Drag Reduction in the Flow of Aqueous Solutions of a Mixture of Cocamidopropyl Betaine and Cocamide DEA
Abstract
:1. Introduction
2. Experimental Apparatus and Procedure
2.1. Test Facility
2.2. Rheometer
2.3. Determination of Cloud Point
2.4. Materials
3. Results and Discussion
3.1. Cloud Point
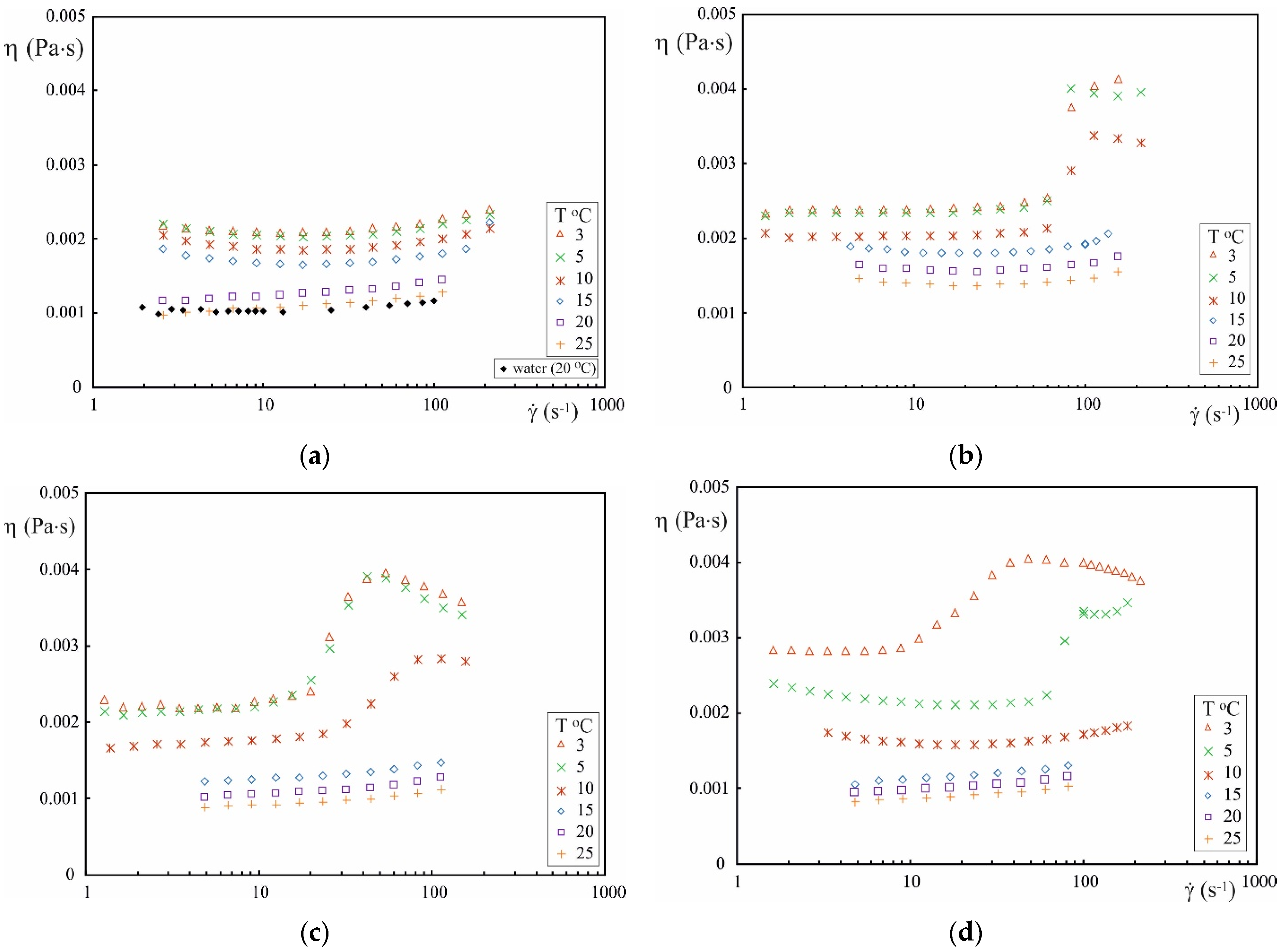
3.2. Rheological Results
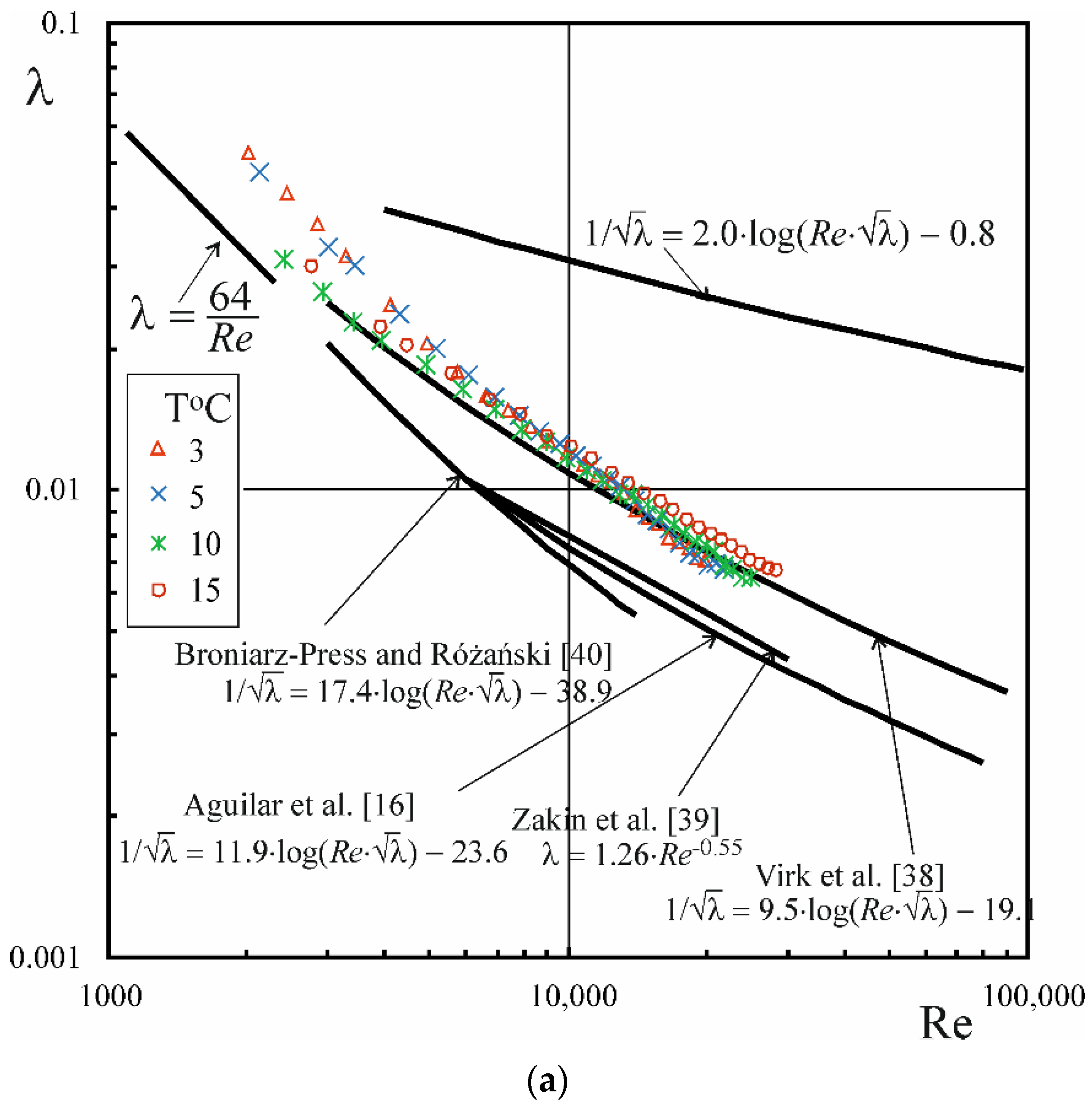
