluidic-based isolation [142], polymer-based precipitation, ultrafiltration, and immunoaffinity column, which can isolate EVs based on their size. All of which supplies plusses and minuses [106].
A study conducted by Buschmann et al. [64] aimed to compare different EVs isolation methods and their miRNA in terms of yield and quality. The isolation protocols used in this study included ultracentrifugation, precipitation, sedimentation, and size exclusion chromatography, along with a commercially available isolation kit. The study concluded that each isolation method provided a method-specific difference, including isolates size, yield, and purity of miRNA, indicating that each method can isolate a particular population of EVs with specific contaminants.
Antibody-based isolation methods employ a chosen biomarker present on particular cells’ released vesicles’ surfaces. This method has its drawbacks, including the inability to isolate vesicles that carry more than one protein biomarker on their surface [143].
9.1. Centrifugation Method
This method is declared to be the golden protocol to isolate EVs. However, its time consuming and depends mainly on the instruments needed. A fast and practical approach is required to facilitate EVs’ isolation [142]. Several studies used similar centrifugation speeds ranging between 1500× g and 125,000× g [144]. In general, the method isolates EVs based on their density. The speed gradually increases to sediment the large cells, cell debris, and apoptotic bodies. The process incorporates several steps starting with a speed as low as 300× g for a short period to pellet the cells, then 2000–10,000× g to remove apoptotic bodies and vesicles larger than EVs. The obtained supernatant will be ultracentrifuge at 100,000–200,000× g in order to pellet EVs. The obtained particles should be subjected to microfiltration to remove impurities [145] (Figure 3). Protein profiling of EVs isolated from urine by two methods was performed by Bijnsdorp et al., [146] where ultracentrifugation and heat chock proteins Vn 96-peptides were performed separately. The proteomic profiling was undertaken by mass-spectrometry analysis. The results indicated that protein profiling of EVs from both methods was similar; also, the Vn96-peptide methods are less time-consuming and can yield higher EVs.
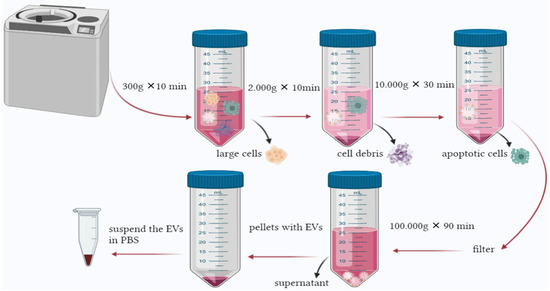
Figure 3.
Ultracentrifugation is the most common method used to isolate EVs. The process is based on increasing the force and time gradually to pellet large cells and contaminants until reaching the final centrifugation, where EVs can be found in the pellet.
Optimization of Ultracentrifugation Isolation Method
A common modification to the ultracentrifugation method is to combine it with a sucrose gradient. The sucrose gradient is the purification step. This method allows the vesicles to float into a laid sucrose gradient. EVs markers can be observed and trapped within the different gradient layers, as reported in many studies [147]; this method was applied by Silverman et al. [148] to isolate and purify EVs derived from frozen neural tissue from both human and murine.
Somiya et al. [101] developed an approach to improve EVs’ yield and purity isolated by ultracentrifugation; the extra step included treating the yield by acid to remove impurities and non-EV protein followed by additional ultracentrifugation.
Before conducting a differential centrifugation process, another treatment was experimented with by treating the tumor sample with enzymes (collagenase D and Dnase) to isolate a pure EVs subpopulation with intact characteristics. Electron microscopy analysis indicated that the isolated EVs are intact, pure, and maintained the morphological characteristics of the vesicles and that enzyme treatment enhanced the isolation of EVs from dense fibrotic tissue [18].
A combination of ultracentrifugation with subsequent agarose gel electrophoresis to isolate dry plant EVs was developed by Woith et al. [149]. The approach included a series of centrifugations, the protein contaminants were eliminated, and the final isolates obtained from 5000× g pellet were applied to agarose gel to remove the soluble contaminants. In order to recover the pure EVs, the gel is sectioned and removed by an additional final centrifugation step. Results indicated that impurities passed through the gel while EVs were retained in the gel pockets due to their smaller size.
Ultracentrifugation methods were compared with Optiprep™ Density Gradient ultracentrifugation in terms of purity and quality of the isolated EVs. The study isolated CNS-derived EVs from microglia, isolated EVs from both methods were characterized using mass spectrometer analysis indicated that Optiprep™ Density Gradient ultracentrifugation provided richer isolates with a limited amount of co-protein contaminants, but the TEM analysis detected aggregated vesicles within the isolates [150].
9.2. Size Exclusion Chromatography Method (SEC)
The method is well-established that separates macromolecules based on their size and volume. Typically, the system includes a stationary phase containing cross-linkage agarose beads commonly used for EV isolation [151].
A study combined size exclusion with ion-exchange chromatography methods to investigate miRNA and protein quality and assess the isolated EVs’ biological activities. The study concluded that the isolated EVs were rich in miRNA and proteins and were biologically active [122]. Bind-elute size exclusion chromatography (BE-SEC) combined with tangential flow filtration (TFF) is an optimized protocol to purify the isolated EVs from residual soluble protein [124].
A study conducted by Takov et al. [152] aimed to evaluate the difference between EV isolation using UC and SEC methods in terms of yield, purity, and quality. NTA used for size and concentration determination, as for the protein count and concentration BCA protein assay kit was used along with proteome profiler human angiogenesis essay, along with other characterization and profiling approaches to conclude that the EVs isolated from ultracentrifugation are less in terms of yields. However, the quality and purity of SEC isolated EVs are questionable.
9.3. Immuno-Affinity Based Column Method
The immuno-affinity based column method is employed to capture EVs based on particular surface markers; the method was reported to isolate pure EVs successfully. However, a significant obstacle is the lack of plant EV particular markers [121]. Still, the affinity-based method is an excellent tool for understanding the function of EVs. A study conducted by Nakai et al. [35] aimed to develop a new affinity-based EV isolation method from biological fluids and conditioned media. The study used T-cells immunoglobulin and mucin containing Tim4 protein; the isolated EVs were reported to be pure, intact, and easily separated from the capturing protein by adding buffer with a chelating agent.
Affinity-based aqueous two-phase systems (ATPS), albeit not commonly used, was described as an efficient and fast isolation method with fewer contaminants, used to isolate small EVs from plants, parasites, and cell culture. It combines two polymers with salt solutions that separate and isolate various biomolecules optimized to remove protein contaminants. The most significant advantage when using this method is that shorter time utilized with pure and intact yield; also, there is no need for specific equipment to use ATPS. The primary disadvantage is the presence of dextran in the final suspension of EVs [153].
10. Characterization of EVs
The size of EVs in general and exosomes, in particular, is so small, making accurate characterization of EVs very challenging. For instance, nanoparticle tracking analysis will not detect particles smaller than 50 nm; on the other hand, flow cytometry will only detect particles larger than 300 nm. Dynamic light scattering will detect particles varied in size between 1 and 6000 nm with overestimating the particle count. Another option is using atomic force microscopy and electron microscopy. However, these methods are time-consuming, costly, and require professionals to operate the device. For example, if the sample concentration is high, the support film will be damaged, preventing EV counting and imaging since the characterization methods are not standardized and integrated. The data available in the literature are diverse and conflicting [71].
