Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems
Abstract
:1. Introduction
2. Adsorption of Molecules on Pristine or Nonmetal Functionalized Systems
2.1. Nanotubes
2.2. Graphene
2.3. Fullerenes
2.4. Graphdiyne
2.5. Hybrid Systems
3. Adsorption on Systems Doped with Transition Metals
3.1. Transition Metals
3.2. Nanotubes Doped with Transition Metals
3.3. Graphene Doped with Transition Metals
3.4. Fullerenes Doped with Transition Metals
3.5. Graphdiyne Doped with Transition Metals
3.6. Hybrid Systems Doped with Transition Metals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Pikhitsa, P.V.; Jiang, H.; Brown, D.P.; Krasheninnikov, A.V.; Anisimov, A.S.; Queipo, P.; Moisala, A.; Gonzalez, D.; Lientschnig, G.; et al. A Novel Hybrid Carbon Material. Nat. Nanotechnol. 2007, 2, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Moisala, A.; Nasibulin, A.G.; Shandakov, S.D.; Jiang, H.; Kauppinen, E.I. On-Line Detection of Single-Walled Carbon Nanotube Formation during Aerosol Synthesis Methods. Carbon 2005, 43, 2066–2074. [Google Scholar] [CrossRef]
- Delgado, J.L.; Herranz, M.; Martín, N. The Nano-Forms of Carbon. J. Mater. Chem. 2008, 18, 1417. [Google Scholar] [CrossRef]
- Falcao, E.H.; Wudl, F. Carbon Allotropes: Beyond Graphite and Diamond. J. Chem. Technol. Biotechnol. 2007, 82, 524–531. [Google Scholar] [CrossRef]
- Langenhorst, F.; Campione, M. Ideal and Real Structures of Different Forms of Carbon, with Some Remarks on Their Geological Significance. J. Geol. Soc. 2019, 176, 337–347. [Google Scholar] [CrossRef]
- Pacheco, M.; Pacheco, J.; Valdivia, R.; Santana, A.; Tu, X.; Mendoza, D.; Frias, H.; Medina, L.; Macias, J. Green Applications of Carbon Nanostructures Produced by Plasma Techniques. MRS Adv. 2017, 2, 2647–2659. [Google Scholar] [CrossRef] [Green Version]
- Lejaeghere, K.; Van Speybroeck, V.; Van Oost, G.; Cottenier, S. Error Estimates for Solid-State Density-Functional Theory Predictions: An Overview by Means of the Ground-State Elemental Crystals. Crit. Rev. Solid State Mater. Sci. 2014, 39, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas Sensing Mechanism of Carbon Nanotubes: From Single Tubes to High-Density Networks. Carbon 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Lu, J.P. Gas Molecule Adsorption in Carbon Nanotubes and Nanotube Bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Liu, Y.; Zheng, Q. Adsorption Equilibrium of Hydrogen Adsorption on Activated Carbon, Multi-Walled Carbon Nanotubes and Graphene Sheets. Cryogenics 2019, 101, 36–42. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen Adsorption in Different Carbon Nanostructures. Carbon 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Dhall, S.; Jaggi, N.; Nathawat, R. Functionalized Multiwalled Carbon Nanotubes Based Hydrogen Gas Sensor. Sens. Actuators A Phys. 2013, 201, 321–327. [Google Scholar] [CrossRef]
- Elyassi, M.; Rashidi, A.; Hantehzadeh, M.R.; Elahi, S.M. Hydrogen Storage Behaviors by Adsorption on Multi-Walled Carbon Nanotubes. J. Inorg. Organomet. Polym. 2017, 27, 285–295. [Google Scholar] [CrossRef]
- Lithoxoos, G.P.; Labropoulos, A.; Peristeras, L.D.; Kanellopoulos, N.; Samios, J.; Economou, I.G. Adsorption of N2, CH4, CO and CO2 Gases in Single Walled Carbon Nanotubes: A Combined Experimental and Monte Carlo Molecular Simulation Study. J. Supercrit. Fluids 2010, 55, 510–523. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from Flue Gas via Multiwalled Carbon Nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Lu, C.; Su, F.; Zeng, W.; Chen, W. Thermodynamics and Regeneration Studies of CO2 Adsorption on Multiwalled Carbon Nanotubes. Chem. Eng. Sci. 2010, 65, 1354–1361. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Chung, A.-J.; Liao, C.-H. CO2 Capture with Amine-Loaded Carbon Nanotubes via a Dual-Column Temperature/Vacuum Swing Adsorption. Appl. Energy 2014, 113, 706–712. [Google Scholar] [CrossRef]
- Inoue, S.; Tomita, Y.; Kokabu, T.; Matsumura, Y. Principles of Detection Mechanism for Adsorbed Gases Using Carbon Nanotube Nanomat. Chem. Phys. Lett. 2018, 709, 77–81. [Google Scholar] [CrossRef]
- Fatemi, S.; Vesali-Naseh, M.; Cyrus, M.; Hashemi, J. Improving CO2/CH4 Adsorptive Selectivity of Carbon Nanotubes by Functionalization with Nitrogen-Containing Groups. Chem. Eng. Res. Des. 2011, 89, 1669–1675. [Google Scholar] [CrossRef]
- Lin, C.-C.; Gupta, S.; Chang, C.; Lee, C.-Y.; Tai, N.-H. Polyethylenimine-Polyethylene Glycol/Multi-Walled Carbon Nanotubes Bilayer Structure for Carbon Dioxide Gas Sensing at Room Temperature. Mater. Lett. 2021, 297, 129941. [Google Scholar] [CrossRef]
- Rahimi, K.; Riahi, S.; Abbasi, M.; Fakhroueian, Z. Modification of Multi-Walled Carbon Nanotubes by 1,3-Diaminopropane to Increase CO2 Adsorption Capacity. J. Environ. Manag. 2019, 242, 81–89. [Google Scholar] [CrossRef]
- Faginas-Lago, N.; Apriliyanto, Y.B.; Lombardi, A. Confinement of CO2 inside Carbon Nanotubes. Eur. Phys. J. D 2021, 75, 161. [Google Scholar] [CrossRef]
- Mukhtar, A.; Mellon, N.; Saqib, S.; Khawar, A.; Rafiq, S.; Ullah, S.; Al-Sehemi, A.G.; Babar, M.; Bustam, M.A.; Khan, W.A.; et al. CO2/CH4 Adsorption over Functionalized Multi-Walled Carbon Nanotubes; an Experimental Study, Isotherms Analysis, Mechanism, and Thermodynamics. Microporous Mesoporous Mater. 2020, 294, 109883. [Google Scholar] [CrossRef]
- Delavar, M.; Asghar Ghoreyshi, A.; Jahanshahi, M.; Khalili, S.; Nabian, N. Equilibria and Kinetics of Natural Gas Adsorption on Multi-Walled Carbon Nanotube Material. RSC Adv. 2012, 2, 4490. [Google Scholar] [CrossRef]
- Babu, D.J.; Lange, M.; Cherkashinin, G.; Issanin, A.; Staudt, R.; Schneider, J.J. Gas Adsorption Studies of CO2 and N2 in Spatially Aligned Double-Walled Carbon Nanotube Arrays. Carbon 2013, 61, 616–623. [Google Scholar] [CrossRef]
