Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data
Abstract
:1. Introduction
2. Osseointegration
- biocompatibility,
- form,
- implant surface,
- implant site,
- surgical technique,
- loading conditions.
3. Bone Modifications after Implant Placement/Insertion
4. General Properties of Titanium
5. Surface Treatments
5.1. Smooth Implants
5.2. Rough Surface
5.2.1. Sandblasting
5.2.2. Surfaces TPS
5.2.3. Surfaces Coated with Hydroxyapatite
5.2.4. Sandblasted and Etched Surfaces
5.2.5. Surfaces Coated with Bioactive Glass
5.2.6. Trademark Surfaces: Tioblast, Osseotite, and TiUnite
5.3. New Surface Treatment That Does Not Alter Roughness and Favors Osseointegration: Hyaluronic Acid
6. Studies on Surface Modifications of Dental Implants Using HA
6.1. Evaluation of TNF-α
6.2. Histomorphometric and Histochemical Analysis
6.3. Effects of Collagen on Healing and Bone Formation in a Rabbit Model as a Coat on Ti Implants
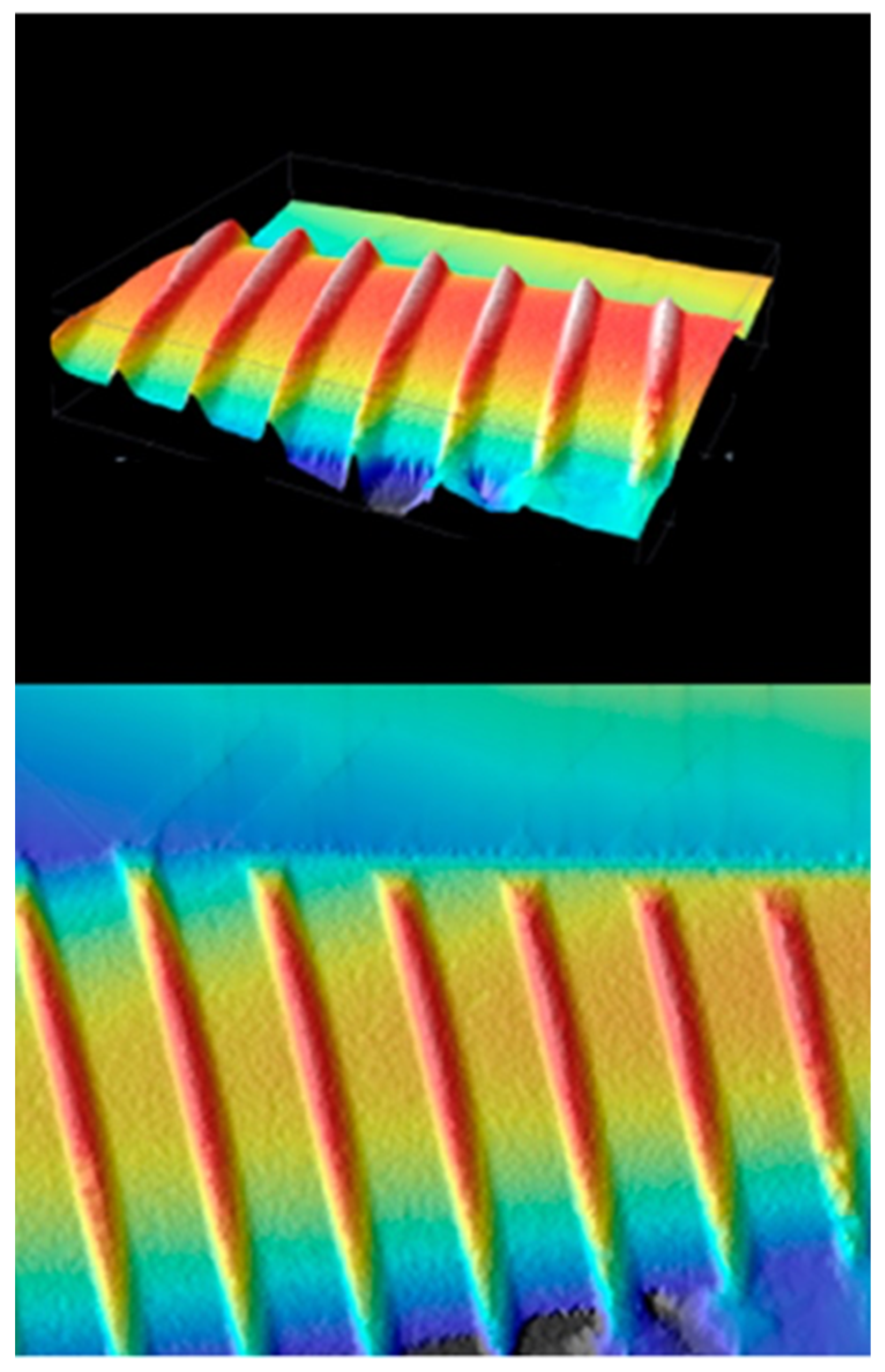
6.3.1. Surface Analysis Using XPS
6.3.2. Surface Analysis Using AFM
6.3.3. Micro-TC Evaluation
6.3.4. Histological Evaluation
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genc, T.; Duruel, O.; Kutlu, H.B.; Dursun, E.; Karabulut, E.; Tozum, T.F. Evaluation of anatomical structures and variations in the maxilla and the mandible before dental implant treatment. Dent. Med Probl. 2018, 55, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Scrascia, R.; Fiorillo, L.; Gaita, V.; Secondo, L.; Nicita, F.; Cervino, G. Implant-Supported Prosthesis for Edentulous Patient Rehabilitation. From Temporary Prosthesis to Definitive with a New Protocol: A Single Case Report. Prosthesis 2020, 2, 10–24. [Google Scholar] [CrossRef] [Green Version]
- De Stefano, R. Psychological Factors in Dental Patient Care: Odontophobia. Medicina 2019, 55, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorillo, L.; Cervino, G.; De Stefano, R.; Iannello, G.; Cicciù, M. Socio-economic behaviours on dental profession: An in Italy google trends investigation. Minerva Stomatol. 2020, 69, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, A.; Kanazawa, H.; Ishige, T.; Kishimoto, S. A missing denture. Lancet 2004, 364, 1884. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Ortensi, L.; Vitali, T.; Bonfiglioli, R.; Grande, F. New Tricks in the Preparation Design for Prosthetic Ceramic Laminate Veeners. Prosthesis 2019, 1, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Cicciù, M. Prosthesis: New Technological Opportunities and Innovative Biomedical Devices. Prosthesis 2019, 1, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Vozza, I.; Barone, A.; Quaranta, M.; De Paolis, G.; Covani, U.; Quaranta, A. A comparison between endodontics and implantology: An 8-year retrospective study. Clin. Implant. Dent. Relat. Res. 2013, 15, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [Green Version]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin. Implant. Dent. Relat. Res. 2018, 20, 199–207. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants: A systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Wang, Q.; Hong, Y.B.; Huang, M.D.; Wang, Q.M.; Teng, W. Constructing self-adhesive and robust functional films on titanium resistant to mechanical damage during dental implanting. Mater. Sci. Eng. C 2020, 110, 110688. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, E.; Magán-Fernández, A.; O’Valle, F.; Bravo, M.; Mesa, F. Hyaluronic acid reduces inflammation and crevicular fluid IL-1β concentrations in peri-implantitis: A randomized controlled clinical trial. J. Periodontal Implant. Sci. 2021, 51, 63–74. [Google Scholar] [CrossRef]
