Influence of Temperature, Photoperiod, and Supplementary Nutrition on the Development and Reproduction of Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Development and Reproduction of S. caerulea at Different Temperatures
2.3. Development and Reproduction of S. caerulea at Different Photoperiods
2.4. Development and Reproduction of S. caerulea under Different Supplementary Nutrition
2.5. Data Analysis
3. Results
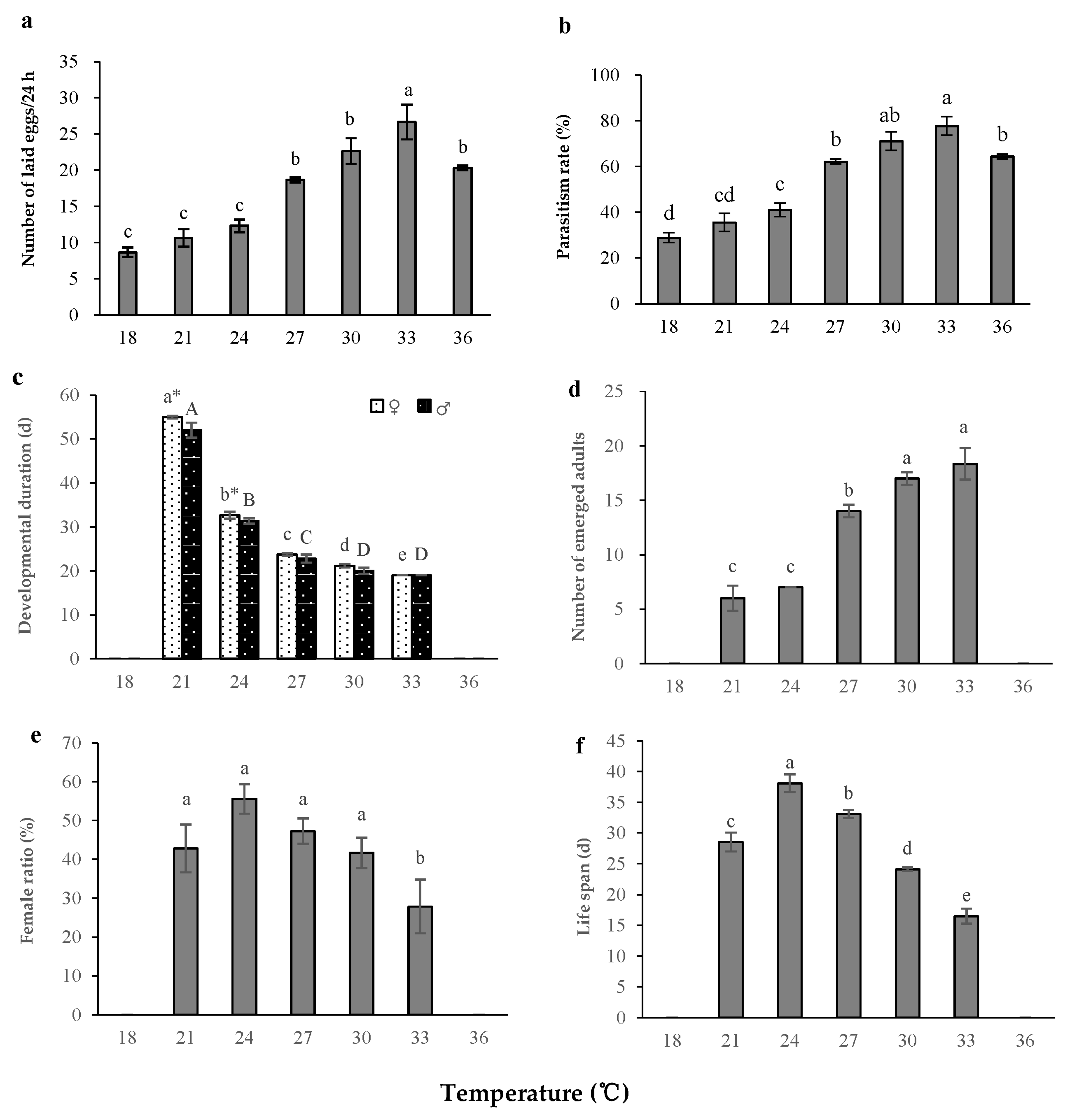
3.1. Effects of Temperature on the Development and Reproduction of S. caerulea
3.2. Effects of Photoperiod on the Development and Reproduction of S. caerulea
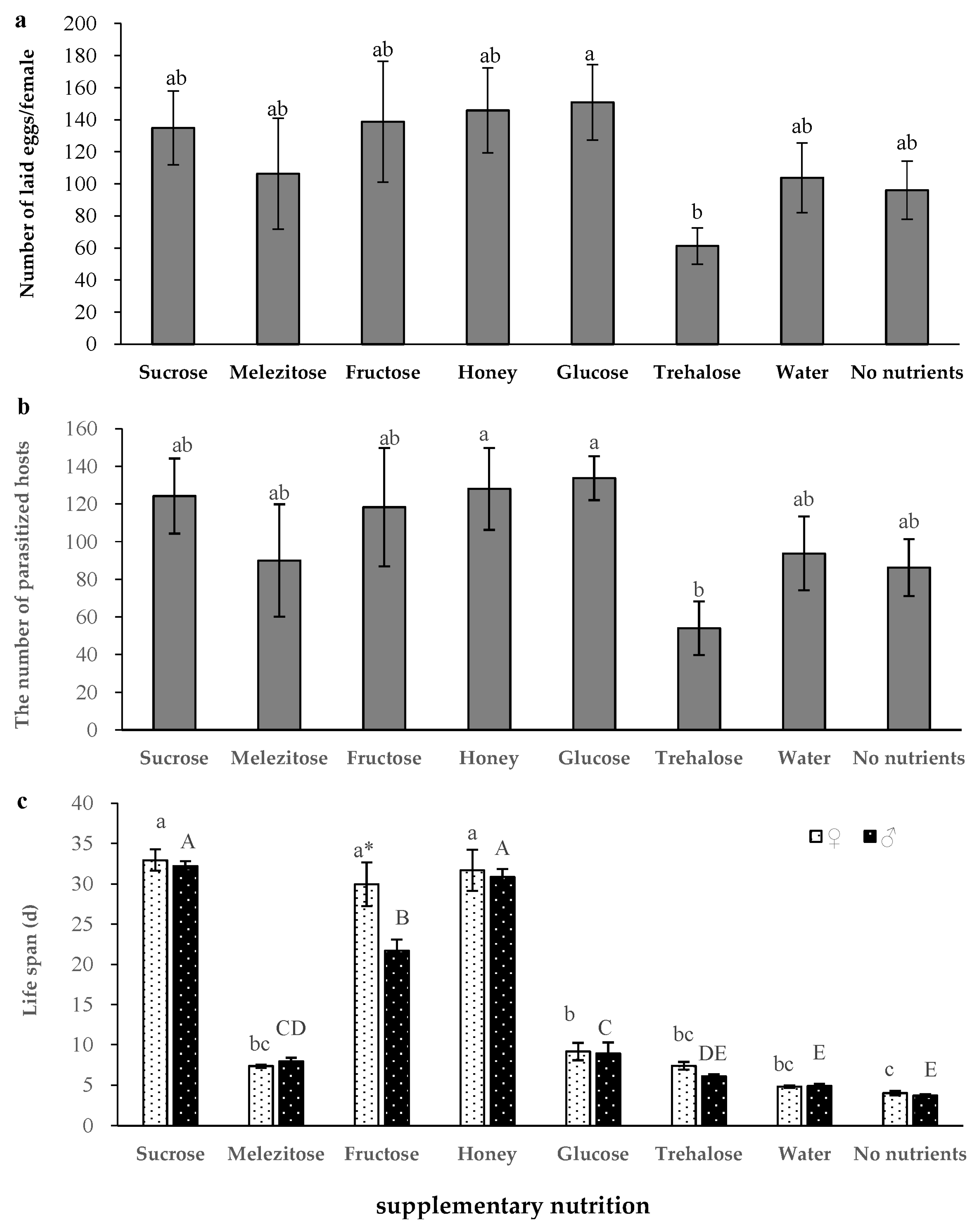
3.3. Effects of Supplementary Nutrition on the Development and Reproduction of S. caerulea
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brändle, M.; Amarell, U.; Auge, H.; Klotz, S.; Brandl, R. Plant and insect diversity along a pollution gradient: Understanding species richness across trophic levels. Biodivers. Conserv. 2001, 10, 1497–1511. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Z.S.; Luo, S.P.; Xu, Z.F. Effect of temperature on development, survival, and fecundity of Microplitis manilae (Hymenoptera: Braconidae). Environ. Entomol. 2012, 41, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Spanoudis, C.G.; Andreadis, S.S. Temperature-dependent survival, development, and adult longevity of the koinobiont endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae) parasitizing Plodia interpunctella (Lepidoptera: Pyralidae). J. Pest Sci. 2012, 85, 75–80. [Google Scholar] [CrossRef]
- Potter, K.A.; Davidowitz, G.; Woods, A.H. Cross-stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 2011, 25, 548–556. [Google Scholar] [CrossRef]
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: Oxford, UK, 2012; pp. 64–101. [Google Scholar]
- Tauber, M.J.; Tauber, C.A. Photoperiodic induction and termination of diapause in an insect: Response to changing day lengths. Science 1970, 167, 170. [Google Scholar] [CrossRef] [PubMed]
- Belozerov, V.N.; Naumov, R.L. Nymphal diapause and its photoperiodic control in the tick Ixodes scapularis (Acari: Ixodidae). Folia Parasitol. 2002, 49, 314–318. [Google Scholar] [CrossRef]