- Liu, L.; Nicholson, D.; Bhatia, S.K. Impact of H2O on CO2 Separation from Natural Gas: Comparison of Carbon Nanotubes and Disordered Carbon. J. Phys. Chem. C 2015, 119, 407–419. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Wu, P.-Y. Adsorption Equilibrium of Sulfur Hexafluoride on Multi-Walled Carbon Nanotubes. J. Hazard. Mater. 2010, 178, 729–738. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 81st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-8493-0481-1. [Google Scholar]
- Kazachkin, D.V.; Nishimura, Y.; Irle, S.; Feng, X.; Vidic, R.; Borguet, E. Temperature and Pressure Dependence of Molecular Adsorption on Single Wall Carbon Nanotubes and the Existence of an “Adsorption/Desorption Pressure Gap”. Carbon 2010, 48, 1867–1875. [Google Scholar] [CrossRef]
- Young, S.-J.; Lin, Z.-D. Ethanol Gas Sensors Composed of Carbon Nanotubes with Au Nanoparticles Adsorbed onto a Flexible PI Substrate. ECS J. Solid State Sci. Technol. 2017, 6, M130–M132. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly Sensitive, Room Temperature Gas Sensor Based on Polyaniline-Multiwalled Carbon Nanotubes (PANI/MWCNTs) Nanocomposite for Trace-Level Ammonia Detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Battie, Y.; Ducloux, O.; Thobois, P.; Dorval, N.; Lauret, J.S.; Attal-Trétout, B.; Loiseau, A. Gas Sensors Based on Thick Films of Semi-Conducting Single Walled Carbon Nanotubes. Carbon 2011, 49, 3544–3552. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced Gas Sensing in Pristine Carbon Nanotubes under Continuous Ultraviolet Light Illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef]
- Huyen, D.N.; Tung, N.T.; Vinh, T.D.; Thien, N.D. Synergistic Effects in the Gas Sensitivity of Polypyrrole/Single Wall Carbon Nanotube Composites. Sensors 2012, 12, 7965–7974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, A.; Yates, J.T.; Simonyan, V.V.; Johnson, J.K.; Huffman, C.B.; Smalley, R.E. Optimization of Xe Adsorption Kinetics in Single Walled Carbon Nanotubes. J. Chem. Phys. 2001, 115, 6691–6698. [Google Scholar] [CrossRef] [Green Version]
- Beheshtian, J.; Peyghan, A.A.; Bagheri, Z. Nitrate Adsorption by Carbon Nanotubes in the Vacuum and Aqueous Phase. Mon. Chem. 2012, 143, 1623–1626. [Google Scholar] [CrossRef]
- Beheshtian, J.; Peyghan, A.A.; Bagheri, Z. Formaldehyde Adsorption on the Interior and Exterior Surfaces of CN Nanotubes. Struct. Chem. 2013, 24, 1331–1337. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, H.; Wang, Q.; Chen, S. Adsorption and Diffusion of Sulfur Dioxide and Nitrogen in Single-Wall Carbon Nanotubes. J. Mol. Graph. Model. 2019, 88, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, S.C.; Shih, Y. Characterization of Volatile Organic Compound Adsorption on Multiwall Carbon Nanotubes under Different Levels of Relative Humidity Using Linear Solvation Energy Relationship. J. Hazard. Mater. 2016, 315, 35–41. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904. [Google Scholar] [CrossRef]
- Casolo, S.; Løvvik, O.M.; Martinazzo, R.; Tantardini, G.F. Understanding Adsorption of Hydrogen Atoms on Graphene. J. Chem. Phys. 2009, 130, 054704. [Google Scholar] [CrossRef]
- Feijó, T.O.; Rolim, G.K.; Corrêa, S.A.; Radtke, C.; Soares, G.V. Thermally Driven Hydrogen Interaction with Single-Layer Graphene on SiO2/Si Substrates Quantified by Isotopic Labeling. J. Appl. Phys. 2020, 128, 225702. [Google Scholar] [CrossRef]
- Sunnardianto, G.K.; Bokas, G.; Hussein, A.; Walters, C.; Moultos, O.A.; Dey, P. Efficient Hydrogen Storage in Defective Graphene and Its Mechanical Stability: A Combined Density Functional Theory and Molecular Dynamics Simulation Study. Int. J. Hydrog. Energy 2021, 46, 5485–5494. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on Graphene: A First-Principles Study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef] [Green Version]
- Bo, Z.; Guo, X.; Wei, X.; Yang, H.; Yan, J.; Cen, K. Density Functional Theory Calculations of NO2 and H2S Adsorption on the Group 10 Transition Metal (Ni, Pd and Pt) Decorated Graphene. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 109, 156–163. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z. DFT Study of NO Adsorption on Pristine Graphene. RSC Adv. 2017, 7, 13082–13091. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhou, Q.; Wang, J.; Xu, L.; Zeng, W. Performance of Intrinsic and Modified Graphene for the Adsorption of H2S and CH4: A DFT Study. Nanomaterials 2020, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Panes-Ruiz, L.A.; Croy, A.; Löffler, M.; Khavrus, V.; Bezugly, V.; Cuniberti, G. Highly Sensitive Room Temperature Ammonia Gas Sensor Using Pristine Graphene: The Role of Biocompatible Stabilizer. Carbon 2021, 173, 262–270. [Google Scholar] [CrossRef]
- Lazar, P.; Karlický, F.; Jurečka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of Small Organic Molecules on Graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Salih, E.; Ayesh, A.I. Co-Doped Zigzag Graphene Nanoribbon Based Gas Sensor for Sensitive Detection of H2S: DFT Study. Superlattices Microstruct. 2021, 155, 106900. [Google Scholar] [CrossRef]
- Karami, Z.; Hamed Mashhadzadeh, A.; Habibzadeh, S.; Ganjali, M.R.; Ghardi, E.M.; Hasnaoui, A.; Vatanpour, V.; Sharma, G.; Esmaeili, A.; Stadler, F.J.; et al. Atomic Simulation of Adsorption of SO2 Pollutant by Metal (Zn, Be)-Oxide and Ni-Decorated Graphene: A First-Principles Study. J. Mol. Model. 2021, 27, 70. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cao, S.; Wu, X.; Yu, B.-Y.; Chen, L.-Y.; Zhang, J.-M. Density Functional Theory Study of Hydrogen Sulfide Adsorption onto Transition Metal-Doped Bilayer Graphene Using External Electric Fields. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114252. [Google Scholar] [CrossRef]
- Gui, Y.; Peng, X.; Liu, K.; Ding, Z. Adsorption of C2H2, CH4 and CO on Mn-Doped Graphene: Atomic, Electronic, and Gas-Sensing Properties. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113959. [Google Scholar] [CrossRef]
- Shukri, M.S.M.; Saimin, M.N.S.; Yaakob, M.K.; Yahya, M.Z.A.; Taib, M.F.M. Structural and Electronic Properties of CO and NO Gas Molecules on Pd-Doped Vacancy Graphene: A First Principles Study. Appl. Surf. Sci. 2019, 494, 817–828. [Google Scholar] [CrossRef]