- Rauso, R.; Federico, F.; Zerbinati, N.; De Cicco, D.; Nicoletti, G.F.; Tartaro, G. Hyaluronic Acid Injections to Correct Lips Deformity Following Surgical Removal of Permanent Implant. J. Craniofacial Surg. 2020, 31, e604–e606. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed]
- Areevijit, K.; Dhanesuan, N.; Luckanagul, J.A.; Rungsiyanont, S. Biocompatibility study of modified injectable hyaluronic acid hydrogel with mannitol/BSA to alveolar bone cells. J. Biomater. Appl. 2021, 35, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Loney, R.W.; Lee, C.J.; Michaud, P.L.; Cook, T.J.A. Use of a dental surveyor to ensure optimal seating of implant overdenture attachments. J. Prosthet. Dent. 2019, 121, 381–383. [Google Scholar] [CrossRef]
- Könönen, M.; Hormia, M.; Kivilahti, J.; Hautaniemi, J.; Thesleff, I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J. Biomed. Mater. Res. 1992, 26, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Lausmaa, J.; Kasemo, B.; Hansson, S. Accelerated oxide growth on titanium implants during autoclaving caused by fluorine contamination. Biomaterials 1985, 6, 23–27. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Aspects of surface physics on titanium implants. Swed. Dent. J. Suppl. 1985, 28, 19–36. [Google Scholar] [PubMed]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: A surface science approach. Int. J. Oral Maxillofac. Implants 1988, 3, 247–259. [Google Scholar] [PubMed]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: On the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res. 1988, 22, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Herford, A.S.; Cervino, G.; Troiano, G.; Lauritano, F.; Laino, L. Tissue fluorescence imaging (VELscope) for quick non-invasive diagnosis in oral pathology. J. Craniofacial Surg. 2017, 28, e112–e115. [Google Scholar] [CrossRef] [PubMed]
- Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, D.; Sinigaglia, S.; Pancera, P.; Faccioni, P.; Portelli, M.; Luciano, U.; Cosola, S.; Penarrocha, D.; Bertossi, D.; Nocini, R.; et al. An overview of socket preservation. J. Boil. Regul. Homeost. Agents 2019, 33, 55–59. [Google Scholar]
- Yilmaz, B.; Salaita, L.G.; Seidt, J.D.; Clelland, N.L.; McGlumphy, E.A. Load to failure of different titanium abutments for an internal hexagon implant. J. Prosthet. Dent. 2015, 114, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pruncu, C.I.; Gupta, M.K.; Mia, M.; Khan, A.M.; Jamil, M.; Pimenov, D.Y.; Sen, B.; Sharma, V.S. Investigations of Machining Characteristics in the Upgraded MQL-Assisted Turning of Pure Titanium Alloys Using Evolutionary Algorithms. Materials 2019, 12, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrangelo, F.; Quaresima, R.; Abundo, R.; Spagnuolo, G.; Marenzi, G. Esthetic and Physical Changes of Innovative Titanium Surface Properties Obtained with Laser Technology. Materials 2020, 13, 1066. