Prevalence and Initial Diagnosis of Cerebral Palsy in Preterm and Term-Born Children in Taiwan: A Nationwide, Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Source of Data
2.2. Study Design and Identification of Study Subjects
2.3. Definition and Diagnosis of Cerebral Palsy
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- McIntyre, S.; Morgan, C.; Walker, K.; Novak, I. Cerebral palsy—Don′t delay. Dev. Disabil. Res. Rev. 2011, 17, 114–129. [Google Scholar] [CrossRef]
- Bax, M.; Tydeman, C.; Flodmark, O. Clinical and MRI correlates of cerebral palsy: The European Cerebral Palsy Study. JAMA 2006, 296, 1602–1608. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.B. Causative factors in cerebral palsy. Clin. Obstet. Gynecol. 2008, 51, 749–762. [Google Scholar] [CrossRef]
- Hollung, S.J.; Vik, T.; Lydersen, S.; Bakken, I.J.; Andersen, G.L. Decreasing prevalence and severity of cerebral palsy in Norway among children born 1999 to 2010 concomitant with improvements in perinatal health. Eur. J. Paediatr. Neurol. 2018, 22, 814–821. [Google Scholar] [CrossRef]
- Himmelmann, K.; McManus, V.; Hagberg, G.; Uvebrant, P.; Krägeloh-Mann, I.; Cans, C. Dyskinetic cerebral palsy in Europe: Trends in prevalence and severity. Arch. Dis. Child. 2009, 94, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.M.; Carlin, J.B.; Reddihough, D.S. Rates of cerebral palsy in Victoria, Australia, 1970 to 2004: Has there been a change? Dev. Med. Child. Neurol. 2011, 53, 907–912. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Touyama, M.; Touyama, J.; Toyokawa, S.; Kobayashi, Y. Trends in the prevalence of cerebral palsy in children born between 1988 and 2007 in Okinawa, Japan. Brain Dev. 2016, 38, 792–799. [Google Scholar] [CrossRef]
- Kim, S.W.; Jeon, H.R.; Shin, J.C.; Youk, T. Incidence of cerebral palsy in Korea and the effect of socioeconomic status: A population-based nationwide study. Yonsei Med. J. 2018, 59, 781–786. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Van den Broeck, C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Dev. Med. Child. Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salokorpi, T.; Rautio, T.; Sajaniemi, N.; Serenius-Sirve, S.; Tuomi, H.; Wendt, L.V. Neurological development up to the age of four years of extremely low birthweight infants born in Southern Finland in 1991–1994. Acta Paediatr. 2001, 90, 218–221. [Google Scholar] [CrossRef]
- Tommiska, V.; Heinonen, K.; Kero, P.; Pokela, M.-L.; Tammela, O.; Järvenpää, A.-L.; Salokorpi, T.; Virtanen, M.; Fellman, V. A national two year follow up study of extremely low birthweight infants born in 1996–1997. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F29–F35. [Google Scholar] [CrossRef] [Green Version]
- Leversen, K.T.; Sommerfelt, K.; Rønnestad, A.; Kaaresen, P.I.; Farstad, T.; Skranes, J.; Støen, R.; Elgen, I.B.; Rettedal, S.; Eide, G.E.; et al. Predicting neurosensory disabilities at two years of age in a national cohort of extremely premature infants. Early Hum. Dev. 2010, 86, 581–586. [Google Scholar] [CrossRef]
- Surveillance of Cerepraly Palsy in Europe (SCPE). Prevalence and characteristics of children with cerebral palsy in Europe. Dev. Med. Child. Neurol. 2002, 44, 633–640. [Google Scholar]
- Australian Cerebral Palsy Register (ACPR). Australian Cerebral Palsy Register Report. 2018. Available online: https://cpregister.com/wp-content/uploads/2019/02/Report-of-the-Australian-Cerebral-Palsy-Register-Birth-Years-1995-2012.pdf (accessed on 27 April 2021).
- Premature Baby Foundation of Taiwan. Available online: https://www.pbf.org.tw/service_02_04 (accessed on 18 March 2021).