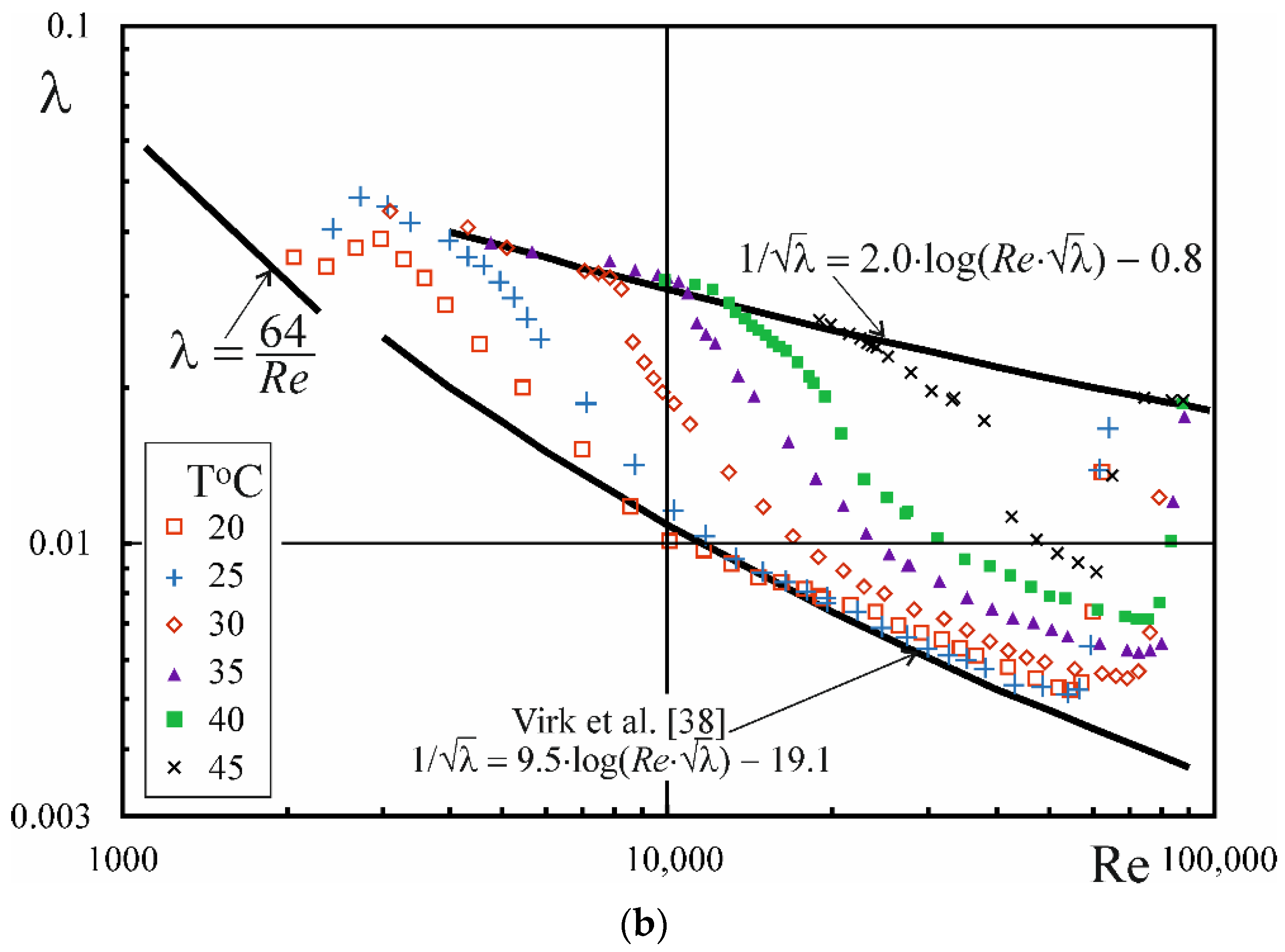
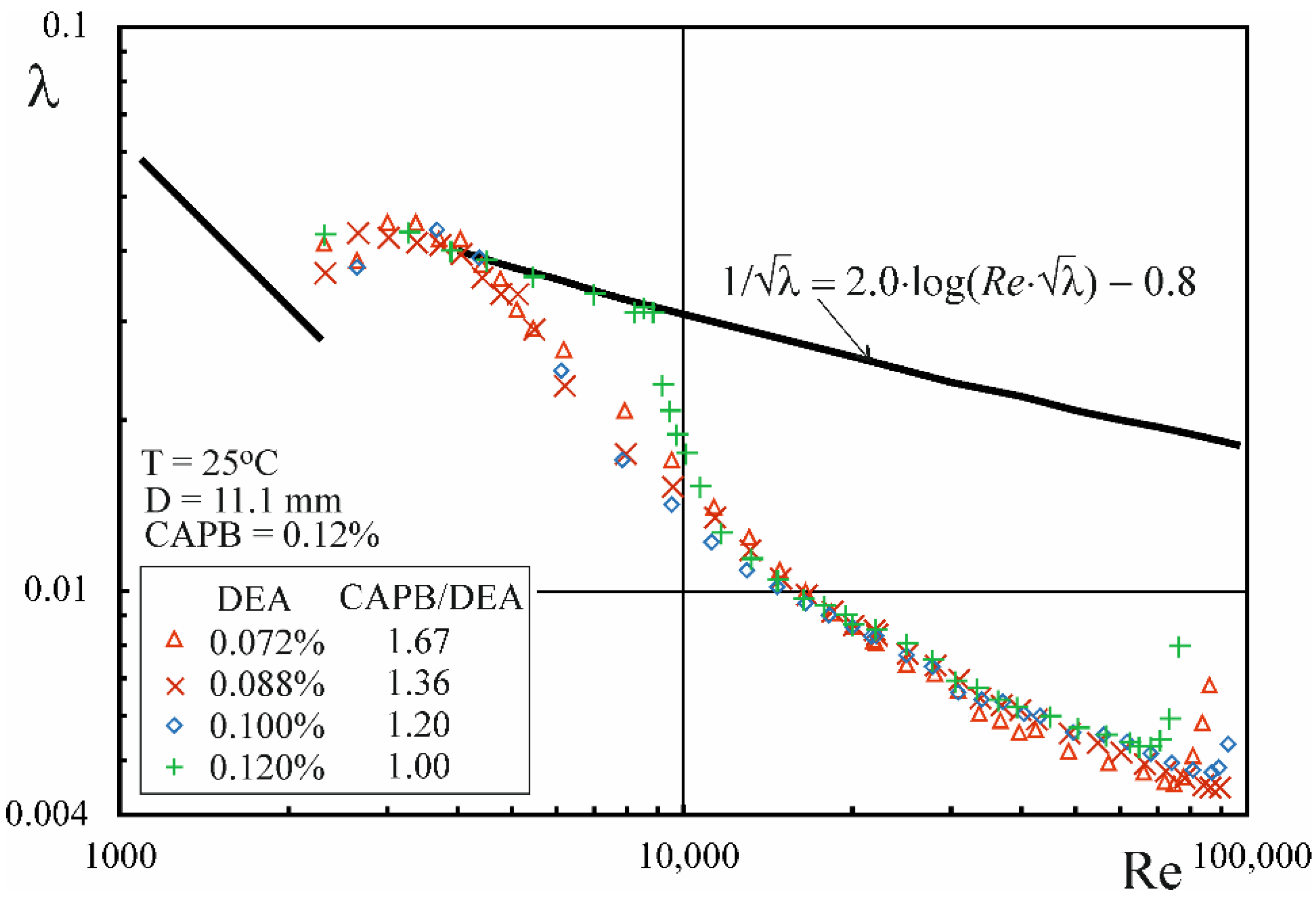
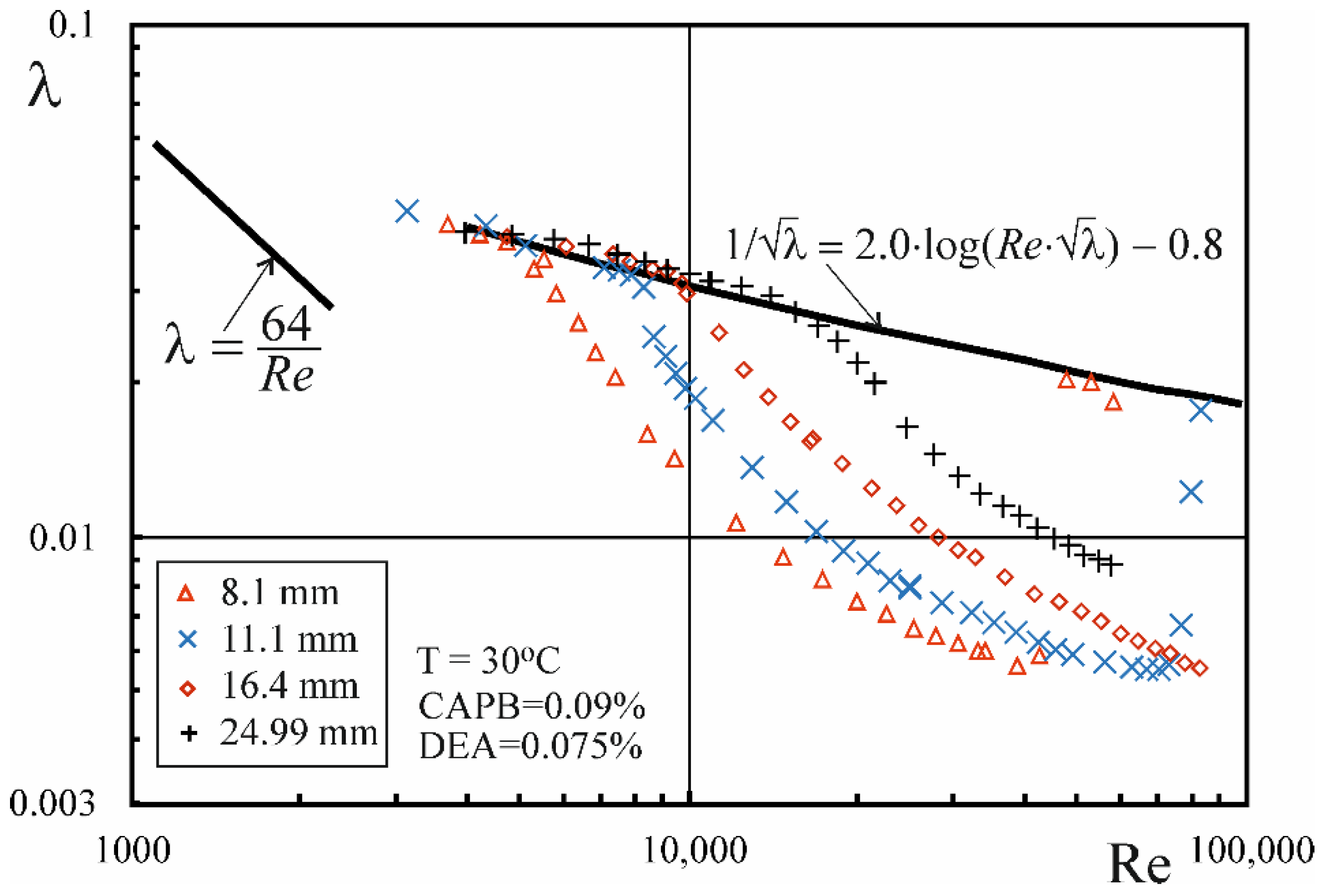
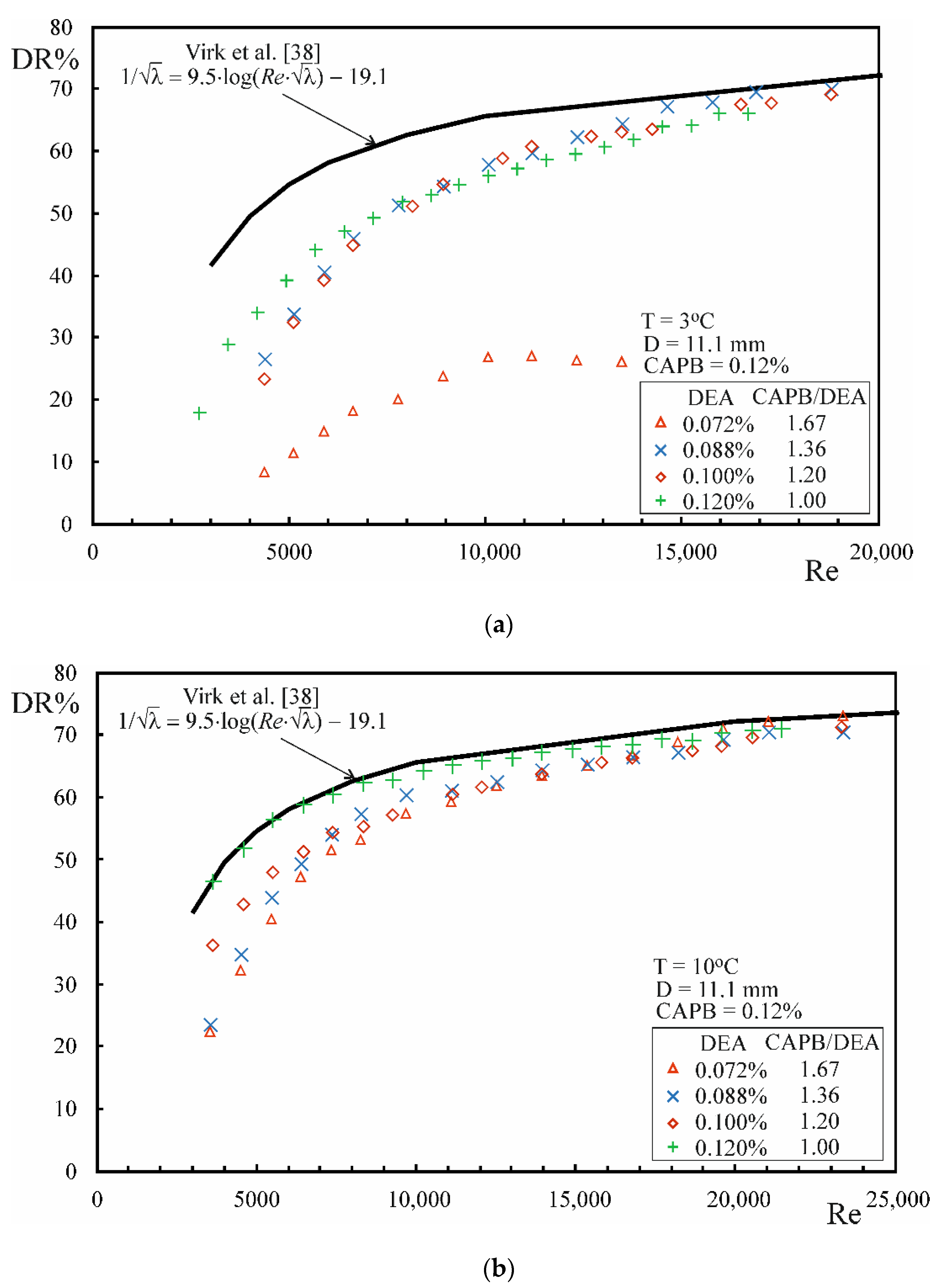
3.3. Drug Reduction
4. Conclusions
- The slope of the viscosity curves generated for aqueous solutions of CAPB/DEA points to the occurrence of the shear thickening range, which is evidence for the formation of shear-induced structure (SIS) and can indicate the presence of wormlike micelles or multilamellar vesicles in the solution.
- The phenomenon of drag reduction occurs during the flow of CAPB/DEA solutions in the temperature range from 3 to 45 °C, whereby the level of drag reduction that approximates the MDRA described by Virk et al. [38] is noted in the temperature range between 3 and 25 °C.
- In the range of higher temperatures, the onset of drag reduction during the flow of CAPB/DEA solutions occurs above a certain critical value of the Rec,0 number, whose value depends on the temperature of the solution, diameter of the pipe and the weight ratio of surfactants. At the same time, the critical value of the wall shear stress τw,c0 corresponding to the critical value of the Rec,0 number is approximately independent of pipe diameter.
