Regulatory T Cells but Not Tumour-Infiltrating Lymphocytes Correlate with Tumour Invasion Depth in Basal Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
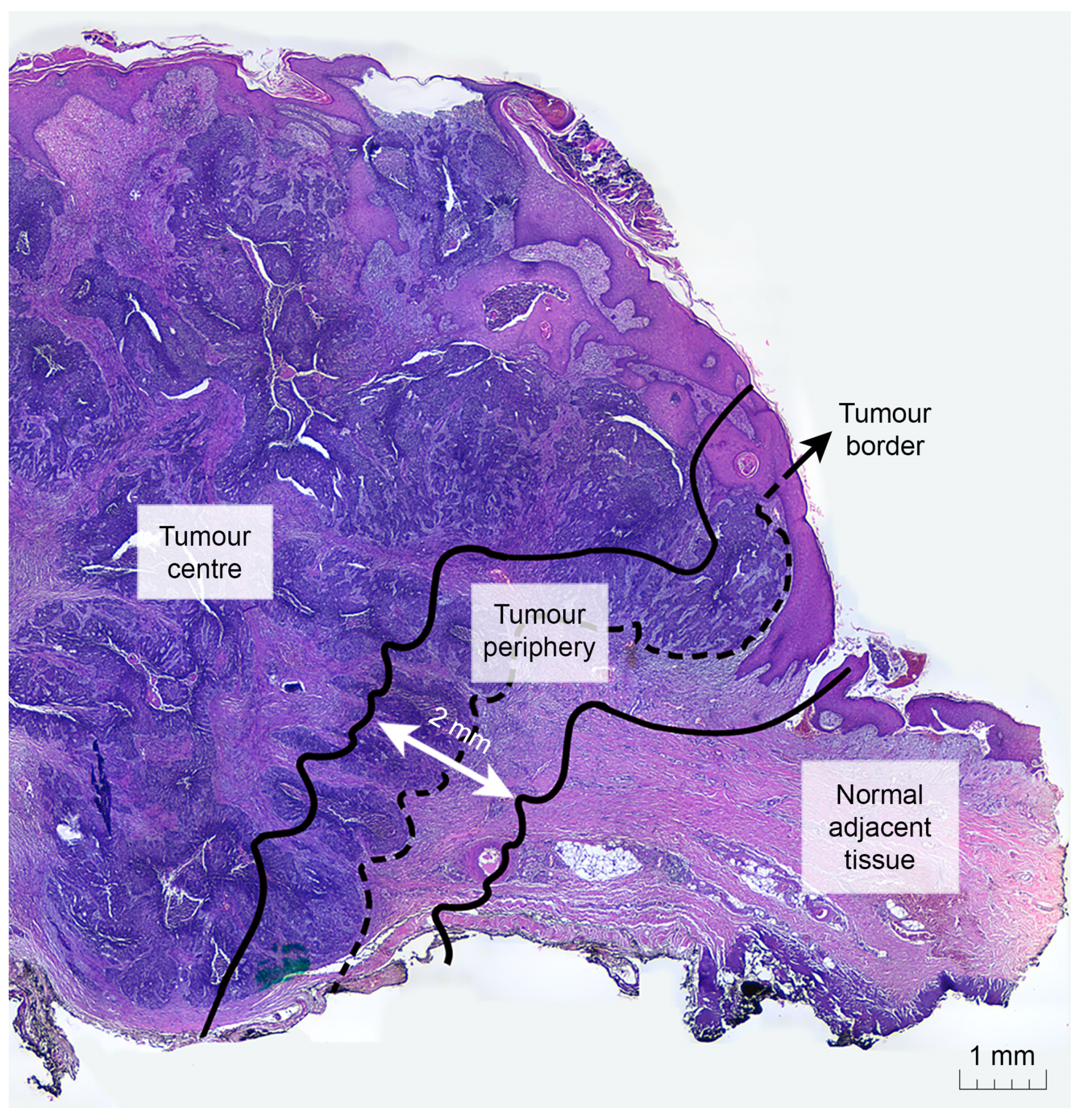
2.1. Sample Specifications and Collection
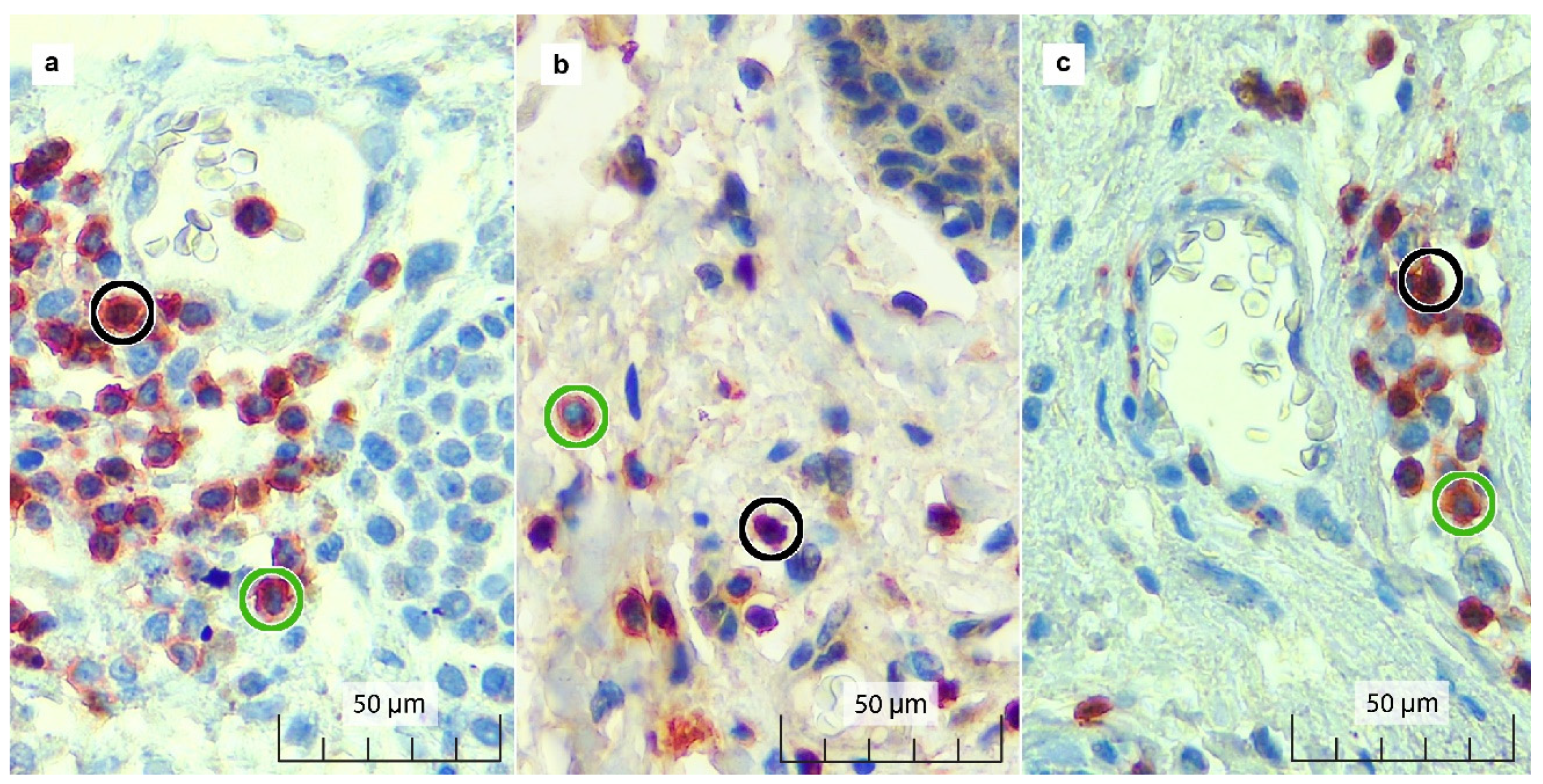
2.2. Immunohistochemistry (IHC): FOXP3/CD3 Double Staining
2.3. Immunostaining and Pathological Parameter Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and Treatment of Basal Cell Carcinoma: European Consensus–Based Interdisciplinary Guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatino, F.; Marra, A.; Liguori, L.; Scognamiglio, G.; Fusciello, C.; Botti, G.; Ferrone, S.; Pepe, S. Resistance to Anti-PD-1-Based Immunotherapy in Basal Cell Carcinoma: A Case Report and Review of the Literature. J. Immunother. Cancer 2018, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.; Nielsen, P.; Gjerdrum, L.; Gniadecki, R. Immunosuppressive Environment in Basal Cell Carcinoma: The Role of Regulatory T Cells. Acta Derm. Venereol. 2016, 96, 917–921. [Google Scholar] [CrossRef] [Green Version]
- van der Veeken, J.; Gonzalez, A.J.; Cho, H.; Arvey, A.; Hemmers, S.; Leslie, C.S.; Rudensky, A.Y. Memory of Inflammation in Regulatory T Cells. Cell 2016, 166, 977–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.P.; Campa, M.J.; Sperlazza, J.; Conlon, D.; Joshi, M.-B.; Harpole, D.H.; Patz, E.F. Tumor Infiltrating Foxp3+ Regulatory T-Cells Are Associated with Recurrence in Pathologic Stage I NSCLC Patients. Cancer 2006, 107, 2866–2872. [Google Scholar] [CrossRef]
- Song, B.; Zhen, S.; Meng, F. T Cell Inflammation Profile after Surgical Resection May Predict Tumor Recurrence in HBV-Related Hepatocellular Carcinoma. Int. Immunopharmacol. 2016, 41, 35–41. [Google Scholar] [CrossRef]