Physicochemical and biochemical characterization of EVs aims to identify the isolated population and elucidate their profile in terms of lipidomics, proteomics, and gene expression analysis [154]. Physicochemical characterization of EVs studies their size [155], morphology [156], cargo, or the number of vesicles; the latter can be achieved by zeta potential analysis [157]. Size characterization is commonly achieved using dynamic light scattering techniques [158], light microscopy, and electron microscopy [21]. Vesicle concentration and size distribution are studied using nanoparticle tracking analysis [159,160] and flow cytometry [105].
Cry electron microscopy characterizes the particles without adding heavy metals to the sample as fixative agents. This method was used in a study to characterize single cell EVs in terms of their morphology, structure, and lipid layer. The cry electron microscopy provided information confirming that single-cell EVs showed diverse characteristics [26].
Flow cytometry has advantages and disadvantages. The functioning concept is detecting the vesicles’ fluorescence and light scattering signal individually, but the technique does not detect all EV subclasses. Therefore, the quality of the results depends on the device’s sensitivity since EVs concentration increases when their size decreases [161].
Additionally, proteomic characterization is a practical approach to detect common EV markers. The most common proteomic characterization is conducted by mass spectrometry [101]. Label-free quantitative proteomics is a mass spectrometry approach that detects common EV protein markers regardless of their morphology, origin, or cellular strain. This method can detect plasma membrane protein, tetraspanin, and GTPase [162]. Liquid chromatography-tandem mass spectrometer (LC-MS/MS) analysis also characterizes EV protein concentration and cargo [150].
Characterization of EVs based on their genetic makeup can be achieved by running a quantitative real-time PCR; the analysis takes place after extracting and amplifying the RNA to detect the genes present and their respective metabolic process [105]. Ex vivo behavior and communication of EVs, especially tumor-derived EVs, are investigated by applying 2D and 3D cell culture models; these two techniques are significant for evaluating RNA-derived EVs. Although, the 3D cell culture model is more competent when evaluating the shape of the vesicles, their behavior, and their extracellular communication [49].
11. Toxicity and Safety Assessment of EVs
Due to the endogenous properties of EVs, they migrate between cells and deliver their biological cargo upon crossing the membrane [86]. EVs can travel long distances through the blood circulation and reach various tissues affecting the signaling process [82], mediating several pathological and physiological functions [83]. On the other hand, EVs play a significant role in anti-inflammatory and antiapoptotic actions [88]. It could either work as protected vesicles that expel invading pathogens from the infected cell or as vector-transmitted virulence factors facilitating the infection [163]. Meaning that the function of EVs upon entering the body depends on the biological status of the host and the receiving cell. There are cases when EVs act as immune responses against infection. Furthermore, it worked as a signaling pathway for pathogens [164].
Studying the efficacy of EVs in therapy is significant, especially in preclinical development. However, evaluating their risk and toxicity is essential in developing their therapeutic performance. Attention must be directed towards the possibility of immunogenicity or toxicity of therapeutic EVs [165]. Therefore, an in-depth understanding of EVs’ overall toxicity and biodiversity following in vivo studies is required before using them as therapeutic agents or drug carriers, primarily if the EVs are originated from xenogeneic sources [166].
Somiya et al. [101] evaluated the safety of milk-derived EVs; the paper stated that no systematic toxicity or immunogenicity was detected. The mice were given an intravenous injection containing EVs derived from milk. A blood sample analysis confirmed the safety of these EVs, and no toxicity markers from the kidney nor the liver were observed.
A study conducted in vitro evaluation of the toxicity of mesenchymal and bovine milk-derived EVs stated that there is no genotoxic response to either EVs, but collagen-induced platelet aggregation in a dose-related manner was recorded. In vivo investigation was conducted to assess the safety of engineered HEK293T cell-derived EVs via intravenous administration for 21 days. The results provided no signs of toxic effect, immune changes, and no alteration of the EVs [167].
According to a study conducted by Roccaro et al. [168]. EVs were isolated from Mesenchymal stem cells obtained from multiple melanoma patients. The results indicated that EVs promote tumor growth by dissemination and metastasis to distant multiple melanoma niches. Besides, EVs are associated with stimulating or suppressing the immune response and play a role in regulating the suitable microenvironment for the tumor’s biological process [163].
Plant-derived EVs pose several advantages over mammalian-derived EVs. Therefore, the field experts recommended an accurate assessment of their toxicity and safety for clinical application [169]. Engineering EVs into a therapeutic tool is valuable, ensuring quality and safety. in the time being, most available data on the safety and toxicity of EVs in vitro and in vivo are using mesenchymal stromal cells-derived EVs. Not enough attention was dedicated to other EVs from different sources [166].
Therefore, evaluating the toxicity and safety of EVs in vitro and in vivo will set the dose for future clinical use and safe consumption recommendations for human application. It provides fundamental insight into pharmacodynamics, therapeutic benefits, purity, ideal storage, shelf-life, and quality control (Figure 4). These quality and safety control processes will innovate the possibilities of applying plant-derived EVs in therapy and establish regulations and standards for production and development.
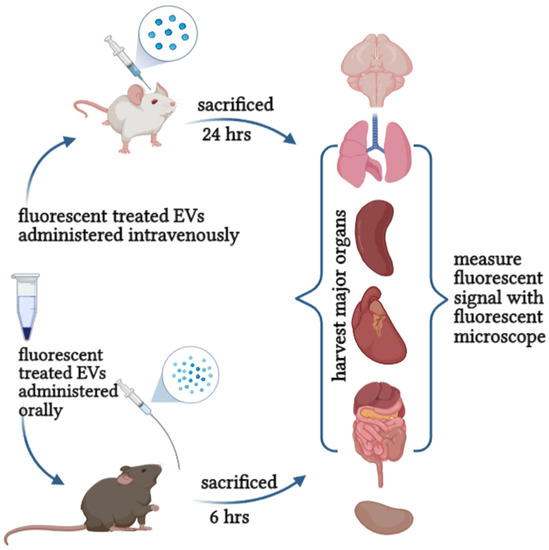
Figure 4.
Evaluation the safety and toxicity of EVs can be achieved either orally or intravenously.
Although, there are no set standards or parameters to evaluate the safety and toxicity of EVs. However, understanding their uptake and behavior locally and systematically is vital to assess their safety [170].
12. Storage Recommendations and Stability of EVs
EVs’ storage conditions significantly impact their therapeutic value and stability [171]. Many studies showed uncertainty regarding the storage stability of EVs under different conditions, particularly prolonged storage. Mainly focusing on the degradation of protein using western plot analysis but failed to focus on their functional ability after its uptake by other cells. Sterzenbach et al. [172] tested the biological function of EVs after storage; they indicated that EVs showed a degree of sensitivity and fragility compared to newly isolated ones; there was also a reduction of EV ability to transfer their protein to the receptor cell [172]. Rutering et al. [173] isolated EVs from bovine milk and stored them for 18 months at −80 °C, the study claimed that this storage condition had little effect on the particle size, and no coagulation was observed. Additionally, the impact of storage on the biological activity of EVs was assessed by anti-proliferation activity. The results indicated no loss of activity due to prolonged storage. The recommended storing condition of EVs as was published by ISEV is that EVs should be suspended in phosphate buffer saline and stored at −80 °C because a greater temperature causes a reduction of the number of isolated EVs [174].