- Jia, X.; An, L. The Adsorption of Nitrogen Oxides on Noble Metal-Doped Graphene: The First-Principles Study. Mod. Phys. Lett. B 2019, 33, 1950044. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Zhou, L.; Ning, P.; Tang, L. Density Functional Theory Analysis of Selective Adsorption of AsH3 on Transition Metal-Doped Graphene. J. Mol. Model. 2019, 25, 145. [Google Scholar] [CrossRef]
- Khodadadi, Z. Evaluation of H2S Sensing Characteristics of Metals–Doped Graphene and Metals-Decorated Graphene: Insights from DFT Study. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 261–268. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Wang, F.; Zhang, W.; Tang, S.; Ma, J.; Gong, H.; Zhang, J. Adsorption of Phosgene Molecule on the Transition Metal-Doped Graphene: First Principles Calculations. Appl. Surf. Sci. 2017, 425, 340–350. [Google Scholar] [CrossRef]
- Promthong, N.; Tabtimsai, C.; Rakrai, W.; Wanno, B. Transition Metal-Doped Graphene Nanoflakes for CO and CO2 Storage and Sensing Applications: A DFT Study. Struct. Chem. 2020, 31, 2237–2247. [Google Scholar] [CrossRef]
- Chuvilin, A.; Kaiser, U.; Bichoutskaia, E.; Besley, N.A.; Khlobystov, A.N. Direct Transformation of Graphene to Fullerene. Nat. Chem. 2010, 2, 450–453. [Google Scholar] [CrossRef]
- Manna, A.K.; Pati, S.K. Stability and Electronic Structure of Carbon Capsules with Superior Gas Storage Properties: A Theoretical Study. Chem. Phys. 2013, 426, 23–30. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, I.; Ahmad, R. Influence of Electric Field on CO2 Removal by P-Doped C60-Fullerene: A DFT Study. Chem. Phys. Lett. 2020, 742, 137155. [Google Scholar] [CrossRef]
- Tascón, J.M.D.; Bottani, E.J. Ethylene Physisorption on C60 Fullerene. Carbon 2004, 42, 1333–1337. [Google Scholar] [CrossRef]
- Furuuchi, N.; Shrestha, R.; Yamashita, Y.; Hirao, T.; Ariga, K.; Shrestha, L. Self-Assembled Fullerene Crystals as Excellent Aromatic Vapor Sensors. Sensors 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Ahmad, R.; Ahmad, I. Removal of Nitrous and Carbon Mono Oxide from Flue Gases by Si-Coordinated Nitrogen Doped C60-Fullerene: A DFT Approach. Mol. Catal. 2021, 509, 111674. [Google Scholar] [CrossRef]
- Haghgoo, S.; Nekoei, A.-R. Metal Oxide Adsorption on Fullerene C60 and Its Potential for Adsorption of Pollutant Gases; Density Functional Theory Studies. RSC Adv. 2021, 11, 17377–17390. [Google Scholar] [CrossRef]
- Bubenchikov, M.A.; Bubenchikov, A.M.; Usenko, O.V.; Tsyrenova, V.B.; Budaev, S.O. Ability of Fullerene to Accumulate Hydrogen. EPJ Web Conf. 2016, 110, 01077. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Deng, S. Hydrogen Adsorption on Partially Truncated and Open Cage C60 Fullerene. Carbon 2010, 48, 3471–3476. [Google Scholar] [CrossRef]
- Kaiser, A.; Leidlmair, C.; Bartl, P.; Zöttl, S.; Denifl, S.; Mauracher, A.; Probst, M.; Scheier, P.; Echt, O. Adsorption of Hydrogen on Neutral and Charged Fullerene: Experiment and Theory. J. Chem. Phys. 2013, 138, 074311. [Google Scholar] [CrossRef] [Green Version]
- Kalateh, K.; Cordshooli, G.A.; Kheirollahpoor, S. Hydrogen Adsorption,Structural, Electronic, and Spectroscopic Properties of C32, B16N16, and B8C24 by DFT Calculations. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 459–465. [Google Scholar] [CrossRef]
- Barajas-Barraza, R.E.; Guirado-López, R.A. Endohedral Nitrogen Storage in Carbon Fullerene Structures: Physisorption to Chemisorption Transition with Increasing Gas Pressure. J. Chem. Phys. 2009, 130, 234706. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Janebi, H. B-, N-Doped and BN Codoped C60 Heterofullerenes for Environmental Monitoring of NO and NO2: A DFT Study. Mol. Phys. 2020, 118, e1631495. [Google Scholar] [CrossRef]
- Acosta-Gutiérrez, S.; Bretón, J.; Llorente, J.M.G.; Hernández-Rojas, J. Optimal Covering of C60 Fullerene by Rare Gases. J. Chem. Phys. 2012, 137, 074306. [Google Scholar] [CrossRef]
- Siadati, S.A.; Vessally, E.; Hosseinian, A.; Edjlali, L. Possibility of Sensing, Adsorbing, and Destructing the Tabun-2D-Skeletal (Tabun Nerve Agent) by C20 Fullerene and Its Boron and Nitrogen Doped Derivatives. Synth. Met. 2016, 220, 606–611. [Google Scholar] [CrossRef]
- Najafi, M. Density Functional Study of Cyanogen (C2N2) Sensing Using OH Functionalized Fullerene (C60) and Germanium-Fullerene (Ge60). Vacuum 2016, 134, 88–91. [Google Scholar] [CrossRef]
- Bashiri, S.; Vessally, E.; Bekhradnia, A.; Hosseinian, A.; Edjlali, L. Utility of Extrinsic [60] Fullerenes as Work Function Type Sensors for Amphetamine Drug Detection: DFT Studies. Vacuum 2017, 136, 156–162. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-Pot Green Synthesis of Magnetic Fullerene Nanocomposite for Adsorption Characteristics. J. Water Process Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- El Mahdy, A.M. Density Functional Investigation of CO and NO Adsorption on TM-Decorated C60 Fullerene. Appl. Surf. Sci. 2016, 383, 353–366. [Google Scholar] [CrossRef]
- Khan, A.A.; Ahmad, R.; Ahmad, I.; Su, X. Selective Adsorption of CO2 from Gas Mixture by P-Decorated C24N24 Fullerene Assisted by an Electric Field: A DFT Approach. J. Mol. Graph. Model. 2021, 103, 107806. [Google Scholar] [CrossRef]
- Wu, P.; Du, P.; Zhang, H.; Cai, C. Graphdiyne as a Metal-Free Catalyst for Low-Temperature CO Oxidation. Phys. Chem. Chem. Phys. 2014, 16, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Tang, Y.; Chen, W.; Li, Y.; Li, R.; Ma, Y.; Dai, X. Graphdiyne Coordinated Transition Metals as Single-Atom Catalysts for Nitrogen Fixation. Phys. Chem. Chem. Phys. 2020, 22, 9216–9224. [Google Scholar] [CrossRef]
- Ebadi, M.; Reisi-Vanani, A. Methanol and Carbon Monoxide Sensing and Capturing by Pristine and Ca-Decorated Graphdiyne: A DFT-D2 Study. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 125, 114425. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Guo, M.; Yun, J. Adsorption of Hydrogen and Oxygen on Graphdiyne and Its BN Analog Sheets: A Density Functional Theory Study. Comput. Mater. Sci. 2019, 160, 197–206. [Google Scholar] [CrossRef]