- Liu, S.; Foulkes, W.D.; Leung, S.; Gao, D.; Lau, S.; Kos, Z.; Nielsen, T.O. Prognostic Significance of FOXP3+ Tumor-Infiltrating Lymphocytes in Breast Cancer Depends on Estrogen Receptor and Human Epidermal Growth Factor Receptor-2 Expression Status and Concurrent Cytotoxic T-Cell Infiltration. Breast Cancer Res. 2014, 16, 432. [Google Scholar] [CrossRef] [Green Version]
- West, N.R.; Kost, S.E.; Martin, S.D.; Milne, K.; deLeeuw, R.J.; Nelson, B.H.; Watson, P.H. Tumour-Infiltrating FOXP3+ Lymphocytes Are Associated with Cytotoxic Immune Responses and Good Clinical Outcome in Oestrogen Receptor-Negative Breast Cancer. Br. J. Cancer 2013, 108, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Ghatalia, P.; Gordetsky, J.; Kuo, F.; Dulaimi, E.; Cai, K.Q.; Devarajan, K.; Bae, S.; Naik, G.; Chan, T.A.; Uzzo, R.; et al. Prognostic Impact of Immune Gene Expression Signature and Tumor Infiltrating Immune Cells in Localized Clear Cell Renal Cell Carcinoma. J. Immunother. Cancer 2019, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Traverso, P.; Kalli, F.; Conteduca, G.; Tardito, S.; Curto, M.; Grillo, F.; Mastracci, L.; Bernardi, C.; Nasi, G.; et al. Residual Tumor Micro-Foci and Overwhelming Regulatory T Lymphocyte Infiltration Are the Causes of Bladder Cancer Recurrence. Oncotarget 2016, 7, 6424–6435. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasi. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, D.E.; Massi, D.; Scolyer, R.W.R. WHO Classification of Skin Tumours. In Skin Tumours; IARC Press: Lyon, France, 2018; Volume 11. [Google Scholar]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omland, S.H. Local Immune Response in Cutaneous Basal Cell Carcinoma. Dan. Med. J. 2017, 64, B5412. [Google Scholar] [PubMed]