- Lu, J.F.R.; Hsiao, W.C. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff. 2003, 22, 77–88. [Google Scholar] [CrossRef]
- Cheng, T.M. Taiwan′s new national health insurance program: Genesis and experience so far. Health Aff. 2003, 22, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.F.; Chen, P.; Li, C.Y. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology 2010, 52, 155–163. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, Y.H.; Chen, H.F.; Su, Y.H.; Su, H.F.; Li, C.Y. Risk of Parkinson disease onset in patients with diabetes: A 9-year population-based cohort study with age and sex stratifications. Diabetes Care 2012, 35, 1047–1049. [Google Scholar] [CrossRef] [Green Version]
- Cutler, S.J.; Ederer, F. Maximum utilization of the life table method in analyzing survival. J. Chronic Dis. 1958, 8, 699–712. [Google Scholar] [CrossRef]
- Hollung, A.J.; Vik, T.; Wiik, R.; Bakken, I.J.; Andersen, G.L. Completeness and correctness of cerebral palsy diagnoses in two health registers: Implications for estimating prevalence. Dev. Med. Child. Neurol. 2017, 59, 402–406. [Google Scholar] [CrossRef]
- Chang, M.J.; Ma, H.I.; Lu, T.H. Estimating the prevalence of cerebral palsy in Taiwan: A comparison of difference case definitions. Res. Dev. Disabil. 2015, 36, 207–212. [Google Scholar] [CrossRef]