13. Discussion and Conclusions
This review provides a concise retelling of the history, concept, development, latest findings, and recommendations related to extracellular vesicles. We recommended this paper to those new to the field needing to understand EVs and those already familiar with it but wanting to keep up with the latest findings. We listed the continuous change in technical developments, new findings, and debates along the review lines.
Starting from the early history of these riveting nanoparticles, we observed that it is well established that contrary to the previous common concept that classifies EVs as a mere cellular waste carrier, the field’s advancement unveiled that these nano-sized vesicles play an essential role in several processes [175]. One of the most is their involvement in cellular communication, transportation of miRNA, protein, lipids [60], and as a significant player in several pathological processes, including infection and metastasis [176], agreeing that EVs are an appealing tool for non-invasive rapid diagnosis that can be exploited in the case of asymptomatic diseases [177]. Another vital fact is that EVs play an essential role in embryonic growth, development, and maturation. Such as in breast milk-derived EVs, which transports EVs from the mother to the newborn [178]—confirming their contribution to pathological or physiological processes.
The reported number of searches on EVs is elevated yearly, providing more knowledge and clues to understand these nanoparticles better.
This review focused on the most recent advancement in extracellular vesicles, their function, the latest update on EVs cargo, and their contribution and involvement in physiological and pathological processes [179]. We highlighted some of the technical challenges, current findings and, breakthrough developments in research related to EVs isolation, characterization, and drug loading technology. That has been addressed by many papers [180], giving hope for more findings and better results.
A fact affirms an increased count of the number of EVs circulating in the system during infection, providing early diagnostic tools significant in cancer or sudden brain ailment [83]. Several researchers concluded that cancer cells release EVs and therefore are found circulating in the body carrying their genetic code [181]; these EVs act as a messenger, communicating with nearby or distant cells [182] during metastasis, making the surrounding environment ideal for the pre-metastatic niche [183].
ISEV visited the topic in a position paper, stated that EVs had already been used in human trials to treat cancer in early 2000 [177]. Furthermore, the available results point to the importance of the role played by plant-derived EVs in cancer studies as therapeutic and drug carriers [71]. The cargo of plant EVs is substantial and similar to mammal EVs, not to mention their use in therapy is meaningful to those who are strictly vegan or religious individuals. All are essential contributions to the fight against cancer.
Some studies used plant-derived EVs to understand their function and potential use as a drug carrier [184]. Plants EVs also act similarly to mammal EVs, albeit with a slight difference in the biogenesis, circulate, allocate and carry specific cargo [185]. The significance of plant EVs is underestimated since plants are readily available from biological origins [186]. Not to mention that the toxicity of plant source materials is not substantial, with no considerable reported side effects [187]. As a result of this review, mammal and plant EVs are similar in function, characteristics, and cargo, and can act in physiological and pathological conditions. Using these EVs in future research can majorly contribute to the field, especially if the safety of plant EVs were to be confirmed. The already-existing body of evidence on the use of plant-derived EVs for nano-delivery offers a wide range of possibilities for their future use in health and therapeutics.
Since there are several obstacles, the researchers face this field [188]. We highlighted the latest advancements, recommendations, and contributions from ISEV, the leaders in this field. ISEV stated in an early published position paper that EVs’ field faces continuous challenges technically, including the expanding number of EVs isolation methods with low yield [189]. Advising that, it is essential to accurately state whether the studied EVs are used for clinical application as the active drug component, or it is simply the drug carriers following ISEV guidelines [177].
Additionally, based on the recommendations from ISEV that were published on their position paper titled ‘Applying extracellular vesicles-based therapeutics in clinical trials an ISEV position paper’. The paper mentioned that using EVs in therapy must comply with the established regulations. Clinical investigation and quality control are crucial aspects of EV production in therapy. It is essential to accurately state whether the used EVs are the active drug component or used as a drug carrier. Their statement was:
EV-based therapeutics can be defined as biological medicine and belong to the pharmaceutical class of biologicals. Regulatory frameworks for manufacturing and clinical trials exist in Europe, Australia, and United States, but special guidelines targeting EV-based therapeutics may be needed.
However, more extensive studies are needed to fully understand the former as an active component in therapy; we recommend that added attention be directed towards plant-derived EVs, their function, cargo, and characteristics that were the subjects of a shy number of articles compared to mammal EVs. More safety assessment studies and quality control standards are additional clinical application recommendations. We observed that the lack of standard isolation, characterization protocols, and quality control hinders these studies’ advancement in either purity or yield of EVs for clinical use [190], making it hard to link and compare the available findings. Then again, the field recognized these issues [65] and they have been addressed on several occasions, emphasizing the need to standardize the isolation protocols. Although there is so much more to learn about EVs, what scientists have discovered so far is fascinating and promising. Nevertheless, this preliminary data offers additional insights into other possible benefits of EVs for a better future.
Author Contributions
Conceptualization: N.B.A., A.F.A.R. and D.J.O.; supervision: A.F.A.R., D.J.O., K.W.C., N.I. and J.B.F.; funding acquisition: D.J.O.; writing—original draft preparation: A.F.A.R. and N.B.A.; writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Malaysian Ministry of Higher Education under the Fundamental Research Grant Scheme (grant no: FRGS/1/2019/STG05/MAHSA/02/2) on the project entitled Bioprospecting of Oil Palm-Derived Exosome for Nanotherapeutic Delivery: A Study on Exosome Stability and Uptake Mechanism.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-Cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef]