- Chen, X.; Gao, P.; Guo, L.; Zhang, S. Graphdiyne as a Promising Material for Detecting Amino Acids. Sci. Rep. 2015, 5, 16720. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, V.; Srimathi, U.; Chandiramouli, R. First-Principles Insights on Detection of Dimethyl Amine and Trimethyl Amine Vapors Using Graphdiyne Nanosheets. Comput. Theor. Chem. 2018, 1123, 119–127. [Google Scholar] [CrossRef]
- Srimathi, U.; Nagarajan, V.; Chandiramouli, R. Investigation on Graphdiyne Nanosheet in Adsorption of Sorafenib and Regorafenib Drugs: A DFT Approach. J. Mol. Liq. 2019, 277, 776–785. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. Investigation of NH3 Adsorption Behavior on Graphdiyne Nanosheet and Nanotubes: A First-Principles Study. J. Mol. Liq. 2018, 249, 24–32. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Princy Maria, J.; Nagarajan, V.; Chandiramouli, R. Graphdiyne Nanosheets as a Sensing Medium for Formaldehyde and Formic Acid—A First-Principles Outlook. Comput. Theor. Chem. 2020, 1176, 112751. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively Nitrogen-Doped Carbon Materials as Superior Metal-Free Catalysts for Oxygen Reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Chen, Y.; Liu, X.; Xu, W.; Zhao, Y.; Zhang, M.; Zhang, C. A First-Principles Study of Gas Molecule Adsorption on Hydrogen-Substituted Graphdiyne. Phys. Lett. A 2020, 384, 126332. [Google Scholar] [CrossRef]
- Xu, P.; Na, N.; Mohamadi, A. Investigation the Application of Pristine Graphdiyne (GDY) and Boron-Doped Graphdiyne (BGDY) as an Electronic Sensor for Detection of Anticancer Drug. Comput. Theor. Chem. 2020, 1190, 112996. [Google Scholar] [CrossRef]
- Yuan, J.; Mohamadi, A. Study the Adsorption Process of 5-Fluorouracil Drug on the Pristine and Doped Graphdiyne Nanosheet. J. Mol. Model. 2021, 27, 32. [Google Scholar] [CrossRef]
- Feng, Z.; Ma, Y.; Li, Y.; Li, R.; Tang, Y.; Dai, X. Oxygen Molecule Dissociation on Heteroatom Doped Graphdiyne. Appl. Surf. Sci. 2019, 494, 421–429. [Google Scholar] [CrossRef]
- Rangel, E.; Ramirez-de-Arellano, J.M.; Magana, L.F. Variation of Hydrogen Adsorption with Increasing Li Doping on Carbon Nanotubes: Variation of Hydrogen Adsorption with Increasing Li Doping on CNTs. Phys. Status Solidi B 2011, 248, 1420–1424. [Google Scholar] [CrossRef]
- Rangel, E.; Ramírez-Arellano, J.M.; Carrillo, I.; Magana, L.F. Hydrogen Adsorption around Lithium Atoms Anchored on Graphene Vacancies. Int. J. Hydrog. Energy 2011, 36, 13657–13662. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Lim, J.; Kim, W.Y. Study of Li Adsorption on Graphdiyne Using Hybrid DFT Calculations. ACS Appl. Mater. Interfaces 2019, 11, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Su, G.; Ding, H.; Ma, Y.; Li, Y.; Tang, Y.; Dai, X. Atomic Alkali Metal Anchoring on Graphdiyne as Single-Atom Catalysts for Capture and Conversion of CO2 to HCOOH. Mol. Catal. 2020, 494, 111142. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Wang, G.; Bao, X. Electrochemical CO2 Reduction on Graphdiyne: A DFT Study. Green Chem. 2021, 23, 1212–1219. [Google Scholar] [CrossRef]
- Feng, Z.; Tang, Y.; Ma, Y.; Li, Y.; Dai, Y.; Ding, H.; Su, G.; Dai, X. Theoretical Investigation of CO2 Electroreduction on N (B)-Doped Graphdiyne Mononlayer Supported Single Copper Atom. Appl. Surf. Sci. 2021, 538, 148145. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Weng, K.; Jiang, J.; Ong, W.; Zhang, P.; Zhao, X.; Li, N. Highly Sensitive and Selective Gas Sensor Using Heteroatom Doping Graphdiyne: A DFT Study. Adv. Electron. Mater. 2021, 7, 2001244. [Google Scholar] [CrossRef]
- Cao, J.; Li, N.; Zeng, X. Exploring the Synergistic Effect of B–N Doped Defective Graphdiyne for N2 Fixation. New J. Chem. 2021, 45, 6327–6335. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Z.-Z. Single-Layer Graphdiyne-Covered Pt(111) Surface: Improved Catalysis Confined under Two-Dimensional Overlayer. J. Nanopart. Res. 2018, 20, 136. [Google Scholar] [CrossRef]
- Khan, S.; Yar, M.; Kosar, N.; Ayub, K.; Arshad, M.; Zahid, M.N.; Mahmood, T. First-Principles Study for Exploring the Adsorption Behavior of G-Series Nerve Agents on Graphdyine Surface. Comput. Theor. Chem. 2020, 1191, 113043. [Google Scholar] [CrossRef]
- Sajid, H.; Khan, S.; Ayub, K.; Mahmood, T. Effective Adsorption of A-Series Chemical Warfare Agents on Graphdiyne Nanoflake: A DFT Study. J. Mol. Model. 2021, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sajid, H.; Ayub, K.; Mahmood, T. Sensing of Toxic Lewisite (L1, L2, and L3) Molecules by Graphdiyne Nanoflake Using Density Functional Theory Calculations and Quantum Theory of Atoms in Molecule Analysis. J. Phys. Org. Chem. 2021, 34, e4181. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Vahedi, A.M.; Ardjmand, M.; Ahangari, M.G. Investigation of Heavy Metal Atoms Adsorption onto Graphene and Graphdiyne Surface: A Density Functional Theory Study. Superlattices Microstruct. 2016, 100, 1094–1102. [Google Scholar] [CrossRef]
- Seif, A.; López, M.J.; Granja-DelRío, A.; Azizi, K.; Alonso, J.A. Adsorption and Growth of Palladium Clusters on Graphdiyne. Phys. Chem. Chem. Phys. 2017, 19, 19094–19102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ma, C.; Chen, K.; Li, H.; Wang, P. Interaction between Nanobuds and Hydrogen Molecules: A First-Principles Study. Phys. Lett. A 2009, 374, 87–90. [Google Scholar] [CrossRef]
- Wu, C.-D.; Fang, T.-H.; Lo, J.-Y. Effects of Pressure, Temperature, and Geometric Structure of Pillared Graphene on Hydrogen Storage Capacity. Int. J. Hydrog. Energy 2012, 37, 14211–14216. [Google Scholar] [CrossRef]
- Hassani, A.; Hamed Mosavian, M.T.; Ahmadpour, A.; Farhadian, N. Hybrid Molecular Simulation of Methane Storage inside Pillared Graphene. J. Chem. Phys. 2015, 142, 234704. [Google Scholar] [CrossRef]