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; D’Amico, C.; Campagna, P.; Terranova, A.; Militi, A. Dental Materials Implant Alloys: An X-ray Fluorescence Analysis On Fds76®. Minerva Stomatol. 2021, 69, 370–376. [Google Scholar] [CrossRef]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017, 60, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [Green Version]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Wazen, R.M.; Lefebvre, L.P.; Baril, E.; Nanci, A. Initial evaluation of bone ingrowth into a novel porous titanium coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. Nanoporosity Stimulates Cell Spreading and Focal Adhesion Formation in Cells with Mutated Paxillin. ACS Appl. Mater. Interfaces 2020, 12, 14924–14932. [Google Scholar] [CrossRef]
- Variola, F.; Zalzal, S.F.; Leduc, A.; Barbeau, J.; Nanci, A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomed. 2014, 9, 2319–2325. [Google Scholar] [CrossRef] [Green Version]
- Bueno Rde, B.; Adachi, P.; Castro-Raucci, L.M.; Rosa, A.L.; Nanci, A.; Oliveira, P.T. Oxidative nanopatterning of titanium surfaces promotes production and extracellular accumulation of osteopontin. Braz. Dent. J. 2011, 22, 179–184. [Google Scholar] [CrossRef]
- Ariganello, M.B.; Guadarrama Bello, D.; Rodriguez-Contreras, A.; Sadeghi, S.; Isola, G.; Variola, F.; Nanci, A. Surface nanocavitation of titanium modulates macrophage activity. Int. J. Nanomed. 2018, 13, 8297–8308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conserva, E.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Meto, A.; Colombari, B.; Blasi, E.; Bellini, P.; de Pol, A.; Consolo, U.; et al. Evaluation of Biological Response of STRO-1/c-Kit Enriched Human Dental Pulp Stem Cells to Titanium Surfaces Treated with Two Different Cleaning Systems. Int. J. Mol. Sci. 2019, 20, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meto, A.; Conserva, E.; Liccardi, F.; Colombari, B.; Consolo, U.; Blasi, E. Differential Efficacy of Two Dental Implant Decontamination Techniques in Reducing Microbial Biofilm and Re-Growth onto Titanium Disks In Vitro. Appl. Sci. 2019, 9, 3191. [Google Scholar] [CrossRef] [Green Version]
- Quaranta, A.; Poli, O.; Vozza, I. A case report of a TPS dental implant rigidly connected to a natural tooth: 19-year follow-up. Ann. Stomatol. 2013, 4, 263–268. [Google Scholar]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Kubo, T.; Kajihara, S.; Makihara, Y.; Oue, H.; Oki, Y.; Perrotti, V.; Piattelli, A.; Akagawa, Y.; Tsuga, K. A stability evaluation of a novel titanium dental implant/interconnected porous hydroxyapatite complex under functional loading conditions. Dent. Mater. J. 2017, 36, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odusote, J.K.; Danyuo, Y.; Baruwa, A.D.; Azeez, A.A. Synthesis and characterization of hydroxyapatite from bovine bone for production of dental implants. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019836829. [Google Scholar] [CrossRef]