- Zerbino, M.S.; Altier, N.A.; Panizzi, A.R. Effect of photoperiod and temperature on nymphal development and adult reproduction of Piezodorus guildinii (Heteroptera: Pentatomidae). Fla. Entomol. 2013, 96, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Rechav, Y. Biological and ecological studies of the parasitoid Chelonus inanitus (Hym.: Braconidae) in Israel. Entomophaga 1978, 23, 95–102. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.Y.; Yang, Z.Q.; Duan, J.J. Effects of photoperiod and light intensity on wing dimorphism and development in the parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Strange-George, J.E.; Kulhanek, C.A.; Wäckers, F.L.; Heimpel, G.E. Sugar feeding by the aphid parasitoid Binodoxys communis: How does honeydew compare with other sugar sources. J. Insect Physiol. 2008, 54, 481–491. [Google Scholar] [CrossRef]
- Hanan, A.; He, X.Z.; Wang, Q. Host feeding and oviposition strategy of Eretmocerus warrae (Aphelinidae Hymenoptera) under different host densities. N. Zealand Plant Prot. 2012, 65, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Tena, A.; Pekas, A.; Waeckers, F.L.; Urbaneja, A. Energy reserves of parasitoids depend on honeydew from non-hosts. Ecol. Entomol. 2013, 38, 278–289. [Google Scholar] [CrossRef]
- Rivero, A.; Casas, J. Incorporating physiology into parasitoid behavioral ecology: The allocation of nutritional resources. Res. Popul. Ecol. 1999, 41, 39–45. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhu, P.Y.; Gurr, G.M.; Zheng, X.S.; Read, D.M.Y.; Heong, K.L.; Yang, Y.J.; Xu, H.X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dov, Y.; Miller, D.R. ScaleNet: Systematic Database of the Scale Insects of the World (Version December 2004). In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Species 2000: Leiden, The Netherlands, 2019. [Google Scholar]
- Abdul-Rassoul, M.S.; Mallo, I.M. First record of nigra scale, Parasaissetia nigra (Nietner, 1861) (Hemiptera; Coccidae) as a pest of fig trees in Iraq. Bull. Iraq Nat. Hist. Mus. 2016, 14, 171–178. [Google Scholar]
- Yin, S.S.; Chuan, X.X.; Bai, Y.B.; Li, W.W.; Zhang, X.Y.; Duan, B.T.; Li, J.T.; Luo, K. Research Status and Prospect on Biology, Ecology and Control of Parasaissetia nigra. Chin. J. Trop. Agric. 2017, 37, 41–46. [Google Scholar]
- Myartseva, S.N.; Ruíz-Cancino, E.; Coronado-Blanco, J.M. Parasaissetia nigra (Hemiptera: Coccidae) and its parasitoids from the genus Coccophagus (Hymenoptera: Aphelinidae), with description of a new species from Tamaulipas, México. Fla. Entomol. 2014, 97, 1015–1020. [Google Scholar] [CrossRef]