- Baykasoglu, C.; Mert, H.; Deniz, C.U. Grand Canonical Monte Carlo Simulations of Methane Adsorption in Fullerene Pillared Graphene Nanocomposites. J. Mol. Graph. Model. 2021, 106, 107909. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, Z.; Baykasoglu, C.; Kirca, M. Sandwiched Graphene-Fullerene Composite: A Novel 3-D Nanostructured Material for Hydrogen Storage. Int. J. Hydrog. Energy 2016, 41, 6403–6411. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and Closure of Graphitic Networks under Electron-Beam Irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- Goclon, J.; Bankiewicz, B.; Kolek, P.; Winkler, K. Role of Nitrogen Doping in Stoichiometric and Defective Carbon Nano-Onions: Structural Diversity from DFT Calculations. Carbon 2021, 176, 198–208. [Google Scholar] [CrossRef]
- Hussain, M.A.; Vijay, D.; Sastry, G.N. Buckybowls as Adsorbents for CO2, CH4, and C2H2: Binding and Structural Insights from Computational Study. J. Comput. Chem. 2016, 37, 366–377. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, E.; He, C.; Shi, C.; Du, X.; Hauge, R.H.; Zhao, N. Synthesis of Hollow Carbon Nano-Onions and Their Use for Electrochemical Hydrogen Storage. Carbon 2012, 50, 3513–3521. [Google Scholar] [CrossRef]
- Nikmaram, F.R.; Khoddamzadeh, A. Chemical Shielding of Doped Nitrogen on C20 Cage and Bowl Fullerenes. J. Struct. Chem. 2017, 58, 173–177. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Zhang, W.-H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 160698. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Chapline, M.G.; Dai, H. Functionalized Carbon Nanotubes for Molecular Hydrogen Sensors. Adv. Mater. 2001, 13, 1384–1386. [Google Scholar] [CrossRef]
- Sayago, I.; Terrado, E.; Aleixandre, M.; Horrillo, M.C.; Fernández, M.J.; Lozano, J.; Lafuente, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; et al. Novel Selective Sensors Based on Carbon Nanotube Films for Hydrogen Detection. Sens. Actuators B Chem. 2007, 122, 75–80. [Google Scholar] [CrossRef]
- Mubeen, S.; Zhang, T.; Yoo, B.; Deshusses, M.A.; Myung, N.V. Palladium Nanoparticles Decorated Single-Walled Carbon Nanotube Hydrogen Sensor. J. Phys. Chem. C 2007, 111, 6321–6327. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.H. Electrodeposition of Pd Nanoparticles on Single-Walled Carbon Nanotubes for Flexible Hydrogen Sensors. Appl. Phys. Lett. 2007, 90, 213107. [Google Scholar] [CrossRef]
- Sippel-Oakley, J.; Wang, H.-T.; Kang, B.S.; Wu, Z.; Ren, F.; Rinzler, A.G.; Pearton, S.J. Carbon Nanotube Films for Room Temperature Hydrogen Sensing. Nanotechnology 2005, 16, 2218–2221. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Chen, Z.; Rajaputra, S.; Singh, V. Hydrogen Sensors Based on Aligned Carbon Nanotubes in an Anodic Aluminum Oxide Template with Palladium as a Top Electrode. Sens. Actuators B Chem. 2007, 124, 12–17. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Han, J.; Ng, H.-T.; Binder, C.; Partridge, C.; Meyyappan, M. Room Temperature Methane Detection Using Palladium Loaded Single-Walled Carbon Nanotube Sensors. Chem. Phys. Lett. 2004, 391, 344–348. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Chen, Y.; Yang, M. A Multi-Walled Carbon Nanotube/Palladium Nanocomposite Prepared by a Facile Method for the Detection of Methane at Room Temperature. Sens. Actuators B Chem. 2008, 132, 155–158. [Google Scholar] [CrossRef]
- Kumar, M.K.; Ramaprabhu, S. Nanostructured Pt Functionlized Multiwalled Carbon Nanotube Based Hydrogen Sensor. J. Phys. Chem. B 2006, 110, 11291–11298. [Google Scholar] [CrossRef]
- Krishnakumar, M.; Ramaprabhu, S. Palladium Dispersed Multiwalled Carbon Nanotube Based Hydrogen Sensor for Fuel Cell Applications. Int. J. Hydrog. Energy 2007, 32, 2518–2526. [Google Scholar] [CrossRef]
- Krishna Kumar, M.; Leela Mohana Reddy, A.; Ramaprabhu, S. Exfoliated Single-Walled Carbon Nanotube-Based Hydrogen Sensor. Sens. Actuators B Chem. 2008, 130, 653–660. [Google Scholar] [CrossRef]
- Kamarchuk, G.V.; Kolobov, I.G.; Khotkevich, A.V.; Yanson, I.K.; Pospelov, A.P.; Levitsky, I.A.; Euler, W.B. New Chemical Sensors Based on Point Heterocontact between Single Wall Carbon Nanotubes and Gold Wires. Sens. Actuators B Chem. 2008, 134, 1022–1026. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A.; Dikonimos, T.; Serra, E.; Giorgi, R. Enhancement of Sensitivity in Gas Chemiresistors Based on Carbon Nanotube Surface Functionalized with Noble Metal (Au, Pt) Nanoclusters. Appl. Phys. Lett. 2007, 90, 173123. [Google Scholar] [CrossRef]
- Espinosa, E.H.; Ionescu, R.; Bittencourt, C.; Felten, A.; Erni, R.; Van Tendeloo, G.; Pireaux, J.-J.; Llobet, E. Metal-Decorated Multi-Wall Carbon Nanotubes for Low Temperature Gas Sensing. Thin Solid Film. 2007, 515, 8322–8327. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.-C.P. Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Partridge, C.; Meyyappan, M.; Li, J. A Carbon Nanotube Sensor Array for Sensitive Gas Discrimination Using Principal Component Analysis. J. Electroanal. Chem. 2006, 593, 105–110. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Na, H.G.; Kang, S.Y.; Choi, S.-W.; Kim, S.S.; Kim, H.W. Selective Detection of Low Concentration Toluene Gas Using Pt-Decorated Carbon Nanotubes Sensors. Sens. Actuators B Chem. 2016, 227, 157–168. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Keawwangchai, S.; Nunthaboot, N.; Ruangpornvisuti, V.; Wanno, B. Density Functional Investigation of Hydrogen Gas Adsorption on Fe−doped Pristine and Stone−Wales Defected Single−walled Carbon Nanotubes. J. Mol. Model. 2012, 18, 3941–3949. [Google Scholar] [CrossRef]
- Rather, S. Hydrogen Uptake of Ti-Decorated Multiwalled Carbon Nanotube Composites. Int. J. Hydrog. Energy 2021, 46, 17793–17801. [Google Scholar] [CrossRef]
- Dixit, S.; Patodia, T.; Sharma, K.B.; Katyayan, S.; Dixit, A.; Jain, S.K.; Agarwal, G.; Tripathi, B. Adsorption Characteristics of MWNTs via Intercalation of Nickel. Mater. Today Proc. 2021, 38, 1233–1236. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Wei, L.; Liang, N.; Wu, X. Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes. Sensors 2013, 13, 15159–15171. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Chen, Q.; Tang, J. A DFT Study of SO2 and H2S Gas Adsorption on Au-Doped Single-Walled Carbon Nanotubes. Phys. Scr. 2014, 89, 065803. [Google Scholar] [CrossRef]