- Maleki Vareki, S. High and Low Mutational Burden Tumors versus Immunologically Hot and Cold Tumors and Response to Immune Checkpoint Inhibitors. J. Immunother. Cancer 2018, 6, 4–8. [Google Scholar] [CrossRef]
- Ren, X.; Guo, S.; Guan, X.; Kang, Y.; Liu, J.; Yang, X. Immunological Classification of Tumor Types and Advances in Precision Combination Immunotherapy. Front. Immunol. 2022, 13, 790113. [Google Scholar] [CrossRef]
- Elsharkawy, S.S.; Elrheem, M.A.; Elrheem, S.A. The Tumor Infiltrating Lymphocytes (TILs): Did We Find the Missed Piece of the Huge Puzzle? Open J. Obstet. Gynecol. 2021, 11, 146–161. [Google Scholar] [CrossRef]
- Peng, G.-L.; Li, L.; Guo, Y.-W.; Yu, P.; Yin, X.-J.; Wang, S.; Liu, C.-P. CD8+ Cytotoxic and FoxP3+ Regulatory T Lymphocytes Serve as Prognostic Factors in Breast Cancer. Am. J. Transl. Res. 2019, 11, 5039–5053. [Google Scholar]
- Candido, J.; Hagemann, T. Cancer-Related Inflammation. J. Clin. Immunol. 2013, 33, 79–84. [Google Scholar] [CrossRef]
- Seminerio, I.; Descamps, G.; Dupont, S.; de Marrez, L.; Laigle, J.-A.; Lechien, J.; Kindt, N.; Journe, F.; Saussez, S. Infiltration of FoxP3+ Regulatory T Cells Is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 227. [Google Scholar] [CrossRef] [Green Version]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhu, M.; Shen, Y.; Zhong, Z.; Wu, B. The Prognostic Value of Intratumoral and Peritumoral Tumor-Infiltrating FoxP3+Treg Cells in of Pancreatic Adenocarcinoma: A Meta-Analysis. World J. Surg. Oncol. 2021, 19, 300. [Google Scholar] [CrossRef] [PubMed]
- Kaporis, H.G.; Guttman-Yassky, E.; Lowes, M.A.; Haider, A.S.; Fuentes-Duculan, J.; Darabi, K.; Whynot-Ertelt, J.; Khatcherian, A.; Cardinale, I.; Novitskaya, I.; et al. Human Basal Cell Carcinoma Is Associated with Foxp3+ T Cells in a Th2 Dominant Microenvironment. J. Investig. Dermatol. 