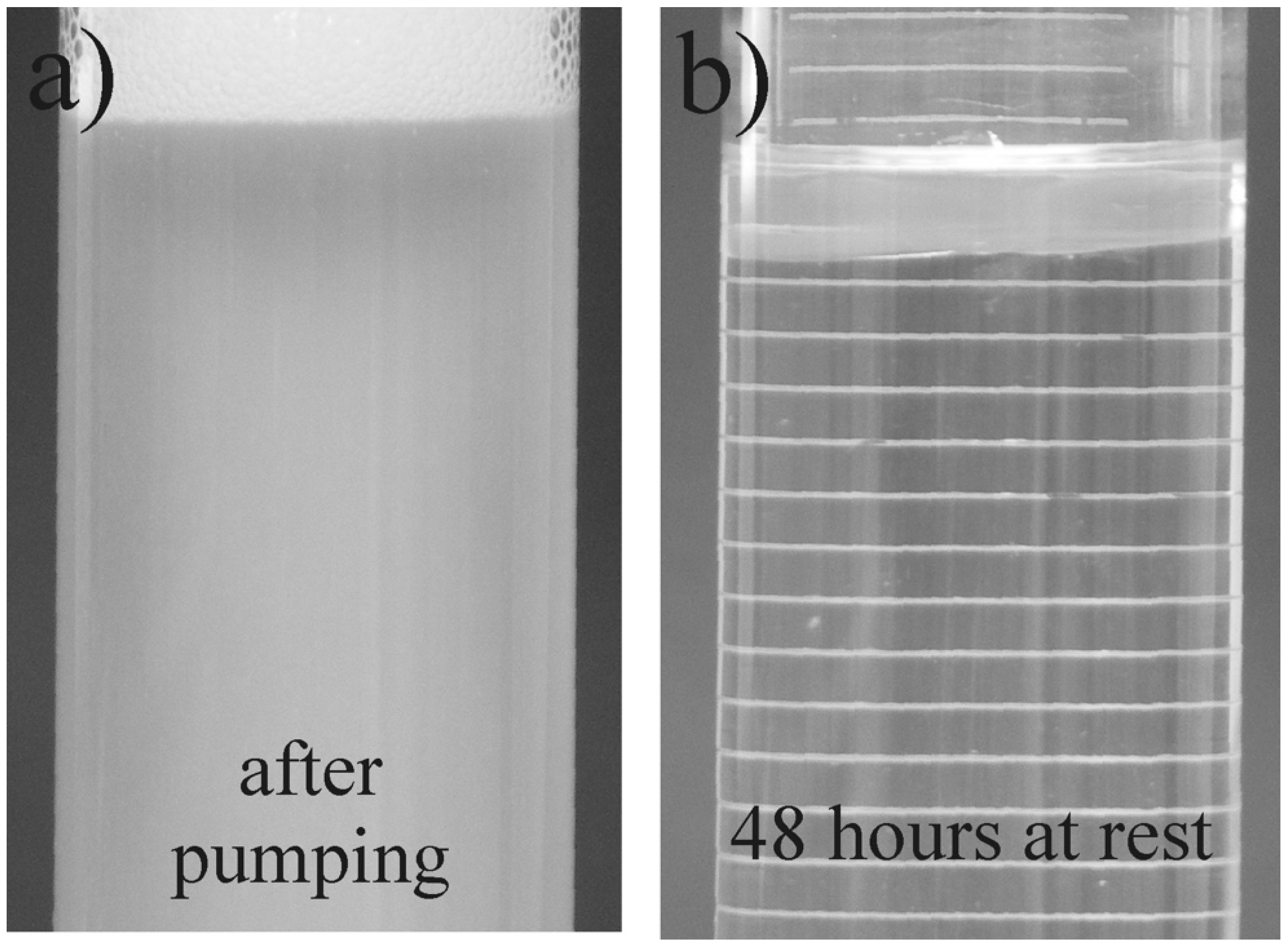
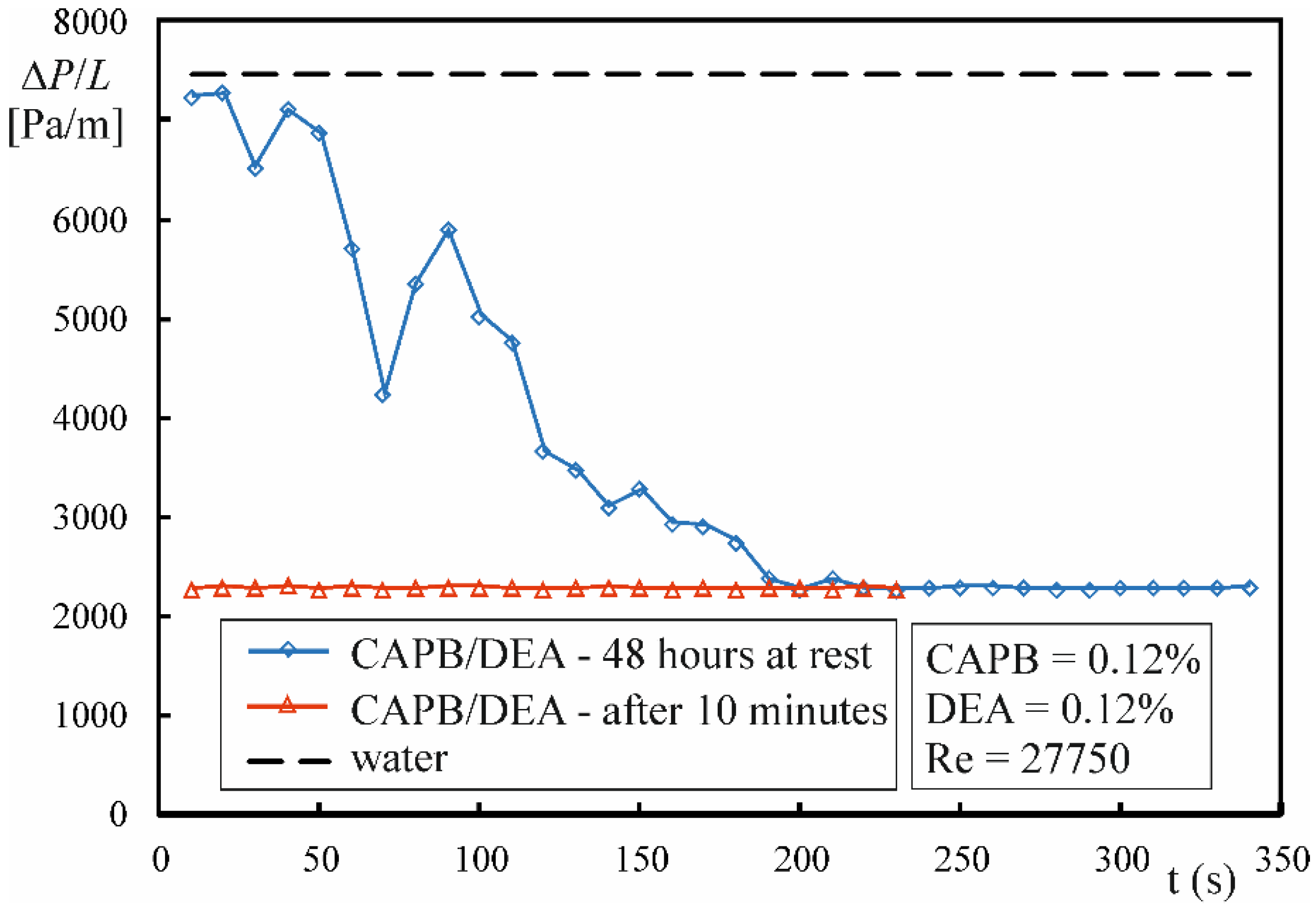
- The presence of a surfactant-rich disperse phase is a precondition for the emergence of drag reduction during the flow of CAPB/DEA solutions. The critical value of the wall shear stress τw,c0 is most likely responsible for the transformation of solution microstructure during the flow.