- Lam, A.D.K.-T.; Lin, Z.-D.; Lu, H.-Y.; Young, S.-J. Carbon Nanotubes with Adsorbed Au Nanoparticles for Sensing Propanone Gas. Microsyst. Technol. 2019, 1–4. [Google Scholar] [CrossRef]
- Li, S.; Jiang, J. Adsorption Behavior Analyses of Several Small Gas Molecules onto Rh-Doped Single-Walled Carbon Nanotubes. Appl. Phys. A 2017, 123, 669. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Ru-Doped Single Walled Carbon Nanotubes as Sensors for SO2 and H2S Detection. Chemosensors 2021, 9, 120. [Google Scholar] [CrossRef]
- Li, K.; Wang, W.; Cao, D. Metal (Pd, Pt)-Decorated Carbon Nanotubes for CO and NO Sensing. Sens. Actuators B Chem. 2011, 159, 171–177. [Google Scholar] [CrossRef]
- Yoosefian, M. Powerful Greenhouse Gas Nitrous Oxide Adsorption onto Intrinsic and Pd Doped Single Walled Carbon Nanotube. Appl. Surf. Sci. 2017, 392, 225–230. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Yao, Q.; Miao, Y.; Tang, J. Rh-Doped Carbon Nanotubes as a Superior Media for the Adsorption of O2 and O3 Molecules: A Density Functional Theory Study. Carbon Lett. 2018, 28, 55–59. [Google Scholar] [CrossRef]
- Young, S.J.; Lin, Z.D. Acetone Gas Sensors Composed of Carbon Nanotubes with Adsorbed Au Nanoparticles on Plastic Substrate. Microsyst. Technol. 2018, 24, 3973–3976. [Google Scholar] [CrossRef]
- Pei, P.; Whitwick, M.B.; Kureshi, S.; Cannon, M.; Quan, G.; Kjeang, E. Hydrogen Storage Mechanism in Transition Metal Decorated Graphene Oxide: The Symbiotic Effect of Oxygen Groups and High Layer Spacing. Int. J. Hydrog. Energy 2020, 45, 6713–6726. [Google Scholar] [CrossRef]
- Singla, M.; Jaggi, N. Enhanced Hydrogen Sensing Properties in Copper Decorated Nitrogen Doped Defective Graphene Nanoribbons: DFT Study. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 131, 114756. [Google Scholar] [CrossRef]
- Ni, J.; Quintana, M.; Song, S. Adsorption of Small Gas Molecules on Transition Metal (Fe, Ni and Co, Cu) Doped Graphene: A Systematic DFT Study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113768. [Google Scholar] [CrossRef]
- Zitoune, H.; Adessi, C.; Benchallal, L.; Samah, M. Quantum Transport Properties of Gas Molecules Adsorbed on Fe Doped Armchair Graphene Nanoribbons: A First Principle Study. J. Phys. Chem. Solids 2021, 153, 109996. [Google Scholar] [CrossRef]
- Kuang, A.; Mo, M.; Kuang, M.; Wang, B.; Tian, C.; Yuan, H.; Wang, G.; Chen, H. The Comparative Study of XO2 (X = C, N, S) Gases Adsorption and Dissociation on Pristine and Defective Graphene Supported Pt13. Mater. Chem. Phys. 2020, 247, 122712. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Formaldehyde Gas Sensing Properties of Transition Metal-Doped Graphene: A First-Principles Study. J. Mater. Sci. 2021, 56, 12256–12269. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, X. First-Principles Investigation of Carbon Dioxide Adsorption on MN4 Doped Graphene. AIP Adv. 2020, 10, 125013. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Liu, S.; Zhao, X.; Huang, K. High-Capacity Hydrogen Storage Medium: Ti Doped Fullerene. Appl. Phys. Lett. 2011, 98, 023107. [Google Scholar] [CrossRef]
- Germán, E.; Alonso, J.A.; Janssens, E.; López, M.J. C60Con Complexes as Hydrogen Adsorbing Materials. Int. J. Hydrog. Energy 2021, 46, 20594–20606. [Google Scholar] [CrossRef]
- Arshadi, S.; Anisheh, F. Theoretical Study of Cr and Co- Porphyrin-Induced C70 Fullerene: A Request for a Novel Sensor of Sulfur and Nitrogen Dioxide. J. Sulfur Chem. 2017, 38, 357–371. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Lai, N. Endohedral Metallofullerenes Mn@C60 (M = Mn, Co, Ni, Cu; n = 2–5) as Electrocatalysts for Oxygen Reduction Reaction: A First-Principles Study. J. Mater. Sci. 2020, 55, 11382–11390. [Google Scholar] [CrossRef]
- Zou, L.; Zhu, Y.; Cen, W.; Jiang, X.; Chu, W. N-Doping in Graphdiyne on Embedding of Metals and Its Effect in Catalysis. Appl. Surf. Sci. 2021, 557, 149815. [Google Scholar] [CrossRef]
- Panigrahi, P.; Dhinakaran, A.K.; Naqvi, S.R.; Gollu, S.R.; Ahuja, R.; Hussain, T. Light Metal Decorated Graphdiyne Nanosheets for Reversible Hydrogen Storage. Nanotechnology 2018, 29, 355401. [Google Scholar] [CrossRef]
- Yu, H.; Hui, L.; Xue, Y.; Liu, Y.; Fang, Y.; Xing, C.; Zhang, C.; Zhang, D.; Chen, X.; Du, Y.; et al. 2D Graphdiyne Loading Ruthenium Atoms for High Efficiency Water Splitting. Nano Energy 2020, 72, 104667. [Google Scholar] [CrossRef]
- Zhai, X.; Yan, H.; Ge, G.; Yang, J.; Chen, F.; Liu, X.; Yang, D.; Li, L.; Zhang, J. The Single-Mo-Atom-Embedded-Graphdiyne Monolayer with Ultra-Low Onset Potential as High Efficient Electrocatalyst for N2 Reduction Reaction. Appl. Surf. Sci. 2020, 506, 144941. [Google Scholar] [CrossRef]
- Jasin Arachchige, L.; Xu, Y.; Dai, Z.; Zhang, X.L.; Wang, F.; Sun, C. Double Transition Metal Atoms Anchored on Graphdiyne as Promising Catalyst for Electrochemical Nitrogen Reduction Reaction. J. Mater. Sci. Technol. 2021, 77, 244–251. [Google Scholar] [CrossRef]
- Chen, X.; Gao, P.; Guo, L.; Wen, Y.; Fang, D.; Gong, B.; Zhang, Y.; Zhang, S. High-Efficient Physical Adsorption and Detection of Formaldehyde Using Sc- and Ti-Decorated Graphdiyne. Phys. Lett. A 2017, 381, 879–885. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Shen, X.; Gao, X.; Chen, Y.; Liu, H.; Liu, Y.; Yin, D.; Liu, Y.; Xu, W.; et al. Graphdiyne-Templated Palladium-Nanoparticle Assembly as a Robust Oxygen Generator to Attenuate Tumor Hypoxia. Nano Today 2020, 34, 100907. [Google Scholar] [CrossRef]
- He, T.; Zhang, L.; Kour, G.; Du, A. Electrochemical Reduction of Carbon Dioxide on Precise Number of Fe Atoms Anchored Graphdiyne. J. CO2 Util. 2020, 37, 272–277. [Google Scholar] [CrossRef]
- Liu, X.; Tang, W.; Liu, S.; Chen, X.; Li, Y.; Hu, X.; Qiao, L.; Zeng, Y. CO Oxidation on Ni and Cu Embedded Graphdiyne as Efficient Noble Metal-Free Catalysts: A First-Principles Density-Functional Theory Investigation. Appl. Surf. Sci. 2021, 539, 148287. [Google Scholar] [CrossRef]