- Myartseva, S.N.; Ruíz-Cancino, E.; Coronodo-Blanco, J.M. Primer registro de Diversinervus elegans Silvestri (Hymenoptera: Encyrtidae) en el estado de Tamaulipas, México, y otros parasitoides de la escama Parasaissetia nigra (Nietner)(Hemiptera: Coccidae). Entomol. Mex. 2017, 16, 777–780. [Google Scholar]
- Shen, S.Z.; Zhang, F.P.; Fu, Y.G.; Li, L.; Zhu, J.H. Factors affecting mating in Coccophagus japonicus Compere. J. Environ. Entomol. 2017, 39, 1135–1141. [Google Scholar]
- Zhang, Y.Z.; Huang, D.W.; Fu, Y.G.; Peng, Z.Q. A new species of Metaphycus mercet (Hymenoptera: Encyrtidae) from China, parasitoid of Parasaissetia nigra (Nietner) (Homoptera: Coccoidea). Entomol. N. 2017, 118, 68–72. [Google Scholar] [CrossRef]
- Wen, L.N.; Fu, Y.G.; Zhang, F.P.; Jin, Q.A.; Zhang, J.B. Effects of temperature on the development and reproduction of Metaphycus parasaissetiae Zhang and Huang. Chin. Bull. Entomol. 2010, 47, 151–155. [Google Scholar]
- Wang, J.Q.; Xu, L.Y.; Li, F.C.; Zheng, Y.P.; Deng, Y.X.; Zhang, Y.K.; Zhu, G.Y.; Li, G.H. Effect of temperature on emergence rate and sex ratio of Diversinervus elegans Silvestri. J. Environ. Entomol. 2019, 41, 161–166. [Google Scholar]
- Li, X.; Fu, Y.G.; Chen, J.Y.; Wang, J.Y.; Zhu, J.H.; Zhang, F.P. Effects of temperature and photoperiod on the development and reproduction of endoparasitoid wasp Coccophagus japonicus Compere. J. Plant Prot. 2021, 48, 848–854. [Google Scholar]
- Tena, A.; Soto, A.; Garcia-Marí, F. Parasitoid complex of black scale Saissetia oleae on citrus and olives: Parasitoid species composition and seasonal trend. Biocontrol 2008, 53, 473–487. [Google Scholar] [CrossRef]
- Abd-Rabou, S.N. New records of the soft scale insects hosts associated with the promising parasitoid, Scutellista caerulea (Fonscolombe) (Hymenoptera: Pteromalidae) in Egypt. Egypt. J. Agric. Res. 2011, 89, 1295–1301. [Google Scholar]
- Badary, H.; Abd-Rabou, S. Role of pteromalid parasitoid Scutellista caerulea (Fonscolombe) (Hymenoptera: Pteromalidae) for biological control of the soft scale insects (Hemiptera: Coccidae) in Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Ehler, L.E. Observations on Scutellista cynea Motsch. (Hymenoptera: Pteromalidae). Pan-Pac. Entomol. 1989, 65, 151–155. [Google Scholar]
- Awamelah, R.A.; Al-Antary, T.M.; Bilal, H.M. Survey of natural enemies of fig wax scale Ceroplastes rusci L. (Homoptera: Coccidae) and seasonal abundance of the parasitoid Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae) in Jordan. Jordan J. Agric. Sci. 2009, 5, 434–445. [Google Scholar]