2007, 127, 2391–2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, Z.; Yang, F.; Di, Y.; Li, J.; Zhou, Z.; Pillarisetty, V.G.; Fu, D. FOXP3+ Lymphocyte Density in Pancreatic Cancer Correlates with Lymph Node Metastasis. PLoS ONE 2014, 9, e106741. [Google Scholar] [CrossRef]
- Sun, X.; Feng, Z.; Wang, Y.; Qu, Y.; Gai, Y. Expression of Foxp3 and Its Prognostic Significance in Colorectal Cancer. Int. J. Immunopathol. Pharmacol. 2017, 30, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Berglund, E.; Maaskola, J.; Schultz, N.; Friedrich, S.; Marklund, M.; Bergenstråhle, J.; Tarish, F.; Tanoglidi, A.; Vickovic, S.; Larsson, L.; et al. Spatial Maps of Prostate Cancer Transcriptomes Reveal an Unexplored Landscape of Heterogeneity. Nat. Commun. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Shi, Y.; Yan, B.; Xiao, D.; Lai, W.; Pan, Y.; Jiang, Y.; Chen, L.; Mao, C.; Zhou, J.; et al. LGR5 Expression Is Controled by IKKα in Basal Cell Carcinoma through Activating STAT3 Signaling Pathway. Oncotarget 2016, 7, 27280–27294. [Google Scholar] [CrossRef] [Green Version]
- Faget, J.; Biota, C.; Bachelot, T.; Gobert, M.; Treilleux, I.; Goutagny, N.; Durand, I.; León-Goddard, S.; Blay, J.Y.; Caux, C.; et al. Early Detection of Tumor Cells by Innate Immune Cells Leads to T Reg Recruitment through CCL22 Production by Tumor Cells. Cancer Res. 2011, 71, 6143–6152. [Google Scholar] [CrossRef] [Green Version]
- Kaler, P.; Augenlicht, L.; Klampfer, L. Activating Mutations in β-Catenin in Colon Cancer Cells Alter Their Interaction with Macrophages; the Role of Snail. PLoS ONE 2012, 7, e45462. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous Malignant Melanoma. Am. J. Dermatopathol. 