- Vijay, D.; Sakurai, H.; Subramanian, V.; Sastry, G.N. Where to Bind in Buckybowls? The Dilemma of a Metal Ion. Phys. Chem. Chem. Phys. 2012, 14, 3057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Shi, C.; He, C.; Liu, E.; Zhao, N. Effect of Ni, Fe and Fe-Ni Alloy Catalysts on the Synthesis of Metal Contained Carbon Nano-Onions and Studies of Their Electrochemical Hydrogen Storage Properties. J. Energy Chem. 2014, 23, 324–330. [Google Scholar] [CrossRef]
- Canales, M.; Ramírez-de-Arellano, J.M.; Magana, L.F. Interaction of a Ti-Doped Semi-Fullerene (TiC30) with Molecules of CO and CO2. J. Mol. Model. 2016, 22, 223. [Google Scholar] [CrossRef]
- Canales-Lizaola, M.; Arellano, J.S.; Magaña, L.F. Hydrogen Molecule Adsorption on a Ti-Doped Graphene+ Semi-Fullerene Surface. J. Phys. Conf. Ser. 2019, 1221, 012081. [Google Scholar] [CrossRef]
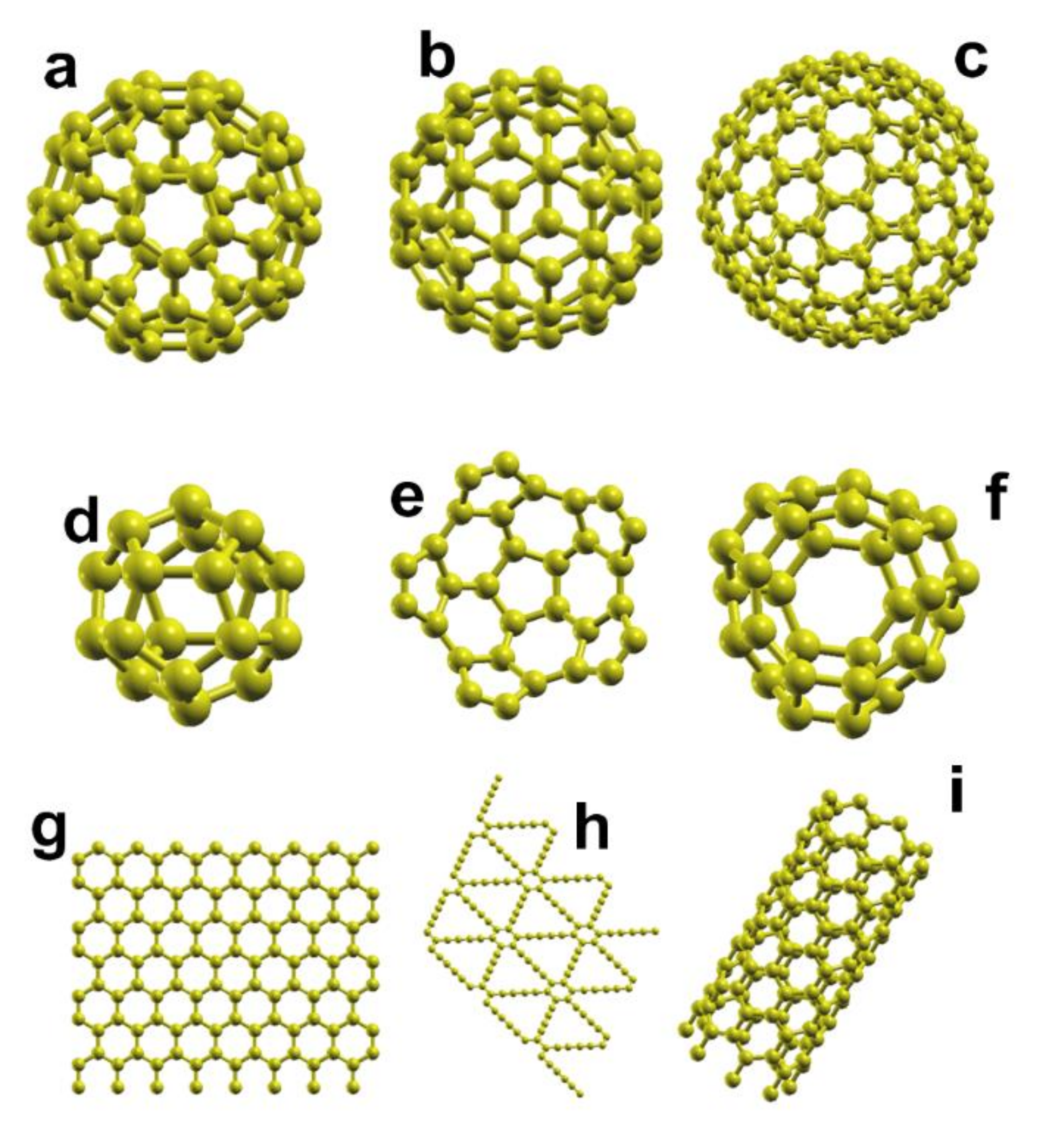
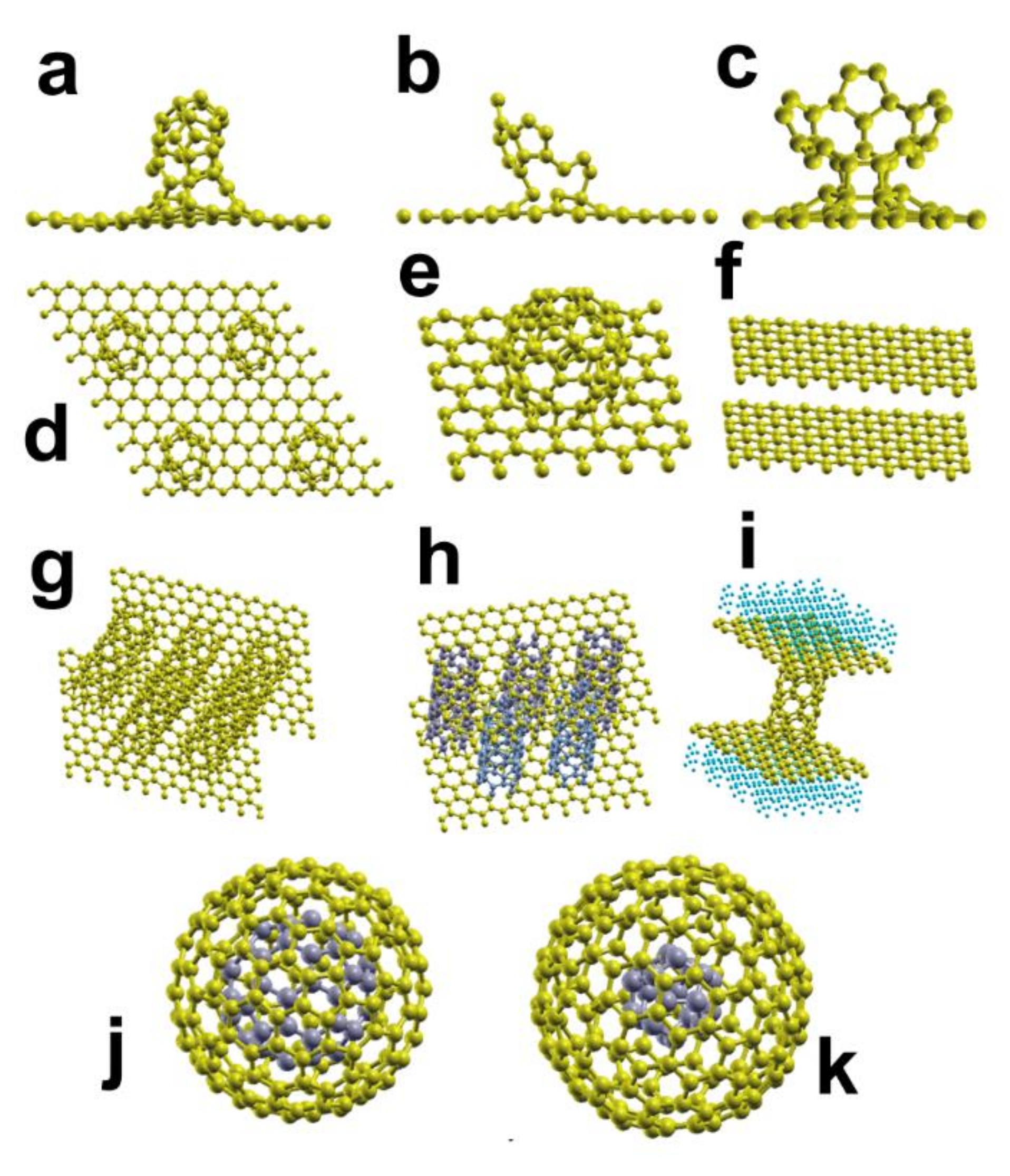
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| CNTs [41] | DFT | NO2 CH4 CO2 | −0.427 −0.122 −0.109 |
| SWCNTs [39] | GCMC, MD | SO2 | −0.464 |
| MWCNTs [11] | Experimental | H2 | [−0.26, −0.046] |
| MWNTs [28] | Experimental | SF6 | [−0.529, −1.285] |
| SWCNTs [37] | DFT | NO3 | −1.30 |
| CNNT [38] | DFT | H2CO | −0.321 |
| SWNTs and bundles [10] | DFT | NO2 O2 H2O NH3 N2 CO2 CH4 Ar | −0.427 −0.306 −0.128 −0.162 −0.123 −0.109 −0.122 −0.082 |
| SWCNTs [12] | Experimental | H2 | −0.056 |
| SWCNTs and SiC-DC [27] | GCMC | CO2 CH4 | −0.005 −0.003 |
| SWCNTs [30] | Exp. and DFT | C3H6O | [−0.255, −0.771] |
| CNTs [31] | Experimental | C2H5OH | Not reported |
| CNTs films [19] | Exp. and GCMC | CO2 | Not reported |
| MWCNTs [40] | Experimental | VOC | Not reported |
| SWCNTs [36] | Exp., GCMC, MD | Xe | Not reported |
| F-MWCNTs [24] | Experimental | CO2 | [−0.084, −0.036] |
| F-MWCNTs [13] | Experimental | H2 | Not reported |
| Oxygen F-CNTs [26] | Experimental | C2, N2 | Not reported |
| PANI/MWCNTs [32] | Experimental | NH3 | Not reported |
| F-MWCNTs and A-MWCNTs [14] | Experimental | H2 | Not reported |
| 1,3-diaminopropane MWCNTs [22] | Experimental | CO2 | Not reported |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| ZGNR [51] | DFT | H2S | −0.364 |
| Pristine G (PG) [52] | DFT | SO2 | −0.157 |
| PG [49] | Exp. and MD | NH3 | Not reported |
| PG [44] | DFT and MD | H2 | Not reported |
| Graphene [48] | DFT | H2S CH4 | −0.038 −0.022 |
| DG (vacancy) [48] | DFT | H2S CH4 | −2.934 −0.154 |
| G-OH [48] | DFT | H2S CH4 | −1.263 −0.047 |
| Single layer G (SLG) [43] | Experimental | H2 | Not reported |
| Bilayer G (BG) [53] | DFT | H2S | −0.360 |
| PG [54] | DFT | CH4 C2H2 CO | −0.086 −0.102 −0.093 |
| PG [46] | DFT | NO2 H2S | [−0.214, −0.185] [−0.201, −0.122] |
| PG [55] | DFT | CO NO | −0.084 −1.166 |
| Vacancy G (VG) [55] | DFT | CO NO | −0.069 −1.203 |
| PG [56] | DFT | NO NO2 | [−0.1280, −0.1176] [−0.1679, −0.1427] |
| PG [57] | DFT | AsH3 CO | Not reported |
| PG [58] | DFT | H2S | −0.360 |
| PG supercell [47] | DFT | NO | [−2.3713, −1.7453] |
| PG [59] | DFT | COCl2 | −0.554 |
| G nanoflakes (GNFs) [60] | DFT | CO CO2 | −1.18 −0.58 |
| PG [45] | DFT | H2O NH3 CO NO2 NO | −0.047 −0.031 −0.014 −0.067 −0.029 |
| PG [42] | DFT | H | [−0.84, −0.75] |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| C60 [70] | Exp. and DFT | H2 | [0.0495, 0.0641] |
| C60 [74] | PES (potential energy surface) | Ne, Ar, Kr, Xe | Not reported |
| C60 [69] | Experimental | H | −0.0247 |
| C60 [72] | DFT | N2 | [−0.28, −0.03] |
| C60 [64] | GCMC | C2H4 | [−0.0207, 0.0207] |
| C60 [79] | DFT | CO NO | −0.006 0.00008 |
| C60 [76] | DFT | C2N2 | −4.78 |
| OH-C60 [76] | DFT | C2N2 | −5.13 |
| C60 [77] | DFT | C9H13N + N C9H13N + H | −0.017 −0.0516 |
| C32 [71] | DFT | H2 | [−0.118, −0.0086] |
| C60 [65] | Experimental | Aromatic vapor | Not reported |
| C60-Nx [73] | DFT | NO NO2 | [−0.35, −0.28] [−1.01, −0.94] |
| C24 + P + N24 [80] | DFT | CO2 | [−0.94, −0.30] |
| C60+P [63] | DFT | CO2 | [−1.97, 0.06] |
| Si@C54N4 [66] | DFT | N2O CO O2 | [−3.50, −0–77] |
| C60 [67] | DFT | N2O CO | −0.169 −0.092 |
| C20 [75] B-C20 [75] N-C20 [75] | DFT | C5H11N2O2P | [−0.054, −0.043] [−1.092, −0.072] [−0.027, −1.65] |
| C460 [62] | DFT | H2 CO2 | −4.37 [−0.49, −0.42] |
| C60 [68] | Modified LJ-potential | H2, He | Not reported |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| GDY [86] | DFT | DMA TMA | [−0.503, −0.757] [−0.607, −0.796] |
| GDY supercell [85] | DFT | C2H5NO2 C5H9NO4 C6H9N3O2 C9H11NO2 | [−1.10, −0.59] [−1.14, −0.54] [−1.46. −0.73] [−1.53, −0.77] |
| GDY, BGDY [92] | DFT | TMZ | [−1.97, −0.95] |
| GDY nanoflakes [105] | DFT | CWA A-230 CWA A-232 CWA A-234 | −0.594 −0.713 −0.745 |
| GDY [81] | DFT | CO O2 | −1.43 −3.27 |
| GDY-NS [87] | DFT | C21H16ClF3N4O3 C21H15ClF4N4O3 | [−0.660, −0.085] [−0.641, −0.081] |
| GDY [89] | DFT | CH2O CH2O2 | [−1.502, −0.342] [−0.945, −0.390] |
| GDY [107] | DFT | Ag Cu Ni Zn | [−0.792926, −1.236] [−0.622651, −2.783] [−2.913467, −3.446] [−0.0196, 0.0356] |
| GDY [84] | DFT | H O | −3.73 −7.53 |
| GDY nanoflakes [106] | DFT | L1 L2 L3 | −0.441 −0.534 −0.567 |
| GDY [97] | Hybrid DFT | Li | −1.82 |
| Ca-GDY [83] | DFT | CH4O CO | [−0.349, −0.122] [−0.128, −0.060] |
| GDY [104] | DFT, QTAIM | CWA GA CWA GB CWA GD CWA GF | −0.707 −0.520 −0.543 −0.382 |
| GDY [88] | DFT | NH3 | [−0.465, −0.435] |
| GDY [108] | DFT | Pd clusters | [−4.0, −3.0] |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| CNO [117] | Experimental | H2O | Not reported |
| CNO [115] | DFT | mN, m = 1–10 | [−0.18, 0.33] |
| Buckybowls [116] | Hybrid-DFT | CO2, CH4, C2H2 | Not reported |
| C20, C20 (bowl) [118] | DFT | N, H | Not reported |
| Nanobuds [109] | DFT | H2 | [0.069, 0.115] |
| Sandwiched G-fullerene + Li [113] | GCMC | H2 | Not reported |
| Pillared-graphene [110] | MD | H2 | Not reported |
| Fullerene pillared-graphene [112] | GCMC | CH4 | Not reported |
| Pillared-graphene [111] | GCMC-MD | CH4 | Not reported |
| Metal | Adsorbed Molecules |
|---|---|