- Li, X.; Niu, L.M.; Fu, Y.G.; Chen, J.Y.; Li, L.; Han, D.Y.; Zhang, F.P.; Zhu, J.H. Observations on Biological Characteristics of Scutellista caerulea Fonscolombe. Chin. J. Biol. Control 2020, 36, 327–334. [Google Scholar]
- Ding, Y.Q.; Li, D.M.; Chen, Y.P. Study on sampling of Locusta migratoria manilensis (Meyen) nymph. J. Plant Prot. 1980, 7, 101–112. [Google Scholar]
- Li, D.M.; Wang, M.M. Research on the method of rapidly estimating developmental threshold temperature and effective accumulated temperature. Chin. J. Appl. Entomol. 1986, 23, 184–187. [Google Scholar]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Zamani, A.A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Effect of temperature on life history of Aphidius colemani and Aphidius matricariae (Hymenoptera: Braconidae), two parasitoids of Aphis gossypii and Myzus persicae (Homoptera: Aphididae). Environ. Entomol. 2007, 36, 263–271. [Google Scholar] [CrossRef]
- Ma, M.R.; Jin, X.; Cui, J.Z.; Xing, Z.L.; Li, J.Q.; Li, H.P. Biological Characteristics of Pediobius yunnanensis Liao (Hymenoptera: Chalcidoidea: Eulophidae), a Parasitoid of Dioryctria rubella Hampson. Chin. J. Biol. Control 2019, 35, 829–834. [Google Scholar]
- Moiroux, J.; Brodeur, J.; Boivin, G. Sex ratio variations with temperature in an egg parasitoid: Behavioural adjustment and physiological constraint. Anim. Behav. 2014, 91, 61–66. [Google Scholar] [CrossRef]
- Chen, W.; Fu, Y.G.; Peng, Z.Q. Influence of Temperature on the Laboratory Population of Parasaissetia nigra Nietner. Chin. J. Trop. Crops. 2010, 31, 809–814. [Google Scholar]
- Zilch, K.C.F.; Jahnke, S.M.; Köhler, A.; Bender, E. Effect of Diet, Photoperiod and Host Density on Parasitism of Anisopteromalus calandrae on the Tobacco Beetle and Biological Parameters of the Parasitoid. Am. J. Plant Sci. 2017, 8, 3218–3232. [Google Scholar] [CrossRef] [Green Version]
- Temori, N.; Iranipour, S.; Banamolaei, P. Effect of Light Intensity and Photoperiod on Development, Fecundity and Longevity of Trissolcus grandis (Hym.: Platygastridae), Egg Parasitoid of Sunn Pest, Eurygaster integriceps Puton (Hem.: Scutelleridae). J. Appl. Res. Plant Prot. 2019, 8, 77–93. [Google Scholar]
- Zhang, F.P.; Fu, Y.G.; Peng, Z.Q.; Wang, B.; Zhang, J.B.; Jin, Q.A. Effects of temperature and photoperiod on the development and reproduction of Coccophagus ceroplastae Howard. Acta Ecol. Sin. 2010, 30, 1280–1286. [Google Scholar]