- Rühling, A.; Hellweg, A.; Kocher, T.; Plagmann, H.C. Removal of HA and TPS implant coatings and fibroblast attachment on exposed surfaces. Clin. Oral Implant. Res. 2001, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, M.; Zanotto, E.D. Bioactive and inert dental glass-ceramics. J. Biomed. Mater. Res. Part A 2017, 105, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traini, T.; Degidi, M.; Strocchi, R.; Caputi, S.; Piattelli, A. Collagen fiber orientation near dental implants in human bone: Do their organization reflect differences in loading? J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 538–546. [Google Scholar] [CrossRef]
- Kaya, O.A.; Muglali, M.; Torul, D.; Kaya, I. Peri-implant bone defects: A 1-year follow-up comparative study of use of hyaluronic acid and xenografts. Niger. J. Clin. Pract. 2019, 22, 1388–1395. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Dalir, E.; Vahedi, P.; Hasanzadeh, A.; Samiei, M. The Potential Applications of Hyaluronic Acid Hydrogels in Biomedicine. Drug Res. 2020, 70, 6–11. [Google Scholar] [CrossRef]
- Hasan, M.; Al-Ghaban, N. The effects of hyaluronic acid on bone-implant interface in RABBITS (immunohistochemical study for TNF-α). IJABR 2017, 7, 733–738. [Google Scholar]
- Yazan, M.; Kocyigit, I.D.; Atil, F.; Tekin, U.; Gonen, Z.B.; Onder, M.E. Effect of hyaluronic acid on the osseointegration of dental implants. Br. J. Oral Maxillofac. Surg. 2019, 57, 53–57. [Google Scholar] [CrossRef]
- Guler, B.; Uraz, A.; Çetiner, D. The chemical surface evaluation of black and white porous titanium granules and different commercial dental implants with energy-dispersive x-ray spectroscopy analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 352–359. [Google Scholar] [CrossRef]
- Germano, F.; Bramanti, E.; Arcuri, C.; Cecchetti, F.; Cicciù, M. Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur. J. Dent. 2013, 7, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Suchánek, J.; Ivančaková, R.K.; Mottl, R.; Browne, K.Z.; Pilneyová, K.C.; Pilbauerová, N.; Schmidt, J.; Suchánková Kleplová, T. Hyaluronic Acid-Based Medical Device for Treatment of Alveolar Osteitis-Clinical Study. Int. J. Environ. Res. Public Health 2019, 16, 3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Cranio-Maxillofac. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef]
- Levine, R.A.; Huynh-Ba, G.; Cochran, D.L. Soft tissue augmentation procedures for mucogingival defects in esthetic sites. Int. J. Oral Maxillofac. Implants 2014, 29, 155–185. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Chang, J.W.; Koo, W.Y.; Kim, E.K.; Lee, S.W.; Lee, J.H. Facial Rejuvenation Using a Mixture of Calcium Hydroxylapatite Filler and Hyaluronic Acid Filler. J. Craniofacial Surg. 2020, 31, e18–e21. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.C.; Dhalkari, C.D.; Indurkar, M.S. Hyaluronic Acid: Ray of Hope for Esthetically Challenging Black Triangles: A Case Series. Contemp. Clin. Dent. 2020, 11, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Meto, A. Immediate Loading of Dental Implants Using Flapless Technique with Electric Welding. Balk. J. Stomatol. 2013, 17, 162–168. [Google Scholar]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Fiorillo, L.; Romano, G.L. Gels in Medicine and Surgery: Current Trends and Future Perspectives. Gels 2020, 6, 48. [Google Scholar] [CrossRef]