- Boychuck, Z.; Anderson, J.; Fehlings, D.; Kirton, A.; Oskoui, M.; Shevell, M.; Majnemer, A. Current referral practices for diagnosis and intervention for children with cerebral palsy: A national environment scan. J. Pediatr. 2020, 216, 173–180. [Google Scholar] [CrossRef]
- Hirvonen, M.; Ojala, R.; Korhonen, P.; Haataja, P.; Eriksson, K.; Gissler, M.; Luukkaala, T.; Tammela, O. Cerebral palsy among children born moderately and late preterm. Pediatrics 2014, 134, e1584–e1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Promotion Administration, Ministry of Health and Welfare in Taiwan. Available online: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1139 (accessed on 18 March 2021).
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
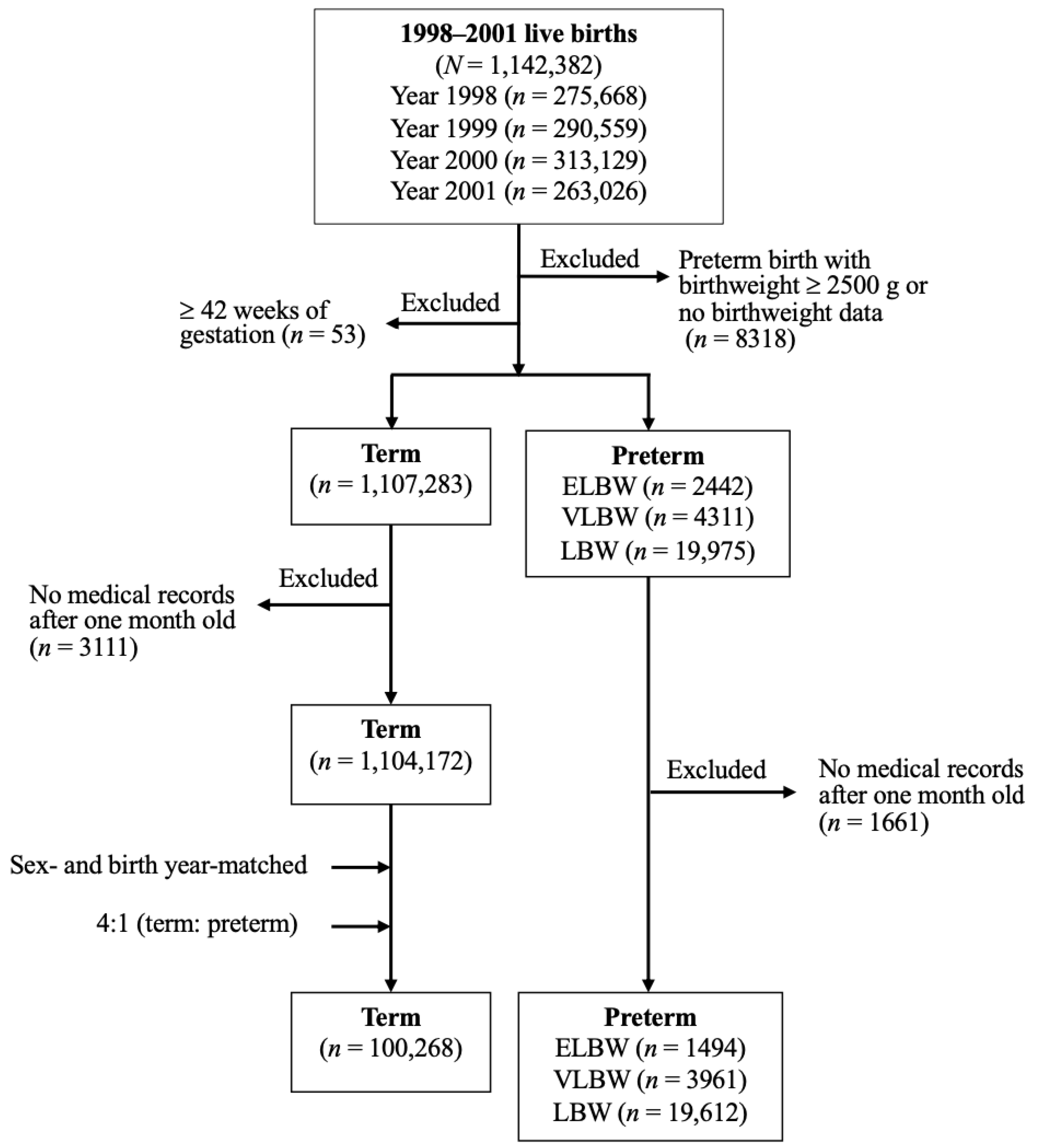
| n (%) | p | ||||
|---|---|---|---|---|---|
| Term | Preterm LBW (1500–2499 g) | Preterm VLBW (1000–1499 g) | Preterm ELBW (<1000 g) | ||
| Total number (n) | 100,268 | 19,612 | 3961 | 1494 | |
| Sex | * | ** | 0.0005 | ||
| Male | 53,660 (53.52) | 10,629 (54.20) | 2040 (51.50) | 746 (49.93) | |
| Female | 46,608 (46.48) | 8983 (45.80) | 1921 (48.50) | 748 (50.07) | |
| Region | ** | * | 0.0006 | ||
| North | 49,857 (49.72) | 9779 (49.86) | 1976 (48.89) | 753 (50.40) | |
| Central | 28,301 (28.23) | 5633 (28.72) | 1049 (26.48) | 373 (24.97) | |