- Zhang, J.J.; Zhang, X.; Du, W.M.; Wang, X.M.; Ruan, C.C. Effects of Photoperiods on Development and Reproduction of Trichogramma dendrolimi Matsumura. J. Jilin Agric. Univ. 2019, 41, 17–22. [Google Scholar]
- Schmale, I.; Wäckers, F.L.; Cardona, C.; Dorn, S. Control potential of three hymenopteran parasitoid species against the bean weevil in stored beans: The effect of adult parasitoid nutrition on longevity and progeny production. Biol. Control 2001, 21, 134–139. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, X.F.; Ye, G.Y.; Li, X.H. Effects of different diets on the ovary and oogenesis development of Telenomus theophilae Wu et Chen. Entomol. J. East China 2006, 15, 112–115. [Google Scholar]
- Zhang, Y.B.; Liu, W.X.; Wang, W.; Wan, F.H.; Li, Q. Lifetime gains and patterns of accumulation and mobilization of nutrients in females of the synovigenic parasitoid, Diglyphus isaea Walker (Hymenoptera: Eulophidae), as a function of diet. J. Insect Physiol. 2011, 57, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Chen, Z.Z.; Duan, B.S.; Zheng, J.T.; Zhang, T.X. Effects of female diet and age on offspring sex ratio of the solitary parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera, Pteromalidae). Rev. Bras. De Entomol. 2012, 56, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lu, S.L.; Liu, W.X.; Cheng, L.S.; Zhang, Y.B.; Wan, F.H. Effects of five naturally occurring sugars on the longevity, oogenesis, and nutrient accumulation pattern in adult females of the synovigenic parasitoid Neochrysocharis formosa (Hymenoptera: Eulophidae). Neotrop. Entomol. 2014, 43, 564–573. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The influence of temperature variation on life history parameters and thermal performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian citrus psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Zheng, X.S.; Xie, G.; Chen, G.H.; Lu, Z.X.; Gurr, G. Relevance of the ecological traits of parasitoid wasps and nectariferous plants for conservation biological control: A hybrid meta-analysis. Pest Manag. Sci. 2020, 76, 1881–1892. [Google Scholar] [CrossRef]
- Wanner, H.; Gu, H.; Dorn, S. Nutritional value of floral nectar sources for flight in the parasitoid wasp, Cotesia glomerata. Physiol. Entomol. 2006, 31, 127–133. [Google Scholar] [CrossRef]