- Brenna, S.; Altmeppen, H.C.; Mohammadi, B.; Rissiek, B.; Schlink, F.; Ludewig, P.; Krisp, C.; Schlüter, H.; Failla, A.V.; Schneider, C.; et al. Characterization of Brain-Derived Extracellular Vesicles Reveals Changes in Cellular Origin after Stroke and Enrichment of the Prion Protein with a Potential Role in Cellular Uptake. J. Extracell. Vesicles 2020, 9, 1809065. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Varela, M.; de Menezes-Neto, A.; Perez-Zsolt, D.; Gámez-Valero, A.; Seguí-Barber, J.; Izquierdo-Useros, N.; Martinez-Picado, J.; Fernández-Becerra, C.; del Portillo, H.A. Proteomics Study of Human Cord Blood Reticulocyte-Derived Exosomes. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lötvall, J.; Hoen, E.N.N.-; Piper, M.G.; Skog, J.; Théry, C.; Wauben, M.H.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining Mesenchymal Stromal Cell (MSC)-Derived Small Extracellular Vesicles for Therapeutic Applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [Green Version]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Wąchalska, M.; Graul, M.; Rychłowski, M.; Bieńkowska-Szewczyk, K.; Lipińska, A.D. Alphaherpesvirus Gb Homologs Are Targeted to Extracellular Vesicles, but They Differentially Affect MHC Class II Molecules. Viruses 2020, 12, 429. [Google Scholar] [CrossRef] [Green Version]
- Saenz-Pipaon, G.; San Martín, P.; Planell, N.; Maillo, A.; Ravassa, S.; Vilas-Zornoza, A.; Martinez-Aguilar, E.; Rodriguez, J.A.; Alameda, D.; Lara-Astiaso, D.; et al. Functional and Transcriptomic Analysis of Extracellular Vesicles Identifies Calprotectin as a New Prognostic Marker in Peripheral Arterial Disease (PAD). J. Extracell. Vesicles 2020, 9, 1729646. [Google Scholar] [CrossRef]
- Xiao, Y.; Driedonks, T.; Witwer, K.W.; Wang, Q.; Yin, H. How Does an RNA Selfie Work? EV-Associated RNA in Innate Immunity as Self or Danger. J. Extracell. Vesicles 2020, 9, 1793515. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular Uptake of Extracellular Vesicles Is Mediated by Clathrin-Independent Endocytosis and Macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-Melanogenic Effects of Extracellular Vesicles Derived from Plant Leaves and Stems in Mouse Melanoma Cells and Human Healthy Skin. J. Extracell. Vesicles 2020, 9, 1703480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariscal, J.; Vagner, T.; Kim, M.; Zhou, B.; Chin, A.; Zandian, M.; Freeman, M.R.; You, S.; Zijlstra, A.; Yang, W.; et al. Comprehensive Palmitoyl-Proteomic Analysis Identifies Distinct Protein Signatures for Large and Small Cancer-Derived Extracellular Vesicles. J. Extracell. Vesicles 2020, 9, 1764192. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Desrochers, L.M.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicles: Satellites of Information Transfer in Cancer and Stem Cell Biology. Dev. Cell 2016, 37, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Azuma, E.; Muramatsu, M.; Hamashima, T.; Ishii, Y.; Sasahara, M. Significance of Extracellular Vesicles: Pathobiological Roles in Disease. Cell Struct. Funct. 2016, 41, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Crescitelli, R.; Lässer, C.; Jang, S.C.; Cvjetkovic, A.; Malmhäll, C.; Karimi, N.; Höög, J.L.; Johansson, I.; Fuchs, J.; Thorsell, A.; et al. Subpopulations of Extracellular Vesicles from Human Metastatic Melanoma Tissue Identified by Quantitative Proteomics after Optimized Isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal Targeting and Its Potential Clinical Application. Drug Deliv. Transl. Res. 2021, 12, 18. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and Function of Extracellular Vesicles in Cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond Size and Phosphatidylserine Exposure: Evidence for a Diversity of Apoptotic Cell-Derived Extracellular Vesicles in Vitro. J. Extracell. Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro-Ibáñez, E.; Lässer, C.; Shelke, G.V.; Crescitelli, R.; Jang, S.C.; Cvjetkovic, A.; García-Rodríguez, A.; Lötvall, J. DNA Analysis of Low- and High-Density Fractions Defines Heterogeneous Subpopulations of Small Extracellular Vesicles Based on Their DNA Cargo and Topology. J. Extracell. Vesicles 2019, 8, 1656993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lässer, C.; Jang, S.C.; Lötvall, J. Subpopulations of Extracellular Vesicles and Their Therapeutic Potential. Mol. Asp. Med. 2018, 60, 1–14. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Kumar, S.; Mathivanan, S. Ticket to a Bubble Ride: Cargo Sorting into Exosomes and Extracellular Vesicles. Biochim. Biophys. Acta BBA Proteins Proteom. 2019, 1867, 140203. [Google Scholar] [CrossRef]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Höög, J.L.; Zabeo, D.; Cvjetkovic, A.; Lässer, C. Exosomes Purified from a Single Cell Type Have Diverse Morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Ke, W.; Afonin, K.A. Exosomes as Natural Delivery Carriers for Programmable Therapeutic Nucleic Acid Nanoparticles (NANPs). Adv. Drug Deliv. Rev. 2021, 176, 113835. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Morphological and Molecular Features of Oral Fluid-Derived Exosomes: Oral Cancer Patients versus Healthy Individuals. J. Cancer Res. Clin. Oncol. 2016, 142, 101–110. [Google Scholar] [CrossRef]
- Hong, C.S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of Biologically Active and Morphologically Intact Exosomes from Plasma of Patients with Cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Zempleni, J. Milk Exosomes: Beyond Dietary MicroRNAs. Genes Nutr. 2017, 12, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L. Post Isolation Modification of Exosomes for Nanomedicine Applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlan, R.S.; Pisano, S.; Oliveira, M.I.; Ferrari, M.; Mendes Pinto, I. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol. Med. 2017, 23, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.W.; Nguyen, J. Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. J. Control. Release 2016, 228, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T. OPEN A Novel Affinity-Based Method for the Isolation of Highly Purified Extracellular Vesicles. Nat. Publ. Group 2016, 6, 33935. [Google Scholar] [CrossRef] [Green Version]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.D.; Bosio, A.; Schauss, A.; Wild, S. A Novel Multiplex Bead-Based Platform Highlights the Diversity of Extracellular Vesicles. J. Extracell. Vesicles 2016, 5, 29975. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Sundar, I.K.; Li, D.; Rahman, I.; Sundar, I.K. Small RNA-Sequence Analysis of Plasma-Derived Extracellular Vesicle MiRNAs in Smokers and Patients with Chronic Obstructive Pulmonary Disease as Circulating Biomarkers. J. Extracell. Vesicles 2019, 8, 1684816. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R. The Biology and Function of Urine Exosomes in Bladder Cancer. J. Clin. Investig. 2016, 4, 2362. [Google Scholar] [CrossRef]
- Dhondt, B.; Geeurickx, E.; Tulkens, J.; Van Deun, J.; Lippens, L.; Miinalainen, I.; Rappu, P.; Heino, J.; Lumen, N.; De Wever, O.; et al. Unravelling the Proteomic Landscape of Extracellular Vesicles in Prostate Cancer by Density- Based Fractionation of Urine. J. Extracell. Vesicles 2020, 9, 1736935. [Google Scholar] [CrossRef]