- The lower temperature limit at which drag reduction occurs depends on the CAPB and DEA weight ratio in the solution. However, when the excess of CAPB is too large, it gives rise to an increase in the surface charge of the micelles, leading to their shortening and disappearance of drag reduction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| D | inner diameter, (m) |
| DR | drag reduction ratio, (%) |
| L | distance between the two pressure taps for ΔP measurements, (m) |
| Re | Reynolds number, (UDρ)/η |
| U | bulk velocity, (m/s) |
| Greek letters | |
| ΔP | pressure drop, (Pa) |
| λ | Darcy friction factor |
| ρ | fluid density, (kg/m3) |
| η | shear viscosity, (Pa·s) |
| τ | shear stress, (Pa) |
| τw | wall shear stress, (ΔPD)/(4L), (Pa) |
| shear rate, (s−1) | |
| Subscripts | |
| c | critical |
| s | surfactant |
| t | turbulent flow |
References
- Zakin, J.L.; Lu, B.; Bewersdorff, H.W. Surfactant Drag Reduction. Rev. Chem. Eng. 1998, 14, 253–320. [Google Scholar] [CrossRef]
- Broniarz-Press, L.; Różański, J.; Różańska, S. Drag Reduction Effect in Pipe Systems and Lquid Falling Film Flow. Rev. Chem. Eng. 2007, 23, 149–245. [Google Scholar] [CrossRef]
- Matras, Z.; Malcher, T.; Gzyl-Malcher, B. The Influence of Polymer-Surfactant Aggregates on Drag Reduction. Thin Solid Film 2008, 516, 8848–8851. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. Synergistic Effects of Anionic Surfactant and Nonionic Polymer Additives on Drag Reduction. Chem. Eng. Commun. 2013, 200, 935–958. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. The Role of Surfactants in Mechanical Degradation of Drag-Reducing Polymers. Ind. Eng. Chem. Res. 2013, 52, 1291–1302. [Google Scholar] [CrossRef]
- White, A. Flow Characteristics of Complex Soap Systems. Nature 1967, 214, 585–586. [Google Scholar] [CrossRef]
- Savins, J.G. A Stress-Controlled Drag-Reduction Phenomenon. Rheol. Acta 1967, 6, 323–367. [Google Scholar] [CrossRef]
- Różański, J. Flow of Drag-Reducing Surfactant Solutions in Rough Pipes. J. Non-Newton. Fluid Mech. 2011, 166, 279–288. [Google Scholar] [CrossRef]
- Różański, J. Heat Transfer in the Thermal Entrance Region for Drag Reduction Surfactant Solutions in Pipe Flow. Int. J. Heat Mass Transf. 2012, 55, 1113–1125. [Google Scholar]
- Lin, Z.; Chou, L.; Lu, B.; Zheng, Y.; Davis, H.T.; Scriven, L.E.; Talmon, Y.; Zakin, J.L. Experimental Studies on Drag Reduction and Rheology of Mixed of Cationic Surfactants with Different Alkyl Chain Lengths. Rheol Acta 2000, 39, 354–359. [Google Scholar] [CrossRef]
- Hellsten, M. Drag-Reducing Surfactants. J. Surfactants Deterg. 2002, 5, 65–70. [Google Scholar] [CrossRef]
- Zakin, J.L.; Chiang, J.L. Non-Ionic Surfactants as Drag Reducing Additives. Nature 1972, 239, 26–28. [Google Scholar] [CrossRef]
- Zakin, J.L.; Lui, H.-L. Variables affecting drag reduction by nonionic surfactant additives. Chem. Eng. Commun. 1983, 23, 77–88. [Google Scholar] [CrossRef]
- Hellsten, M.; Harwigsson, I. Use of Alkoxylated Alkanolamide as Friction-Reducing Agent. U.S. Patent No. 5,339,855, 23 August 1994. [Google Scholar]
- Gasljevic, K.; Aguilar, G.; Matthys, E.F. On Two Distinct Types of Drag-Reducing Fluids, Diameter Scaling, and Turbulent Profiles. J. Non-Newton. Fluid Mech. 2001, 96, 405–425. [Google Scholar] [CrossRef]
- Aguilar, G.; Gasljevic, K.; Matthys, E.F. Asymptotes of Maximum Friction and Heat Transfer Reductions for Drag-Reducing Surfactant Solutions. Int. J. Heat Mass Transf. 2001, 44, 2835–2843. [Google Scholar] [CrossRef]