| South | 16,865 (16.82) | 3168 (16.15) | 691 (17.45) | 274 (18.34) | |
| East | 5245 (5.23) | 1032 (5.26) | 245 (6.19) | 94 (6.29) | |
| Urbanization | 0.0655 | ||||
| Urban | 52,615 (52.47) | 10,505 (53.56) | 2044 (51.60) | 763 (51.07) | |
| Suburban | 36,710 (36.61) | 7022 (35.80) | 1493 (37.69) | 559 (37.42) | |
| Rural | 10,943 (10.91) | 2085 (10.63) | 424 (10.70) | 172 (11.51) | |
| Insured Salary Grade (NTD) | *** | *** | *** | <0.0001 | |
| None | 9706 (9.68) | 3542 (18.06) | 773 (19.52) | 256 (17.14) | |
| Low (1–16,500) | 23,562 (23.50) | 4610 (23.51) | 942 (23.78) | 364 (24.36) | |
| Middle (16,501–33,300) | 46,008 (45.89) | 7879 (40.17) | 1587 (40.07) | 594 (39.76) | |
| High (>33,300) | 20,992 (20.94) | 3581 (18.26) | 659 (16.64) | 280 (18.74) | |
| Term n = 100,268 | Preterm LBW (1500–2499 g) n = 19,612 | Preterm VLBW (1000–1499 g) n = 3961 | Preterm ELBW (<1000 g) n = 1494 | p | |
|---|---|---|---|---|---|
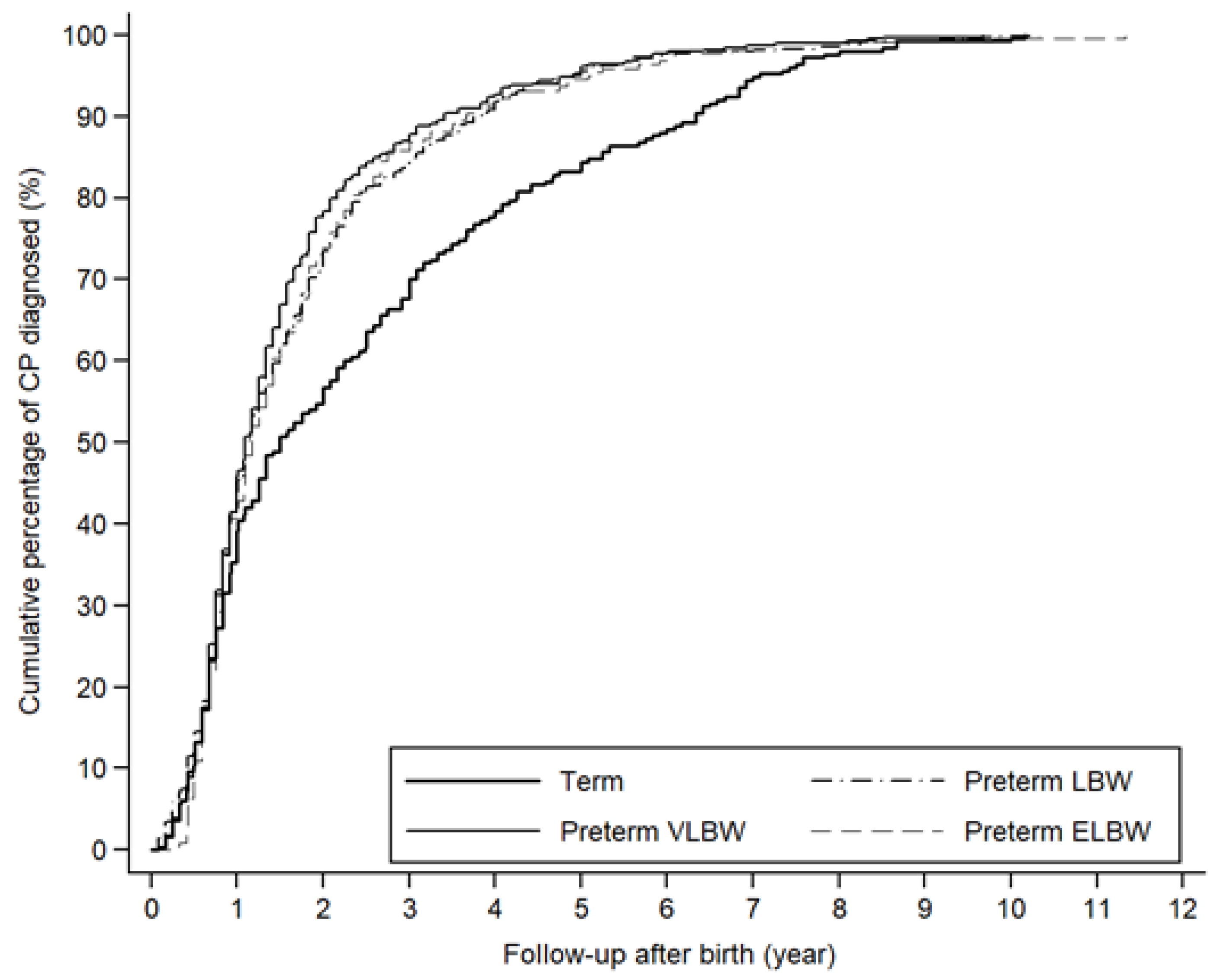
| CP prevalence, n (cases per 1000 neonatal survivors) | 254 (2.53) | 543 (27.69) | 385 (97.20) | 220 (147.26) | <0.0001 |
| AHR a (95% CI) | 1.00 | 11.08 (9.54–12.86) | 40.40 (34.48–47.34) | 62.73 (52.37–75.14) | <0.0001 |
| Cumulative Percentage | Age at First Diagnosis of CP (Year) | p | |||
|---|---|---|---|---|---|