- Burbidge, K.; Zwikelmaier, V.; Cook, B.; Long, M.M.; Lonigro, M.; Ispas, G.; Rademacher, D.J.; Edward, M.; Burbidge, K.; Zwikelmaier, V.; et al. Cargo and Cell-Specific Differences in Extracellular Vesicle Populations Identified by Multiplexed Immunofluorescent Analysis. J. Extracell. Vesicles 2020, 9, 1789326. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Gahan, P.B. MicroRNA Shuttle from Cell-to-Cell by Exosomes and Its Impact in Cancer. Non-Coding RNA 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurj, A.; Zanoaga, O.; Braicu, C.; Lazar, V.; Tomuleasa, C.; Irimie, A.; Berindan-Neagoe, I. A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells. Cancers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Go, G.; Kim, D.; Lee, J.; Park, S.; Di Vizio, D.; Gho, Y.S. Quantitative Proteomic Analysis of Trypsin-Treated Extracellular Vesicles to Identify the Real-Vesicular Proteins. J. Extracell. Vesicles 2020, 9, 1757209. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.P.; Hill, B.L.; Haque, M.W.; Raad, J.; Bonder, C.S.; Harshyne, L.A.; Rodeck, U.; Luginbuhl, A.; Wahl, J.K.; Tsai, K.Y.; et al. MiRNA- and Cytokine-Associated Extracellular Vesicles Mediate Squamous Cell Carcinomas. J. Extracell. Vesicles 2020, 9, 1790159. [Google Scholar] [CrossRef]
- Williams, C.; Royo, F.; Aizpurua-olaizola, O.; Pazos, R.; Reichardt, N.; Falcon-perez, J.M.; Williams, C.; Royo, F.; Aizpurua-olaizola, O.; Pazos, R. Glycosylation of Extracellular Vesicles: Current Knowledge, Tools and Clinical Perspectives. J. Extracell. Vesicles 2018, 7, 1442985. [Google Scholar] [CrossRef]
- Thippabhotla, S.; Zhong, C.; He, M. 3D Cell Culture Stimulates the Secretion of in Vivo like Extracellular Vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef] [Green Version]
- Bahr, M.M.; Amer, M.S.; Abo-el-sooud, K.; Ahmed, N.; El-tookhy, O.S. Preservation Techniques of Stem Cells Extracellular Vesicles: A Gate for Manufacturing of Clinical Grade Therapeutic Extracellular Vesicles and Long-Term Clinical Trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs [OPEN]. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, J.G.; Franklin, J.L.; Weaver, A.M.; Vickers, K.; Zhang, B.; Coffey, R.J.; Ansel, K.M.; Blelloch, R.; Goga, A.; Huang, B.; et al. Biogenesis, Delivery, and Function of Extracellular RNA. J. Extracell. Vesicles 2015, 4, 27494. [Google Scholar] [CrossRef]
- Jabalee, J.; Towle, R.; Garnis, C. The Role of Extracellular Vesicles in Cancer: Cargo, Function, and Therapeutic Implications. Cells 2018, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Morad, G.; Moses, M.A. Brainwashed by Extracellular Vesicles: The Role of Extracellular Vesicles in Primary and Metastatic Brain Tumour Microenvironment. J. Extracell. Vesicles 2019, 8, 1627164. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Beer, K.B.; Wehman, A.M. Mechanisms and Functions of Extracellular Vesicle Release in Vivo—What We Can Learn from Flies and Worms. Cell Adhes. Migr. 2017, 11, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.E.; Sneider, A.; Witwer, K.W.; Bergese, P.; Bhattacharyya, S.N.; Cocks, A.; Cocucci, E.; Erdbrügger, U.; Falcon-Perez, J.M.; Freeman, D.W.; et al. Biological Membranes in EV Biogenesis, Stability, Uptake, and Cargo Transfer: An ISEV Position Paper Arising from the ISEV Membranes and EVs Workshop. J. Extracell. Vesicles 2019, 8, 1684862. [Google Scholar] [CrossRef] [Green Version]
- Nogués, L.; Benito-Martin, A.; Hergueta-Redondo, M.; Peinado, H. The Influence of Tumour-Derived Extracellular Vesicles on Local and Distal Metastatic Dissemination. Mol. Asp. Med. 2018, 60, 15–26. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells123. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-Derived Nano-Lipids Loaded with Doxorubicin as a Novel Drug-Delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschmann, D.; Kirchner, B.; Hermann, S.; Märte, M.; Wurmser, C.; Brandes, F.; Kotschote, S.; Bonin, M.; Steinlein, O.K.; Pfaffl, M.W.; et al. Evaluation of Serum Extracellular Vesicle Isolation Methods for Profiling MiRNAs by Next-Generation Sequencing. J. Extracell. Vesicles 2018, 7, 1481321. [Google Scholar] [CrossRef]
- Welsh, J.A.; Pol, E.; Bettin, B.A.; Carter, D.R.F.; Hendrix, A.; Lenassi, M.; Langlois, M.; Llorente, A.; Nes, A.S.; Nieuwland, R.; et al. Towards Defining Reference Materials for Measuring Extracellular Vesicle Refractive Index, Epitope Abundance, Size and Concentration. J. Extracell. Vesicles 2020, 9, 1816641. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Jaular, L.M.; Soueidi, E.; Jouve, M.; Muth, D.C.; Schøyen, T.H.; Seale, T.; Haughey, N.J.; Ostrowski, M.; Théry, C.; et al. Acetylcholinesterase Is Not a Generic Marker of Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1628592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.S. Extracellular Vesicles as a Platform for Membrane-Associated Therapeutic Protein Delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef] [Green Version]
- De Jong, O.G.; Murphy, D.E.; Mäger, I.; Willms, E.; Garcia-guerra, A.; Gitz-francois, J.J.; Lefferts, J.; Gupta, D.; Steenbeek, S.C.; Van Rheenen, J.; et al. Vesicle-Mediated Functional Transfer of RNA. Nat. Commun. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, P.; Deng, L.; Liu, Z.; Wang, X.; Liu, X. Hepatitis A Virus Structural Protein PX Interacts with ALIX and Promotes the Secretion of Virions and Foreign Proteins through Exosome-like Vesicles. J. Extracell. Vesicles 2020, 9, 1716513. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and Isolation Methods for Plant Leaf Nanovesicles and Small Extracellular Vesicles. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102271. [Google Scholar] [CrossRef]
- Cao, X.-H.; Liang, M.-X.; Wu, Y.; Yang, K.; Tang, J.-H.; Zhang, W. Extracellular Vesicles as Drug Vectors for Precise Cancer Treatment. Nanomedicine 2021, 16, 1519–1537. [Google Scholar] [CrossRef]
- Pérez-bermúdez, P.; Blesa, J.; Miguel, J.; Marcilla, A. European Journal of Pharmaceutical Sciences Extracellular Vesicles in Food: Experimental Evidence of Their Secretion in Grape Fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef] [PubMed]
- García-manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-lópez, M.C.; Pazos, C. Therapeutic Biomaterials Based on Extracellular Vesicles: Classification of Bio-Engineering and Mimetic Preparation Routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Ren, Y.; Sayed, M.; Park, J.W.; Egilmez, N.K.; Zhang, H.; Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; Zhang, H.-G. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-Derived Edible Nanoparticle-Based Lipid Nano-Drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timms, K.; Holder, B.; Day, A.; McLaughlin, J.; Westwood, M.; Forbes, K. Isolation and Characterisation of Watermelon (Citrullus Lanatus) Extracellular Vesicles and Their Cargo. bioRxiv 2020, 791111. [Google Scholar] [CrossRef] [Green Version]