- Usui, H.; Kariyama, H.; Saeki, T.; Sugawara, H.; Wakui, F. Effect of Surfactant Molecular Structure on Turbulent Drag Reduction. Kagaku Kogaku Ronbunshu 1998, 24, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Haruki, N.; Inaba, H.; Horibe, A.; Kodama, Y. Flow Resistance and Heat Transfer Characteristics of Organic Brine (Propylene Glycol) Solution by Adding Flow Drag Reduction Additive. Exp. Heat Transf. 2009, 22, 283–299. [Google Scholar] [CrossRef]
- Haruki, N.; Inaba, H.; Horibe, A.; Tanaka, S. Viscosity Measurements of Ethylene Glycol Solution with Flow Drag Reduction Additives. Heat Transf. Asian Res. 2006, 35, 553–557. [Google Scholar] [CrossRef]
- Tamano, S.; Ikarashi, H.; Morinishi, Y.; Taga, K. Drag Reduction and Degradation of Nonionic Surfactant Solutions with Organic Acid in Turbulent Pipe Flow. J. Non-Newton. Fluid Mech. 2015, 215, 1–7. [Google Scholar] [CrossRef]
- Dosunmu, I.T.; Shah, S.N. Turbulent Flow Behavior of Surfactant Solutions in Straight Pipes. J. Petrol Sci. Eng. 2014, 124, 323–330. [Google Scholar] [CrossRef]
- Harwigsson, I.; Hellsten, M. Environmentally Acceptable Drag-Reducing Surfactants for District Heating and Cooling. J. Am. Oil Chem. Soc. 1996, 73, 921–928. [Google Scholar] [CrossRef]
- Qi, Y.; Kawaguchi, Y.; Christensen, R.N.; Zakin, J.L. Enhancing Heat-Transfer Ability of Drag Reducing Surfactant Solutions with Static Mixer and Honeycombs. Int. J. Heat Mass Transf. 2003, 46, 5161–5173. [Google Scholar] [CrossRef]
- Qi, Y.; Kawaguchi, Y.; Lin, Z.; Ewing, M.; Christensen, R.N.; Zakin, J.L. Enhanced Heat Transfer of Drag Reducing Surfactant Solutions with Fluted Tube-in-Tube Heat Exchanger. Int. J. Heat Mass Transf. 2001, 44, 1495–1505. [Google Scholar] [CrossRef]
- Myska, J.; Chara, Z. The Effect of Zwitterionic and Cationic Surfactant in Turbulent Flows. Exp. Fluids 2001, 30, 229–236. [Google Scholar] [CrossRef]
- Inaba, H.; Aly, W.I.A.; Haruki, N.; Horibe, A. Flow and Heat Transfer Characteristic of Drag Reducing Surfactant Solution in Helically Coiled Pipe. Heat Mass Transf. 2005, 41, 940–952. [Google Scholar] [CrossRef]
- Aly, W.I.A.; Inaba, H.; Haruki, N.; Horibe, A. Drag and Heat Transfer Reduction Phenomena of Drag-Reducing Surfactant Solutions in Straight and Helical Pipes. J. Heat Transf. 2006, 128, 800–810. [Google Scholar] [CrossRef]
- Cho, S.-H.; Tae, C.-S.; Zaheeruddin, M. Effect of Fluid Velocity, Temperature, and Concentration of Non-Ionic Surfactants on Drag Reduction. Energy Convers. Manag. 2007, 48, 913–918. [Google Scholar] [CrossRef]
- Różański, J. Pressure Loss and Heat Transfer during the Flow of Surfactant Solutions. Habilitation Dissertation, Poznan University of Technology, Poznan, Poland, 2015. [Google Scholar]
- Keera, S.T.; Deyab, M.A. Effect of Some Organic Surfactants on the Electrochemical Behaviour of Carbon Steel in Formation Water. Coll. Surf. A Physicochem Eng. Asp. 2005, 266, 129–140. [Google Scholar] [CrossRef]
- Mukherjee, P.; Padhan, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Clouding Behaviour in Surfactant Systems. Adv. Coll. Interf. Sci. 2011, 162, 59–79. [Google Scholar] [CrossRef]
- Hu, Y.; Matthys, E.F. Rheological and Rheo-Optical Characterization of Shear-Induced Structure Formation in a Nonionic Drag-Reducing Surfactant Solution. J. Rheol. 1997, 41, 151–166. [Google Scholar] [CrossRef]
- Qi, Y.; Zakin, J.L. Chemical and Rheological Characterization of Drag-Reducing Cationic Surfactant Systems. Ind. Eng. Chem. Res. 2002, 41, 6326–6336. [Google Scholar] [CrossRef]