- Schwarz, F.; Sanz-Martín, I.; Kern, J.S.; Taylor, T.; Schaer, A.; Wolfart, S.; Sanz, M. Loading protocols and implant supported restorations proposed for the rehabilitation of partially and fully edentulous jaws. Camlog Foundation Consensus Report. Clin. Oral Implant. Res. 2016, 27, 988–992. [Google Scholar] [CrossRef]
| Alloy | Chemical Composition |
|---|---|
| Grade I | Ti (2.15 Fe; 0.12 O2) |
| Grade II | Ti (0.20 Fe; 0.18 O2) |
| Grade III | Ti (0.25 Fe; 0.25 O2) |
| Grade IV | Ti (0.30 Fe; 0.35 O2) |
| Grade V | Ti (0.06 Al; 0.04 V) |
| Grade V Dental Implant Sample | Titanium (Ti) (91.9%) Aluminum (Al) (4.05%) Vanadium (V) (3.89%) Iron (Fe) (0.12%) Molibdenum (Mo) (<0.008%) |
| Control Group | Test Group | |
|---|---|---|
| 1 week | Antibody positivity for fibroblasts, osteoblasts and endothelial cells. Negativity for osteoid cells | Antibody positivity for fibroblast, osteoblast, osteocites, adipocites and endothelial cells. Negativity for osteoid cells |
| 2 weeks | Bone marrow stem cells, osteoblasts, osteoclasts and osteocites antibody positivity. No bone trabeculature. | Bone marrow stem cells, osteoblasts, osteoclasts and osteocites antibody positivity. No bone trabeculature. |
| 4 weeks | Bone marrow stem cells, osteoblasts, osteoclasts and osteocites antibody positivity. No new formation bone matrix. | Bone marrow stem cells, osteoblasts, osteoclasts and osteocites antibody positivity. No new formation bone matrix. |
| Area | Control Group | Test Group |
|---|---|---|
| Bone | 2697.7 | 3252.3 |
| Bone with osteoid tissue | 4704.1 | 5887.3 |
| Control Group | Test Group | |
|---|---|---|
| 15 days | Trabecular bone near to dental implant surface. Few inflammatory cells. Mean BIC 22.42 ± 4.5%, BAIT 23 ± 0.8%, BAOT 19 ± 0.8% | Trabecular bone near to dental implant surface. Numerous osteoblast in contact with dental implant surface. Mean BIC 27.5 ± 3.1%, BAIT 31 ± 0.8%, BAOT 21.8 ± 1% |
| 30 days | Mature bone in contact with dental implant surface. Osteoblasts presence and absence of inflammatory exudate. Mean BIC 51.2 ± 3.9%, BAIT 28 ± 0.8%, BAOT 36 ± 0.8% | Mature bone with Haversian organization in contact with dental implant surface. Osteoblasts activity. Mean BIC 55.3 ± 3.2%, BAIT 39 ± 2.2%, BAOT 38 ± 2.2% |
| 60 days | Mature bone in contact with dental implant surface. Osteoblasts activity. Mean BIC 53.32 ± 3.2%, BAIT 35 ± 2.3%, BAOT 36 ± 2.3% | Mature bone organization in contact with dental implant surface. Osteoblasts activity. Mean BIC 63.6 ± 2.9%, BAIT 42 ± 2.3%, BAOT 44 ± 2.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670. https://doi.org/10.3390/ijerph18094670
Cervino G, Meto A, Fiorillo L, Odorici A, Meto A, D’Amico C, Oteri G, Cicciù M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. International Journal of Environmental Research and Public Health. 2021; 18(9):4670. https://doi.org/10.3390/ijerph18094670
Chicago/Turabian StyleCervino, Gabriele, Agron Meto, Luca Fiorillo, Alessandra Odorici, Aida Meto, Cesare D’Amico, Giacomo Oteri, and Marco Cicciù. 2021. "Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data" International Journal of Environmental Research and Public Health 18, no. 9: 4670. https://doi.org/10.3390/ijerph18094670