| Ag | NO2 [133] |
| Au | NH3, NH2 [132,133,135]; SO2, H2S [141]; C2H6O [31]; C3H6O [142,148] |
| Pt | H2, NO2, H2O, NH3 [132]; C7H8 [136]; NO [145] |
| Pd | H2 [120,121,122,123,124,125,128]; NO2 [135], Cl2 [135]; CH4 [126,127]; NO [145]; N2O [146] |
| Fe | H2 [137] |
| Rh | CO [134]; CO, C2H4 SO2 [143]; O2, O3 [147] |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| CNT(APTS) [18] | Experimental | CO2 | Not reported |
| Fe-SWCNTs [137] | DFT | H2 | [−0.238, −0.141] |
| Au-CNTs [31] | Experimental | C2H6O | Not reported |
| Pt-SWCNTs [140] | DFT | SO2 H2S CO | −1.225 −0.977 −1.386 |
| Au-SWCNTs [141] | DFT | SO2 H2S | −1.258 −1.317 |
| Au-CNTs [142] | Experimental | C3H6O | Not reported |
| Rh–CNT [143] | DFT | CO CO2 CH4 C2H4 SO2 | [−3.527, −1.308] −0.348 −0.253 −1.189 −1.158 |
| Pd-, Pt-SWNTs [145] | DFT | CO NO | [−1.8, −1.6] [−1.814, −1.46] |
| Pd-CNT [146] | DFT | N2O | −0.91 |
| Rh–CNT [147] | DFT | O2 O3 | −1.384 [−2.711, −1.824] |
| Pt-MWCNTs [136] | Experimental | C7H8 | Not reported |
| Au-CNTs [148] | Experimental | C3H6O | Not reported |
| Pd-, Pt-, Rh-, Au-SWNTs [134] | Experimental | H2, CH4, CO, H2S | Not reported |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| Zn-ZGNR [51] | DFT | H2S | −2.237 |
| Cu-ZGNR [51] | DFT | H2S | −1.129 |
| Cu/Zn-ZGNR [51] | DFT | H2S | −7.043 |
| Ni-G [52] | DFT | SO2 | −2.297 |
| TM-PG [154] TM = Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Pd, Ag, Pt, Au | DFT | HCHO | [0.83, 2.01] |
| Cu+1N-GNR [150] | DFT | H2 | −0.020 |
| Cu+2N-GNR [150] | DFT | H2 | −0.200 |
| Cu+3N-GNR [150] | DFT | H2 | −0.780 |
| TiO2-GO [149] | Experimental | H2 | Not reported |
| Ni-G [48] | DFT | H2S CH4 | −0.699 −0.099 |
| TM-BG [53] TM = V, Cr, Mn, Fe, Co, Ni | DFT | H2S | [−0.58, −0.18] |
| Pt13-G [153] | DFT | CO2 NO2 SO2 | [−4.217, −2.422] [−3.767, −2.586] [−3.260, −2.238] |
| Pt13-DG [153] | DFT | CO2 NO2 SO2 | [−3.201, −0.916] [−3.345, −2.309] [−2.978, −2.065] |
| Mn-G [54] | DFT | CH4 C2H2 CO | −0.073 −2.424 −1.954 |
| Ni-G [46] | DFT | NO2 H2S | [−2.631, −2.395] [−1.846, −1.811] |
| Pd-G [46] | DFT | NO2 H2S | [−1.586, −1.294] [−1.228, −1.224] |
| Pt-G [46] | DFT | NO2 H2S | [−2.003, −1.804] [−2.034, −1.858] |
| Pd-G [55] | DFT | CO NO | [−1.227, −0.909] [−3.916, −1.308] |
| Ag-G [56] | DFT | NO NO2 | [−6.9262, −6.9101] [−7.8293, −7.7806] |
| Pt-G [56] | DFT | NO NO2 | [−6.2225, −6.1646] [−7.3758, −7.3723] |
| Au-G [56] | DFT | NO NO2 | [−8.4730, −8.3567] [−9.3391, −9.3209] |
| TM-G [57] TM = Ti, Mn, Fe, Co, Ni, Ag | DFT | AsH3 CO | [−0.95, −1.45] [−1.00, 2.02] |
| Ni-G [58] | DFT | H2S | −0.97 |
| Cu-G [58] | DFT | H2S | −1.15 |
| Zn-G [58] | DFT | H2S | −1.16 |
| TM-G [151] TM = Fe, Ni, Co, Cu | DFT | CO2 NO NO2 SO2 | [−0.89, −1.19] [−0.68, −1.23] [−2.06, −2.57] [−0.89, −1.47] |
| Fe- AGNR [152] | DFT | CO CO2 NO NO2 | −2.4 −1.3 −3.1 −3.0 |
| Zr-G [59] Mo-G [59] Ti-G [59] Mn-G [59] Fe-G [59] Co-G [59] | DFT | COCl2 | −0.894 −0.960 −1.065 −1.677 −1.378 −0.828 |
| TM-GNF [60] TM = Sc, Ti, V, Cr, Mn, Fe, Co, N, Cu, Zn | DFT | CO CO2 | [−8.13, −37.56] [ −5.05, −16.11] |
| MN4-G [155] M = Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn | DFT | CO2 | [−0.0032, −0.0125] |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| Ti-C60 [156] | DFT | H2 | −0.14 |
| TM-C60 [79] TM = Cr, Mn, Fe, Co, Ni, Cu; Zn | DFT | CO NO | [−2.94, −1.03] [−6.52, −1.95] |
| Cr-C70 [158] | TD-DFT | NO2 SO2 | [−1.55, −0.605] [−1.178, −0.025] |
| Co-C70 [158] | TD-DFT | NO2 SO2 | [−1.919, −0.806] [−0.627, −0.0047] |
| Mx-C60 [159] M = Mn, Co, Ni, Cu (x = 2–5) | DFT | CH3OH HCOOH CH3CH2OH O2 CO SO2 | [−0.26, −0.17] [−0.19, −0.08] [−0.27, −0.16] [−0.19, −0.10] [−0.35, −0.12] [−0.22, −0.09] |
| Con-C60 [157] (n = 1–8) | DFT | H2 2H | [1.31, 0.60] [1.78, 1.08] |
| TM-C60 [67] TM = Cu, Zn, Ni | DFT | N2O CO | [−2.30, −1.41] [−3.5, −1.56] |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| TM-GDY [98] TM = Li, Na, K, Rb, Cs | DFT | CO2 | [−0.54, −0.21] |
| Cu-GDY [100] Cu-B-GDY [100] Cu-N-GDY [100] | DFT | CO2 | [−0.5, −0.4] [−0.45, −0.3] [−0.5, −0.31] |
| Pt-GDY [103] | DFT | O2 CO | −1.14 −1.56 |
| Ni-GDY [168] | DFT | O2 CO O2 + CO O CO2 | −0.69 −1.68 −1.09 −3.21 −0.08 |
| Cu-GDY [168] | DFT | O2 CO O2 + CO O CO2 | −0.79 −1.25 −1.43 −3.21 −0.37 |
| Sc-GDY [165] Ti-GDY [165] | DFT | HCHO | −2.59 −2.24 |
| TM-GDY [161] TM = Ti, Sc, Li, Na, K, Ca | DFT (GGA) DFT (vdW-DF) DFT (DFT-D3) | 8H2 | [−0.197, −0.10] [−0.77, −0.194] [−0. 345, −0.173] |
| Mo-GDY [163] | DFT | N2 | [−1.35, −0.93] |
| TM-GDY [82] TM = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Y, Zr, Rh, Pd, Ag, La, Hf, Pt | DFT | N2 | [−1.8, +0.12] |
| TM-GDY [160] TM = Cr, Mn, Fe, Co | DFT-D3 | O2 CO | [−2.52, −1.21] [−1.57, −1.25] |
| TM-1N-GDY [160] TM = Cr, Mn, Fe, Co | DFT-D3 | O2 CO | [−2.56, −1.07] [−1.78, −1.21] |
| TM-2N-GDY [160] TM = Cr, Mn, Fe, Co | DFT-D3 | O2 CO | [−2.31, −1.17] [−1.94, −1.39] |
| Ru-GDY [162] | Experimental | H2O | Not reported |
| Fe-GDY [167] | DFT-D3/AIMD | CO2 | Not reported |
| System | Type of Study | Adsorbate | Eads |
|---|---|---|---|
| Ti-C30 [171] | DFT | CO CO2 | [−0.897, −1.673] [−1.605, −1.247] |
| Ti-G-Semifullerene [172] | DFT | H2 | −1.41 |
| Ni-, Fe-CNOs [170] | Experimental | H2 | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-de-Arellano, J.M.; Canales, M.; Magaña, L.F. Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems. Molecules 2021, 26, 5346. https://doi.org/10.3390/molecules26175346
Ramirez-de-Arellano JM, Canales M, Magaña LF. Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems. Molecules. 2021; 26(17):5346. https://doi.org/10.3390/molecules26175346
Chicago/Turabian StyleRamirez-de-Arellano, J. M., M. Canales, and L. F. Magaña. 2021. "Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems" Molecules 26, no. 17: 5346. https://doi.org/10.3390/molecules26175346
APA StyleRamirez-de-Arellano, J. M., Canales, M., & Magaña, L. F. (2021). Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems. Molecules, 26(17), 5346. https://doi.org/10.3390/molecules26175346