- Wang, X.; Ramualde, N.; Aparicio, E.M.; Maspero, M.; Duan, J.J.; Smith, L. Optimal conditions for diapause survival of Aprostocetus fukutai, an egg parasitoid for biological control of Anoplophora chinensis. Insects 2021, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Basri, M.W.; Idris, A.B. Effects of honey and sucrose on longevity and fecundity of Apanteles metesae (Nixon), a major parasitoid of the oil palm bagworm, Metisa plana (Walker). Sains Malays. 2012, 41, 1543–1548. [Google Scholar]

| Variable | B | Wald | OR (95% CI) | p-Value |
|---|---|---|---|---|
| temperature | 0.113 | 59.401 | 1.120 (1.088–1.152) | 0.000 |
| Variable | B | Wald | OR (95% CI) | p-Value |
|---|---|---|---|---|
| temperature | −0.60 | 7.817 | 0.941 (0.902–0.982) | 0.005 |
| Method | Lower Developmental Threshold Temperature (°C) | Effective Accumulative Temperature (Degree-Days) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Linear regression method | 14.18 | 13.93 | 335.86 | 330.61 |
| Optimum seeking method | 15.21 | 14.97 | 307.00 | 302.71 |
| Variable | B | Wald | OR (95% CI) | p-Value |
|---|---|---|---|---|
| temperature | 0.265 | 146.124 | 0.908 (0.802–1.029) | 0.000 |
| Variable | B | Wald | OR (95% CI) | p-Value |
|---|---|---|---|---|
| temperature | −0.96 | 2.298 | 1.120 (1.088–1.152) | 0.130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ye, Z.; Chen, J.; Zhu, J.; Han, D.; Wang, J.; Li, L.; Fu, Y.; Zhang, F. Influence of Temperature, Photoperiod, and Supplementary Nutrition on the Development and Reproduction of Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae). Insects 2023, 14, 82. https://doi.org/10.3390/insects14010082
Li X, Ye Z, Chen J, Zhu J, Han D, Wang J, Li L, Fu Y, Zhang F. Influence of Temperature, Photoperiod, and Supplementary Nutrition on the Development and Reproduction of Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae). Insects. 2023; 14(1):82. https://doi.org/10.3390/insects14010082
Chicago/Turabian StyleLi, Xian, Zhengpei Ye, Junyu Chen, Junhong Zhu, Dongyin Han, Jianyun Wang, Lei Li, Yueguan Fu, and Fangping Zhang. 2023. "Influence of Temperature, Photoperiod, and Supplementary Nutrition on the Development and Reproduction of Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae)" Insects 14, no. 1: 82. https://doi.org/10.3390/insects14010082
APA StyleLi, X., Ye, Z., Chen, J., Zhu, J., Han, D., Wang, J., Li, L., Fu, Y., & Zhang, F. (2023). Influence of Temperature, Photoperiod, and Supplementary Nutrition on the Development and Reproduction of Scutellista caerulea Fonscolombe (Hymenoptera: Pteromalidae). Insects, 14(1), 82. https://doi.org/10.3390/insects14010082






