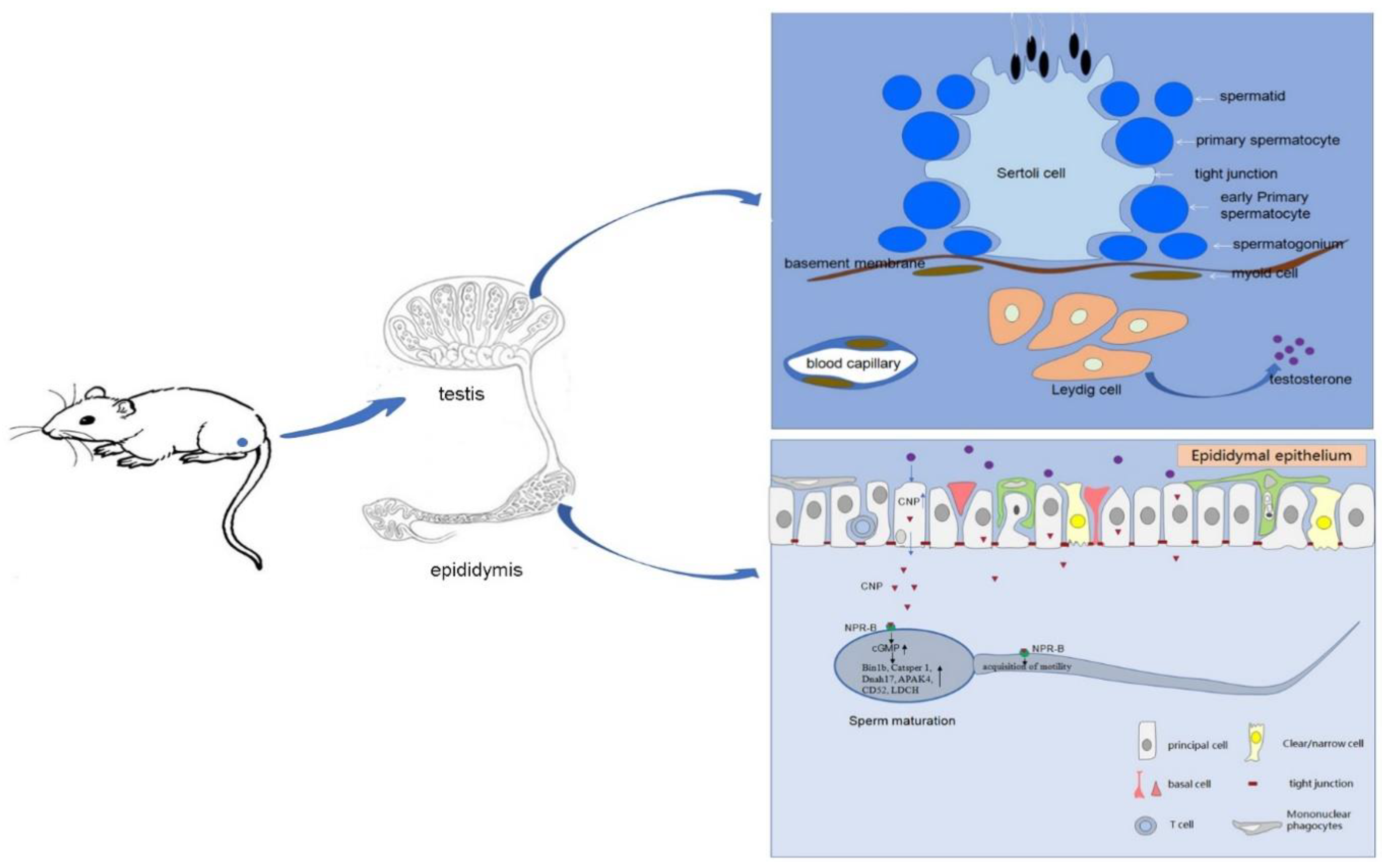
Abstract
C-type natriuretic peptide (CNP) is highly expressed in male reproductive tissues, such as the epididymis. The aim of this study is to explore the role of CNP in the maturation of rat epididymal spermatozoa. First, the expression levels of CNP and its specific natriuretic peptide receptor-B (NPR-B) were detected in various tissues of rats and epididymis at different stages after birth. Then a castrated rat model was established to analyze the relationship between testosterone and CNP/NPR-B expression in the epididymis. Finally, CNP and different inhibitors (NPR-B inhibitors, cGMP inhibitors) were used to incubate epididymal sperm in vitro to examine sperm mobility and expression of sperm maturation-related factors. The results showed CNP/NPR-B mRNAs were expressed in all tissues of rats, but were extremely highly expressed in male genital ducts (seminal vesicle, prostate and epididymis). The expression of CNP/NPR-B in epididymis was the highest at birth and the fifth week after birth. In the epididymis, CNP/NPR-B were highly expressed in the caput and located in the epididymal epithelial cells. After castration, the expression of CNP/NPR-B decreased sharply and was restored quickly after testosterone supplementation. In vitro, CNP could significantly promote the acquisition of epididymal sperm motility through the NPR-B/cGMP pathway and induce the expression of sperm maturation-related factors (such as Bin1b, Catsper 1, Dnah17, Fertilin). This study shows that CNP plays a role in epididymal sperm maturation. The mechanism of CNP is to promote the acquisition of epididymal sperm fluidity through the NPR-B/cGMP signaling pathway and also to regulate sperm maturation-related genes. Moreover, the expression of CNP/NPR-B was regulated by testosterone.