- Rome, S. Biological Properties of Plant-Derived Extracellular Vesicles. Food Funct. 2019, 10, 529–538. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. ScienceDirect Extracellular Vesicles as Key Mediators of Plant—Microbe Interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived MicroRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tong, Y.; Qiu, Y.; Ye, C.; Wu, N.; Xiong, X.; Wang, J.; Han, Y.; Zhou, Y.; Zhang, F.; et al. MiR155-5p in Adventitial Fibroblasts-Derived Extracellular Vesicles Inhibits Vascular Smooth Muscle Cell Proliferation via Suppressing Angiotensin-Converting Enzyme Expression. J. Extracell. Vesicles 2020, 9, 1698795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.S.; Jeong, E.; Boyiadzis, M.; Whiteside, T.L. Increased Small Extracellular Vesicle Secretion after Chemotherapy via Upregulation of Cholesterol Metabolism in Acute Myeloid Leukaemia. J. Extracell. Vesicles 2020, 9, 1800979. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, B.; Rousseau, Q.; De Wever, O.; Hendrix, A. Function of Extracellular Vesicle-Associated MiRNAs in Metastasis. Cell Tissue Res. 2016, 365, 621–641. [Google Scholar] [CrossRef]
- Kranich, J.; Chlis, N.; Rausch, L.; Latha, A.; Kurz, T.; Kia, A.F.; Simons, M.; Theis, J.; Brocker, T.; Kranich, J.; et al. In Vivo Identification of Apoptotic and Extracellular Vesicle-Bound Live Cells Using Image-Based Deep Learning. J. Extracell. Vesicles 2020, 9, 1792683. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, L.; Turchinovich, A.; Mahairaki, V.; Troncoso, C.; Pletniková, O.; Haughey, N.J.; Vella, L.J.; Andrew, F.; Zheng, L.; et al. Influence of Species and Processing Parameters on Recovery and Content of Brain Tissue-Derived Extracellular Vesicles. J. Extracell. Vesicles 2020, 9, 1785746. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Zaragozá, M.C.; Martínez-Martínez, A.; Blanco-López, M.D.; Alegre, J. Circulating Extracellular Vesicles as Potential Biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An Exploratory Pilot Study. J. Extracell. Vesicles 2018, 7, 1453730. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.C.; Guo, S.C.; Zhang, C.Q. Platelet-Derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef] [Green Version]
- Morales-kastresana, A.; Musich, T.A.; Welsh, J.A.; Demberg, T.; Wood, J.C.S.; Bigos, M.; Ross, C.D.; Kachynski, A.; Dean, A.; Felton, E.J.; et al. High-Fidelity Detection and Sorting of Nanoscale Vesicles in Viral Disease and Cancer. J. Extracell. Vesicles 2019, 8, 1597603. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Wu, J.; Fang, D.; Qiu, Y.; Zou, X.; Jia, X.; Yin, Y.; Shen, L.; Mao, L. Exosomes Cloak the Virion to Transmit Enterovirus. Virulence 2020, 11, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Raab-Traub, N.; Dittmer, D.P. Viral Effects on the Content and Function of Extracellular Vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lu, J.; Chen, L.; Bian, H.; Hu, J.; Li, D.; Xia, C.; Xu, H. Tumor-Derived EV-Encapsulated MiR-181b-5p Induces Angiogenesis to Foster Tumorigenesis and Metastasis of ESCC. Mol. Ther. Nucleic Acids 2020, 20, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.P.; Fromm, B.; Andersen, S.D.; Marcilla, A.; Andersen, K.L.; Borup, A.; Williams, A.R.; Jex, A.R.; Gasser, R.B.; Young, N.D.; et al. Exploration of Extracellular Vesicles from Ascaris Suum Provides Evidence of Parasite–Host Cross Talk. J. Extracell. Vesicles 2019, 8, 1578116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia Coli Extracellular Vesicle Proteome Identifies Markers of Purity and Culture Conditions. J. Extracell. Vesicles 2019, 8, 1632099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- András, I.E.; Toborek, M. Extracellular Vesicles of the Blood-Brain Barrier. Tissue Barriers 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C.; Schmitz, G. Exosomes of Pasteurized Milk: Potential Pathogens of Western Diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Galley, D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef] [Green Version]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes from Mothers With Type 1 Diabetes Contain Aberrant Levels of MicroRNAs. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Van Herwijnen, M.J.C.; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Hoen, E.N.M.N.; Wauben, M.H.M. Abundantly Present MiRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Front. Nutr. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of Highly Purified Bovine Milk- Derived Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, J.; Kolhe, R.; Hunter, M.; Isales, C.; Hamrick, M.; Fulzele, S. Stem Cell-Derived Exosomes: A Potential Alternative Therapeutic Agent in Orthopaedics. Stem Cells Int. 2016, 2016, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Hamelin, R.; Demurtas, D.; Ahmed, M.A.; Piemonti, L.; Hirosue, S.; Swartz, M.A.; De Palma, M.; et al. Primary Human and Rat b -Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine- Induced Enhancers of Immunity. Diabetes 2017, 66, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Wang, C.; Long, K.; Zhang, H.; Zhang, J.; Jin, L. Exosomal MicroRNAs in Giant Panda (Ailuropoda Melanoleuca) Breast Milk: Potential Maternal Regulators for the Development of Newborn Cubs. Sci. Rep. 2017, 7, 3507. [Google Scholar] [CrossRef]
- Kobayashi-Sun, J.; Yamamori, S.; Kondo, M.; Kuroda, J.; Ikegame, M.; Suzuki, N.; Kitamura, K.; Hattori, A.; Yamaguchi, M.; Kobayashi, I. Uptake of Osteoblast-Derived Extracellular Vesicles Promotes the Differentiation of Osteoclasts in the Zebrafish Scale. Commun. Biol. 2020, 3, 190. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Ma, H.; Liu, D. Extracellular Vesicles Function as Bioactive Molecular Transmitters in the Mammalian Oviduct: An Inspiration for Optimizing in Vitro Culture Systems and Improving Delivery of Exogenous Nucleic Acids during Preimplantation Embryonic Development. Int. J. Mol. Sci. 2020, 21, 2189. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Luo, S.; Du, Y.; Zhang, Y.; Song, X.; Yuan, X.; Lin, Z.; Li, Y.; Liu, E. Extracellular Vesicles and Melatonin Benefit Embryonic Develop by Regulating Reactive Oxygen Species and 5-methylcytosine. J. Pineal Res. 2020, 68, e12635. [Google Scholar] [CrossRef]
- Rohde, E.V.A.; Pachler, K.; Gimona, M. Manufacturing and Characterization of Extracellular Vesicles from Umbilical Cord À Derived Mesenchymal Stromal Cells for Clinical Testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef]
- Sutaria, D.S.; Badawi, M.; Phelps, M.A.; Schmittgen, T.D. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil Is in Details of Therapeutic Loading. Pharm. Res. 2017, 34, 1053–1066. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Huang, X.; Woodcock, M.; Du, M.; Dittmar, R.; Wang, Y.; Tsai, S.; Kohli, M.; Boardman, L.; Patel, T.; et al. Plasma Extracellular RNA Profiles in Healthy and Cancer Patients. Sci. Rep. 2016, 6, 19413. [Google Scholar] [CrossRef] [Green Version]