2014, 36, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Pyne, J.H.; Myint, E.; Hou, R.; Clark, S.P.; Wong, C.; Gorji, M. Basal Cell Carcinoma with Perineural Invasion: A Prospective Study Examining Subtype, Tumor Surface Diameter, Invasion Depth, and Anatomic Site in 3005 Consecutive Cases. J. Cutan. Pathol. 2020, 47, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, M.; Strickley, J.; Haeberle, M.T.; Brown, T.S. Depth of Invasion of Aggressive and Nonaggressive Basal Cell Carcinoma. J. Clin. Aesthet. Dermatol. 2019, 12, 12–14. [Google Scholar]
- Morgan, F.C.; Ruiz, E.S.; Karia, P.S.; Besaw, R.J.; Neel, V.A.; Schmults, C.D. Factors Predictive of Recurrence, Metastasis, and Death from Primary Basal Cell Carcinoma 2 Cm or Larger in Diameter. J. Am. Acad. Dermatol. 2020, 83, 832–838. [Google Scholar] [CrossRef]
- Mauldin, I.S.; Jo, J.; Wages, N.A.; Yogendran, L.V.; Mahmutovic, A.; Young, S.J.; Lopes, M.B.; Slingluff, C.L.; Erickson, L.D.; Fadul, C.E. Proliferating CD8+ T Cell Infiltrates Are Associated with Improved Survival in Glioblastoma. Cells 2021, 10, 3378. [Google Scholar] [CrossRef]
- Orhan, A.; Vogelsang, R.P.; Andersen, M.B.; Madsen, M.T.; Hölmich, E.R.; Raskov, H.; Gögenur, I. The Prognostic Value of Tumour-Infiltrating Lymphocytes in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2020, 132, 71–84. [Google Scholar] [CrossRef]
- Spector, M.E.; Bellile, E.; Amlani, L.; Zarins, K.; Smith, J.; Brenner, J.C.; Rozek, L.; Nguyen, A.; Thomas, D.; McHugh, J.B.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol.—Head Neck Surg. 2019, 145, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; et al. Basal Cell Skin Cancer, Version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 574–596. [Google Scholar] [CrossRef]