| Term n = 203 | Preterm LBW (1500–2499 g) n = 468 | Preterm VLBW (1000–1499 g) n = 332 | Preterm ELBW (<1000 g) n = 186 | ||
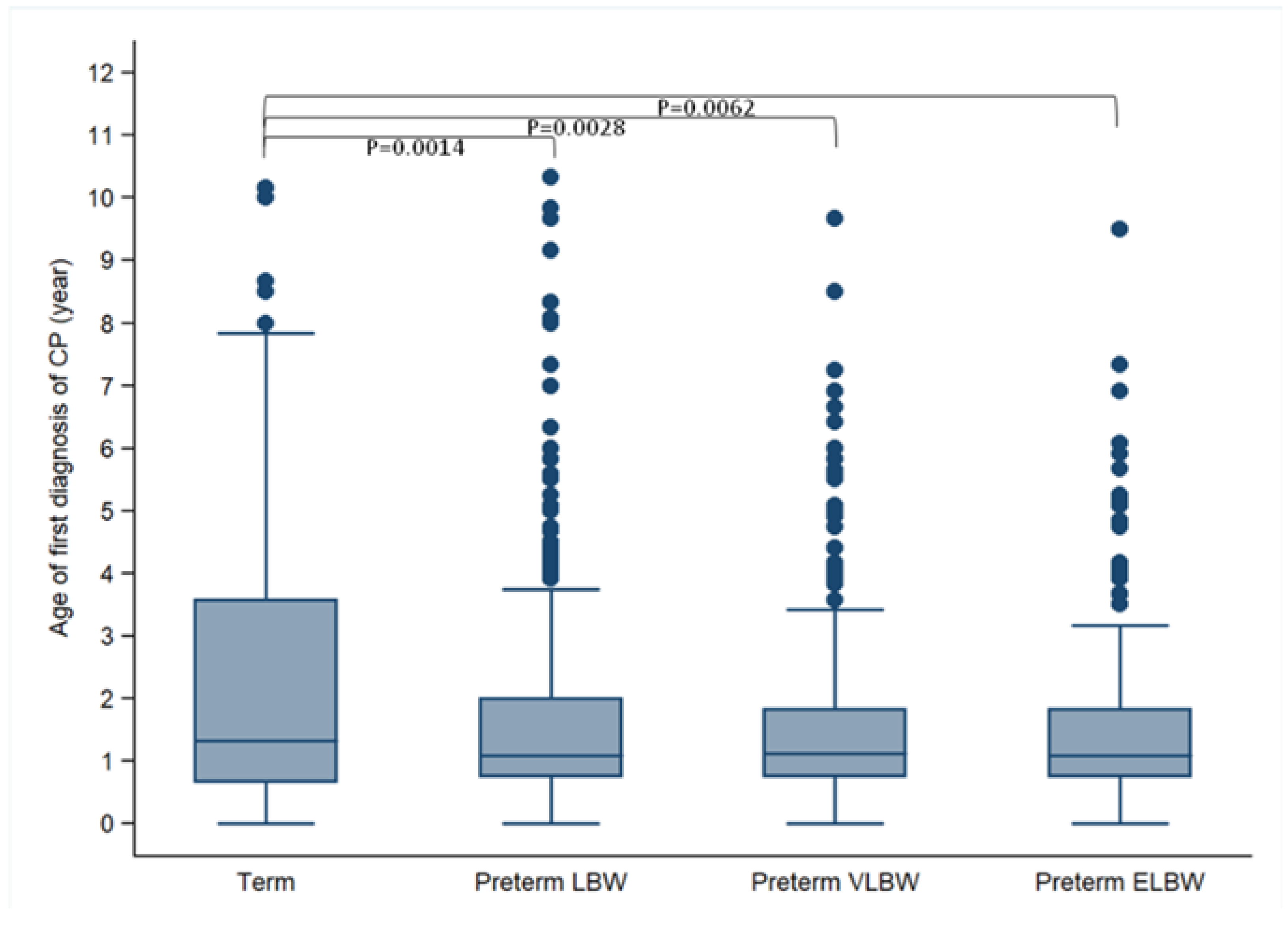
| 25% | 0.67 | 0.75 | 0.75 | 0.75 | -- |
| 50% (median) | 1.33 | 1.08 | 1.12 | 1.08 | -- |
| 75% | 3.58 | 2.00 | 1.84 | 1.83 | -- |
| 90% | 6.33 | 3.75 | 3.42 | 3.66 | -- |
| Mean (SD) | 2.41 (2.26) | 1.64 (1.58) | 1.58 (1.42) | 1.59 (1.46) | 0.0064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-H.; Hwang, Y.-S.; Ho, C.-H.; Lai, M.-C.; Chen, Y.-C.; Tsai, W.-H. Prevalence and Initial Diagnosis of Cerebral Palsy in Preterm and Term-Born Children in Taiwan: A Nationwide, Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 8984. https://doi.org/10.3390/ijerph18178984
Wang H-H, Hwang Y-S, Ho C-H, Lai M-C, Chen Y-C, Tsai W-H. Prevalence and Initial Diagnosis of Cerebral Palsy in Preterm and Term-Born Children in Taiwan: A Nationwide, Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(17):8984. https://doi.org/10.3390/ijerph18178984
Chicago/Turabian StyleWang, Hsin-Hua, Yea-Shwu Hwang, Chung-Han Ho, Ming-Chi Lai, Yu-Chin Chen, and Wen-Hui Tsai. 2021. "Prevalence and Initial Diagnosis of Cerebral Palsy in Preterm and Term-Born Children in Taiwan: A Nationwide, Population-Based Cohort Study" International Journal of Environmental Research and Public Health 18, no. 17: 8984. https://doi.org/10.3390/ijerph18178984