- Zaborowski, M.P.; Lee, K.; Na, Y.J.; Sammarco, A.; Zhang, X.; Iwanicki, M.; Cheah, P.S.; Lin, H.-Y.; Zinter, M.; Chou, C.-Y.; et al. Methods for Systematic Identification of Membrane Proteins for Specific Capture of Cancer-Derived Extracellular Vesicles. Cell Rep. 2019, 27, 255–268.e6. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.C.; Crescitelli, R.; Cvjetkovic, A.; Belgrano, V.; Olofsson Bagge, R.; Sundfeldt, K.; Ochiya, T.; Kalluri, R.; Lötvall, J. Mitochondrial Protein Enriched Extracellular Vesicles Discovered in Human Melanoma Tissues Can Be Detected in Patient Plasma. J. Extracell. Vesicles 2019, 8, 1635420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, P.; Wu, F.; Kang, B.; Sun, X.; Heskia, F.; Pachot, A.; Liang, J.; Li, D. Plasma Extracellular Vesicles Detected by Single Molecule Array Technology as a Liquid Biopsy for Colorectal Cancer. J. Extracell. Vesicles 2020, 9, 1809765. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-S.; Choi, D.-Y.; Hong, B.; Jang, S.; Kim, D.-K.; Lee, J.; Kim, Y.-K.; Pyo Kim, K.; Gho, Y. Quantitative Proteomics of Extracellular Vesicles Derived from Human Primary and Metastatic Colorectal Cancer Cells. J. Extracell. Vesicles 2012, 1, 18704. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Meldolesi, J. Extracellular Vesicles, News about Their Role in Immune Cells: Physiology, Pathology and Diseases. Clin. Exp. Immunol. 2019, 196, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Xu, K.; Zhou, B.; Chen, T.; Huang, Y.; Li, Q.; Wen, F.; Ge, W.; Wang, J.; Yu, S.; et al. A Circulating Extracellular Vesicles-Based Novel Screening Tool for Colorectal Cancer Revealed by Shotgun and Data-Independent Acquisition Mass Spectrometry. J. Extracell. Vesicles 2020, 9, 1750202. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Mao, K.; Xu, J.; Wu, F.; Wang, X.; Wang, S.; Zhou, M.; Duan, L.; Tan, Q.; Ma, G.; et al. Metabolic Characteristics of Large and Small Extracellular Vesicles from Pleural Effusion Reveal Biomarker Candidates for the Diagnosis of Tuberculosis and Malignancy. J. Extracell. Vesicles 2020, 9, 1790158. [Google Scholar] [CrossRef]
- Cheng, L.; Vella, L.J.; Barnham, K.J.; McLean, C.; Masters, C.L.; Hill, A.F. Small RNA Fingerprinting of Alzheimer’s Disease Frontal Cortex Extracellular Vesicles and Their Comparison with Peripheral Extracellular Vesicles. J. Extracell. Vesicles 2020, 9, 1766822. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Hill, M.M.; Trau, M. Extracellular Vesicles as Circulating Cancer Biomarkers: Opportunities and Challenges. Clin. Transl. Med. 2018, 7, 14. [Google Scholar] [CrossRef]
- Popowski, K.; Lutz, H.; Hu, S.; George, A.; George, A. Exosome Therapeutics for Lung Regenerative Medicine. J. Extracell. Vesicles 2020, 9, 1785161. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Leite, D.; Gitaí, G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Kumar, S.; Shuai, B.; et al. Extracellular Vesicles from Human IPSC-Derived Neural Stem Cells: MiRNA and Protein Signatures, and Anti-Inflammatory and Neurogenic Properties. J. Extracell. Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological Roles and Potential Applications of Immune Cell-Derived Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conzelmann, C.; Groß, R.; Zou, M.; Krüger, F.; Görgens, A.; Gustafsson, M.O.; El Andaloussi, S.; Münch, J.; Müller, J.A. Salivary Extracellular Vesicles Inhibit Zika Virus but Not SARS-CoV-2 Infection. J. Extracell. Vesicles 2020, 9, 1808281. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.-W.; Uchiyama, K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and Their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef]
- Takakura, Y.; Matsumoto, A.; Takahashi, Y. Therapeutic Application of Small Extracellular Vesicles (SEVs): Pharmaceutical and Pharmacokinetic Challenges. Biol. Pharm. Bull. 2020, 43, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Goyal, G.; Phukan, A.C.; Hussain, M.; Lal, V.; Modi, M.; Goyal, M.K.; Sehgal, R. Sorting out Difficulties in Immunological Diagnosis of Neurocysticercosis: Development and Assessment of Real Time Loop Mediated Isothermal Amplification of Cysticercal DNA in Blood. J. Neurol. Sci. 2020, 408, 116544. [Google Scholar] [CrossRef]
- Oskouie, M.N.; Aghili Moghaddam, N.S.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic Use of Curcumin-Encapsulated and Curcumin-Primed Exosomes. J. Cell. Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef]
- Bei, Y.; Xu, T.; Lv, D.; Yu, P.; Xu, J.; Che, L.; Das, A.; Tigges, J.; Toxavidis, V.; Ghiran, I.; et al. Exercise-Induced Circulating Extracellular Vesicles Protect against Cardiac Ischemia-Reperfusion Injury. Basic Res. Cardiol. 2017, 112, 38. [Google Scholar] [CrossRef] [PubMed]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A Cost-Effective Polyethylene Glycol-Based Method for the Isolation of Functional Edible Nanoparticles from Ginger Rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwland, R.; Falcon-perez, J.M.; Soekmadji, C.; Carter, D.; Buzas, E.I.; Nieuwland, R.; Falcon-perez, J.M.; Soekmadji, C. Essentials of Extracellular Vesicles: Posters on Basic and Clinical Aspects of Extracellular Vesicles. J. Extracell. Vesicles 2018, 8, 1548234. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest Advances in Extracellular Vesicles: From Bench to Bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes Carrying Doxorubicin Exhibit Potent Anticancer Activity against Human Lung Cancer Cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020, 8, 586130. [Google Scholar] [CrossRef]
- Kwon, S.; Shin, S.; Do, M.; Oh, B.H.; Song, Y.; Bui, V.D.; Lee, E.S.; Jo, D.-G.; Cho, Y.W.; Kim, D.-H.; et al. Engineering Approaches for Effective Therapeutic Applications Based on Extracellular Vesicles. J. Control. Release 2021, 330, 15–30. [Google Scholar] [CrossRef]
- Ogawa, Y.; Akimoto, Y.; Ikemoto, M.; Goto, Y.; Ishikawa, A.; Ohta, S.; Takase, Y.; Kawakami, H.; Tsujimoto, M.; Yanoshita, R. Stability of Human Salivary Extracellular Vesicles Containing Dipeptidyl Peptidase IV under Simulated Gastrointestinal Tract Conditions. Biochem. Biophys. Rep. 2021, 27, 101034. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental PH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef] [Green Version]
- Underdown, B.J.; Dorrington, K.J. Studies on the Structural and Conformational Basis for the Relative Resistance of Serum and Secretory Immunoglobulin a to Proteolysis. J. Immunol. 1974, 112, 949–959. [Google Scholar] [PubMed]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Kondratov, K.; Nikitin, Y.; Fedorov, A.; Kostareva, A.; Mikhailovskii, V.; Isakov, D.; Ivanov, A.; Golovkin, A. Heterogeneity of the Nucleic Acid Repertoire of Plasma Extracellular Vesicles Demonstrated Using High-Sensitivity Fluorescence-Activated Sorting. J. Extracell. Vesicles 2020, 9, 1743139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, e8545347. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Maxouri, O.; Kardar, A.; Schelfhorst, T.; Piersma, S.R.; Pham, T.V.; Vis, A.; van Moorselaar, R.J.; Jimenez, C.R. Feasibility of Urinary Extracellular Vesicle Proteome Profiling Using a Robust and Simple, Clinically Applicable Isolation Method. J. Extracell. Vesicles 2017, 6, 1313091. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-Derived Extracellular Vesicles from Superoxide Dismutase 1 (SOD1) G93A ALS Mice Originate from Astrocytes and Neurons and Carry Misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef] [Green Version]