- Ciurea, M.E.; Cernea, D.; Georgescu, C.C.; Cotoi, O.S.; Pătraşcu, V.; Pârvănescu, H.; Popa, D.; Pârvănescu, V.; Ciurea, R.N.; Mercuţ, R. Expression of CXCR4, MMP-13 and β-Catenin in Different Histological Subtypes of Facial Basal Cell Carcinoma. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2013, 54, 939–951. [Google Scholar]
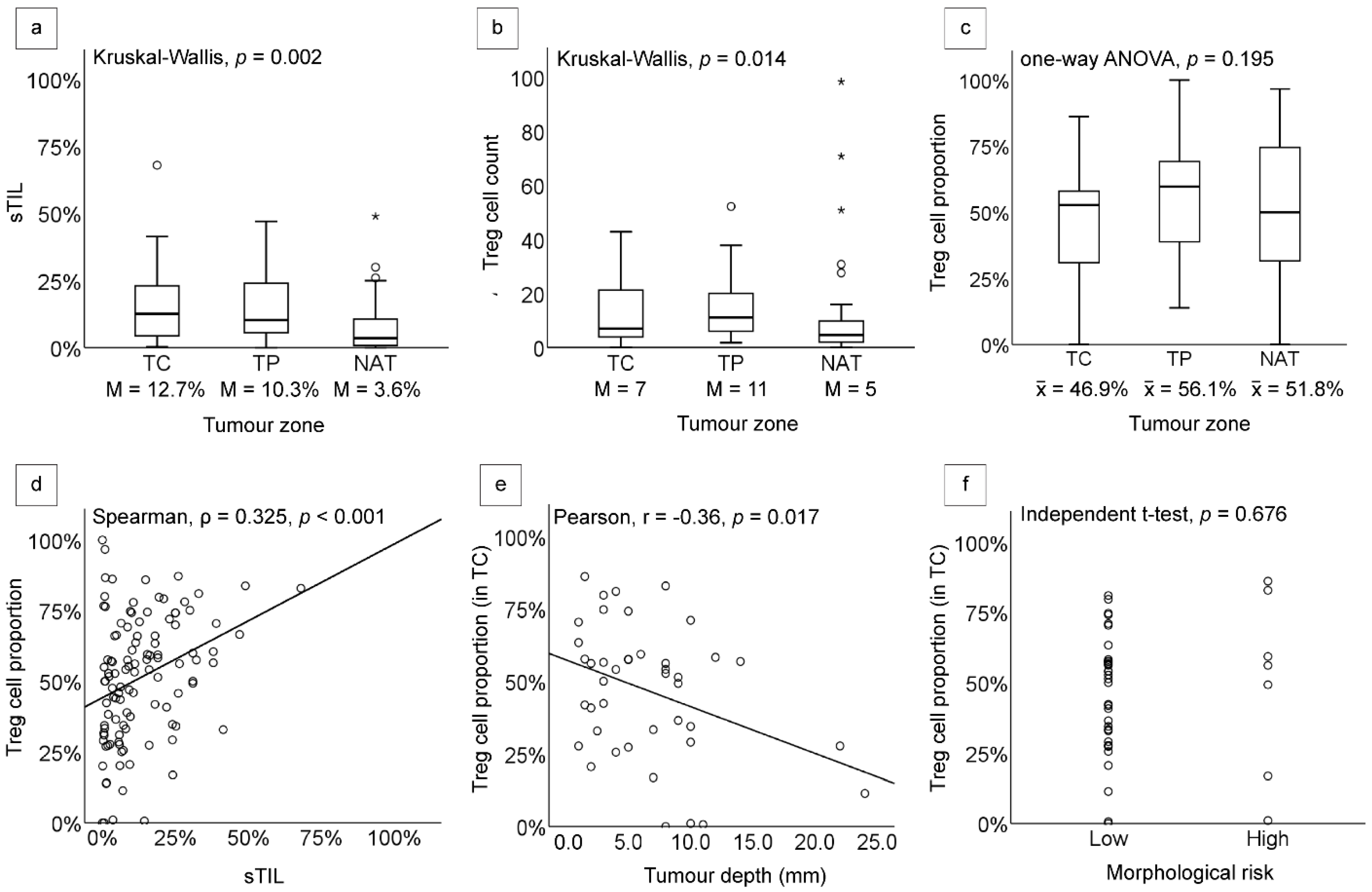
| sTILs | Treg Cell Count | Treg Cell Proportion | |||||
|---|---|---|---|---|---|---|---|
| Median (%) | p Value | Median (Cell) | p Value | Mean (%) | p Value | ||
| Tumour zone | 0.002 (Kruskal–Wallis) | 0.014 (Kruskal–Wallis) | 0.195 (ANOVA) | ||||
| TC | 12.7 | 7 | 46.9 | ||||
| TP | 10.3 | 11 | 56.1 | ||||
| NAT | 3.6 | 5 | 51.8 | ||||
| Tumour depth * | 0.076 (Spearman’s ρ) | 0.027 (Spearman’s ρ) | 0.017 (Pearson’s r) | ||||
| Morphological risk ** | |||||||
| Low | 11.1 | 0.573 (Mann–Whitney) | 9 | 0.392 (Mann–Whitney) | 46.3 | 0.676 (Independent t-test) | |
| High | 19.0 | 6 | 50.2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferronika, P.; Dhiyani, S.A.; Budiarti, T.; Widodo, I.; Rinonce, H.T.; Anwar, S.L. Regulatory T Cells but Not Tumour-Infiltrating Lymphocytes Correlate with Tumour Invasion Depth in Basal Cell Carcinoma. Diagnostics 2022, 12, 2987. https://doi.org/10.3390/diagnostics12122987
Ferronika P, Dhiyani SA, Budiarti T, Widodo I, Rinonce HT, Anwar SL. Regulatory T Cells but Not Tumour-Infiltrating Lymphocytes Correlate with Tumour Invasion Depth in Basal Cell Carcinoma. Diagnostics. 2022; 12(12):2987. https://doi.org/10.3390/diagnostics12122987
Chicago/Turabian StyleFerronika, Paranita, Safira Alya Dhiyani, Tri Budiarti, Irianiwati Widodo, Hanggoro Tri Rinonce, and Sumadi Lukman Anwar. 2022. "Regulatory T Cells but Not Tumour-Infiltrating Lymphocytes Correlate with Tumour Invasion Depth in Basal Cell Carcinoma" Diagnostics 12, no. 12: 2987. https://doi.org/10.3390/diagnostics12122987