- Lerouge, S.; Berret, J.F. Shear-Induced Transitions and Instabilities in Surfactant Wormlike Micelles. Adv. Polym. Sci. 2010, 230, 1–71. [Google Scholar]
- Gasljevic, K.; Matthys, E.F. Friction and Heat Transfer in Drag-Reducing Surfactant Solution Flow Through Curved Pipes and Elbows. Eur. J. Mech. B/Fluids 2009, 28, 641–650. [Google Scholar] [CrossRef]
- Tuan, N.A.; Mizunuma, H. High-Shear Drag Reduction of Surfactant Solutions. J. Non-Newton. Fluid Mech. 2013, 198, 71–77. [Google Scholar] [CrossRef]
- Gasljevic, K.; Hoyer, K.; Matthys, E.F. Temporary Degradation and Recovery of Drag-Reducing Surfactant Solutions. J. Rheol. 2007, 51, 645–667. [Google Scholar] [CrossRef]
- Virk, P.S.; Mickley, H.S.; Smith, K.A. The Ultimate Asymptote and Mean Flow Structure in Toms Phenomenon. J. Appl. Mech. 1970, 37, 488–493. [Google Scholar] [CrossRef]
- Zakin, J.L.; Myska, J.; Chara, Z. New Limiting Drag Reduction and Velocity Profile Asymptotes for Nonpolymeric Additives Systems. AIChE J. 1996, 42, 3544–3546. [Google Scholar] [CrossRef]
- Broniarz-Press, L.; Różański, J. Region of Maximal Drag Reduction in Flow of Surfactant Solutions. Chem. Proc. Eng. 2004, 28, 733–738. [Google Scholar]
- Tamano, S.; Itoh, M.; Kato, K.; Kokota, K. Turbulent Drag Reduction in Nonionic Surfactant Solutions. Phys. Fluids 2010, 22, 055102–055112. [Google Scholar] [CrossRef]
- Virk, P.S.; Wagger, D.L. Aspects of Mechanisms in Type B Drag Reduction. In Structure of Turbulence and Drag Reduction; Gyr, A., Ed.; IUTAM Symposium: Zurich, Switzerland, 1989; pp. 202–215. [Google Scholar]
- MacKintosh, F.C.; Safran, S.A.; Pincus, P.A. Self-Assembly of Linear Aggregates: The Effect on Electrostatics on Growth. Europhys. Lett. 1990, 12, 697–702. [Google Scholar] [CrossRef] [Green Version]
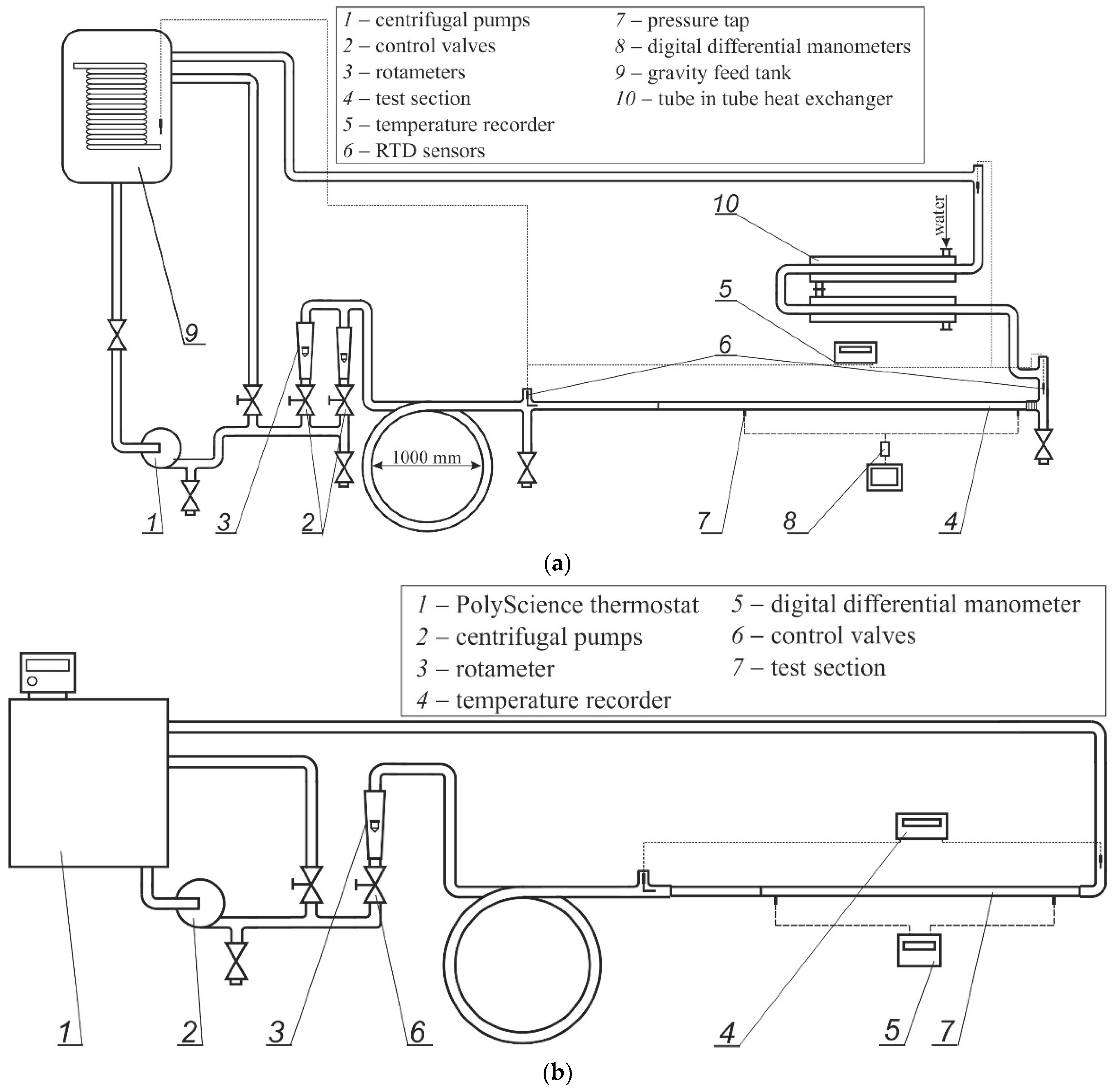

| Inner Diameter (mm) | Pipe Length (mm) | Length of Developing Region (mm) | Length of Measuring Region (mm) |
|---|---|---|---|
| 8.1 | 4000 | 500 | 3000 |
| 11.1 | 4000 | 900 | 2600 |
| 16.4 | 4000 | 900 | 2600 |
| 16.4 | 4956 | 1353 | 3300 |
| CAPB (wt%) | DEA (wt%) | CP (°C) |
|---|---|---|
| 0.09 | 0.075 | 9 |
| 0.12 | 0.072 | 24 |
| 0.12 | 0.088 | 17 |
| 0.12 | 0.100 | 4 |
| 0.12 | 0.120 | Entire range of temperature changes |
| DEA (wt%) | Rec,0 | τw,c0 (Pa) | Rec,I | τw,cI (Pa) |
|---|---|---|---|---|
| 0.072 | 4050 | 0.57 | 74,600 | 21.88 |
| 0.088 | 4050 | 0.56 | - | - |
| 0.100 | 4380 | 0.65 | - | - |
| 0.120 | 8860 | 2.67 | 67,730 | 26.44 |
| D | 8.1 (mm) | 11.1 (mm) | 16.4 (mm) | 24.99 (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Rec,0 | τw,c0 (Pa) | Rec,0 | τw,c0 (Pa) | Rec,0 | τw,c0 (Pa) | Rec,0 | τw,c0 (Pa) |
| 30 | 4743 | 1.12 | 7852 | 1.29 | 9721 | 0.93 | 15565 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różański, J.; Różańska, S.; Mitkowski, P.T.; Szaferski, W.; Wagner, P.; Frankiewicz, A. Drag Reduction in the Flow of Aqueous Solutions of a Mixture of Cocamidopropyl Betaine and Cocamide DEA. Energies 2021, 14, 2683. https://doi.org/10.3390/en14092683
Różański J, Różańska S, Mitkowski PT, Szaferski W, Wagner P, Frankiewicz A. Drag Reduction in the Flow of Aqueous Solutions of a Mixture of Cocamidopropyl Betaine and Cocamide DEA. Energies. 2021; 14(9):2683. https://doi.org/10.3390/en14092683
Chicago/Turabian StyleRóżański, Jacek, Sylwia Różańska, Piotr Tomasz Mitkowski, Waldemar Szaferski, Patrycja Wagner, and Adrianna Frankiewicz. 2021. "Drag Reduction in the Flow of Aqueous Solutions of a Mixture of Cocamidopropyl Betaine and Cocamide DEA" Energies 14, no. 9: 2683. https://doi.org/10.3390/en14092683