- Woith, E.; Melzig, M. Extracellular Vesicles from Fresh and Dried Plants—Simultaneous Purification and Visualization Using Gel Electrophoresis. Int. J. Mol. Sci. 2019, 20, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab, T.; Raffo-Romero, A.; Van Camp, C.; Lemaire, Q.; Le Marrec-Croq, F.; Drago, F.; Aboulouard, S.; Slomianny, C.; Lacoste, A.-S.; Guigon, I.; et al. Proteomic Characterisation of Leech Microglia Extracellular Vesicles (EVs): Comparison between Differential Ultracentrifugation and OptiprepTM Density Gradient Isolation. J. Extracell. Vesicles 2019, 8, 1603048. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Kırbaş, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B. Optimized Isolation of Extracellular Vesicles from Various Organic Sources Using Aqueous Two-Phase System. Sci. Rep. 2019, 9, 19159. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Shehata Draz, M.; Zarghooni, M.; Sanati-Nezhad, A.; Ghavami, S.; Shafiee, H.; Akbari, M. Microfluidic Approaches for Isolation, Detection, and Characterization of Extracellular Vesicles: Current Status and Future Directions. Biosens. Bioelectron. 2017, 91, 588–605. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Choi, J.; Wang, J.; Concepcion, W.; Thakor, A.S. A Novel Approach to Deliver Therapeutic Extracellular Vesicles Directly into the Mouse Kidney via Its Arterial Blood Supply. Cells 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhadj, Z.; He, B.; Deng, H.; Song, S.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A Combined “Eat Me/Don’t Eat Me” Strategy Based on Extracellular Vesicles for Anticancer Nanomedicine. J. Extracell. Vesicles 2020, 9, 1806444. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J Extracell. Vesicle 2022, 11, e12162. [Google Scholar] [CrossRef]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.-L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef]
- Palviainen, M.; Saraswat, M.; Varga, Z.; Kitka, D.; Neuvonen, M.; Puhka, M.; Joenväärä, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular Vesicles from Human Plasma and Serum Are Carriers of Extravesicular Cargo—Implications for Biomarker Discovery. PLoS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef]
- Kornilov, R.; Puhka, M.; Mannerström, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppänen-Kaijansinkko, R.; Kaur, S. Efficient Ultrafiltration-Based Protocol to Deplete Extracellular Vesicles from Fetal Bovine Serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.; van der Pol, E.; Pálmai, M.; Garcia-Diez, R.; Gollwitzer, C.; Krumrey, M.; Fraikin, J.-L.; Gasecka, A.; Hajji, N.; van Leeuwen, T.G.; et al. Hollow Organosilica Beads as Reference Particles for Optical Detection of Extracellular Vesicles. J Thromb Haemost 2018, 16, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.S.; Garcia-Ceron, D.; Rajapaksha, H.; Faou, P.; Bleackley, M.R.; Anderson, M.A. Protein Markers for Candida Albicans EVs Include Claudin-like Sur7 Family Proteins. J. Extracell. Vesicles 2020, 9, 1750810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakhage, A.A.; Zimmermann, A.-K.; Rivieccio, F.; Visser, C.; Blango, M.G. Host-Derived Extracellular Vesicles for Antimicrobial Defense. microLife 2021, 2, uqab003. [Google Scholar] [CrossRef]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L.; et al. Comprehensive Toxicity and Immunogenicity Studies Reveal Minimal Effects in Mice Following Sustained Dosing of Extracellular Vesicles Derived from HEK293T Cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Johnson, J.; Wu, Y.-W.; Blyth, C.; Lichtfuss, G.; Goubran, H.; Burnouf, T. Prospective Therapeutic Applications of Platelet Extracellular Vesicles. Trends Biotechnol. 2021, 39, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, B. Extracellular Vesicles-Based Drug Delivery System for Cancer Treatment. Cogent Med. 2019, 6, 1635806. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.; Gudapati, V.; Lim, H. Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Acute Lung Injury and Other Inflammatory Lung Diseases. Expert Opin. Biol. Ther. 2017, 16, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Biodistribution, Safety and Toxicity Profile of Engineered Extracellular Vesicles—ProQuest. Available online: https://www.proquest.com/openview/56e05c2ceacc312939f67724684f1704/1?pq-origsite=gscholar&cbl=2030046 (accessed on 20 January 2022).
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. STEM CELLS Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chopp, M. Exosome Therapy for Stroke. Stroke 2018, 49, 1083–1090. [Google Scholar] [CrossRef]
- Sterzenbach, U.; Putz, U.; Low, L.H.; Silke, J.; Tan, S.S.; Howitt, J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol. Ther. 2017, 25, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Rutering, J.; Ilmer, M.; Recio, A.; Coleman, M.; Vykoukal, J.; Alt, E.; Orleans, N. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.; Amreddy, N.; Pareek, V.; Chinnappan, M.; Ahmed, R.; Mehta, M.; Razaq, M.; Munshi, A.; Ramesh, R. Progress in Extracellular Vesicle Biology and Their Application in Cancer Medicine. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerreiro, E.M.; Øvstebø, R.; Thiede, B.; Costea, D.E.; Søland, T.M.; Galtung, H.K. Cancer Cell Line-Specific Protein Profiles in Extracellular Vesicles Identified by Proteomics. PLoS ONE 2020, 15, e0238591. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human Milk Extracellular Vesicle MiRNA Expression and Associations with Maternal Characteristics in a Population-Based Cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Roy, S.; Fleming, T.; Leong, H.S.; Schuurmans, C. The Emerging Role of Extracellular Vesicles in the Glioma Microenvironment: Biogenesis and Clinical Relevance. Cancers 2020, 12, 1964. [Google Scholar] [CrossRef]
- Zhi, K.; Kumar, A.; Raji, B.; Kochat, H.; Kumar, S. Formulation, Manufacturing and Regulatory Strategies for Extracellular Vesicles-Based Drug Products for Targeted Therapy of Central Nervous Systerm Diseases. Expert Rev. Precis. Med. Drug Dev. 2020, 12, 469–481. [Google Scholar] [CrossRef]
- LeClaire, M.; Gimzewski, J.; Sharma, S. A Review of the Biomechanical Properties of Single Extracellular Vesicles. Nano Sel. 2021, 2, 1–15. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Monaco, A.; Licitra, F.; Perillo, B.; Migliaccio, A.; Castoria, G. Communication between Cells: Exosomes as a Delivery System in Prostate Cancer. Cell Commun. Signal. 2021, 19, 110. [Google Scholar] [CrossRef]
- An, T.; Qin, S.; Xu, Y.; Tang, Y.; Huang, Y.; Situ, B.; Inal, J.M.; Zheng, L. Exosomes Serve as Tumour Markers for Personalized Diagnostics Owing to Their Important Role in Cancer Metastasis. J. Extracell. Vesicles 2015, 4, 27522. [Google Scholar] [CrossRef] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Kameli, N.; Dragojlovic-Kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus Cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.A.; Gazeau, F. Engineering and Loading Therapeutic Extracellular Vesicles for Clinical Translation: A Data Reporting Frame for Comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.K.; Van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; De Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and Opportunities in the Functional Analysis of Extracellular Vesicle RNA—An ISEV Position Paper. J. Extracell. Vesicles 2001, 6, 1286095. [Google Scholar] [CrossRef] [Green Version]
- Freitas, D.; Balmaña, M.; Poças, J.; Campos, D.; Osório, H.; Konstantinidi, A.; Vakhrushev, S.Y.; Magalhães, A.; Reis, C.A. Different Isolation Approaches Lead to Diverse Glycosylated Extracellular Vesicle Populations. J. Extracell. Vesicles 2019, 8, 1621131. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and Efficiency Assessment of Six Extracellular Vesicle Isolation Methods by Nano-Flow Cytometry. J. Extracell. Vesicles 2020, 9, 1697028. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).