1. Introduction
The epididymis is a fundamental reproductive organ, responsible for sperm maturation, transportation and storage. The gametes produced from the testes are functionally immature and then acquire motility and fertilizing abilities during transportation in the epididymis [1]. The epididymis secretes vesicles and forms a protein matrix to provide a suitable environment for sperm maturation [2]. Therefore, the sperm protein, lipid, and small RNA content in the epididymis change greatly when they interact with these substances in the epididymal lumen [3]. For example, some secretory proteins such as P26h, sperm associated antigen 11, heat-shock protein 1 are deposited on the surface of sperm, while spermatozoal proteins (cyritestin, fertilin, CE9 and others) are also changed in this intraluminal milieu [4]. Furthermore, genetic alterations of epididymal genes can lead to decreased sperm motility, morphological abnormalities of spermatozoa, and subfertility [5].
C-type natriuretic peptide (CNP), the third member of the natriuretic peptide family, generates intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) through the binding of its specific natriuretic peptide receptor-B (NPR-B), followed by various molecular effects [6]. CNP has been shown to play a key role in female reproduction by preventing precocious meiotic maturation [7]. In male reproduction, some studies have demonstrated that CNP was extremely highly expressed in seminal plasma and epididymis [8,9,10]. Sogawa et al. [11] showed that adult NPR-B (slw/slw) mice exhibited infertility with apparently normal spermatogenesis, though the mechanism was unclear. Knockdown of NPR-B by RNAi lead to dysfunction of mouse Leydig cells via S-phase cell cycle arrest and testosterone secretion decrease [12]. CNP could regulate expression of ABP and TRF in Sertoli cells through the NPR-B/cGMP/PKG signaling pathways [13]. Kong et al. [14] found that CNP secreted by oviductal ampulla attracted sperm accumulation in the capillary through Npr2 on the midpiece of flagellum but could not attract spermatozoa from Npr2 mutant mice. Our previous studies have shown that CNP could promote sperm motility [15], induce sperm hyperactivation and acrosome reaction by the cGMP/PKG signalling pathway, via Ca2+ influx and tyrosine phosphorylation [14]. Moreover, the concentration of CNP in the semen of asthenospermia patients was lower than that of healthy people. Additionally, CNP could improve sperm motility and the reproductive function of asthenozoospermia patients [16]. The results indicated that CNP is closely related to the male reproductive function. However, the significance of this especially high CNP expression in the epididymis and its role is unclear.
In combination with the high expression of CNP in the epididymis and its influence on sperm function (such as motility and capacitation), we suspect that infertility of NPR-B (−/−) male mice may be due to the maturation defect of sperm in the epididymis, which may, in turn, lead to sperm motility disability and infertility. This study will first clarify the expression level of CNP/NPR-B in various rat tissues and their location in the epididymis. Then, a castration rat model will be established to explore the relationship between CNP/NPR-B expression and testosterone. Finally, this study will investigate the effect of CNP on sperm motility and maturation in order to elucidate the important role of CNP in the process of epididymal sperm maturation.
2. Materials and Methods
2.1. Animals
Male Sprague–Dawley (SD) rats (weighing 400 ± 20 g, 10–12 W) were purchased from the animal center of Tongji Medical College (Wuhan, Hubei, China). They were raised in a 12 h dark and 12 h light cycle and were free to obtain food and water. They were treated according to the guidelines of the Animal Research Committee [17] and approved by the Centre of Experimental Animals of Huazhong University of Science and Technology (No. S1188).
2.2. Establishment of Castrated Rat Model
Castrated rats were modeled as previously described [18]. Briefly, testicular artery ligation and bilateral testes removal were performed on 10–12 w male SD rats. Castrated rats were divided into nine groups (four in each group). The first five groups were executed after 0, 1, 3, 5, and 7 days of castration. The latter four groups were injected with androgens (3 mg/kg/d) 10 days after castration and were executed 1, 3, 5, and 7 days after the start of androgens. RNA was extracted from epididymal tissue of each group. The expression of CNP and NPR-B in rat epididymis was detected by fluorescence quantitative PCR. Serum testosterone was detected by ELISA, similar to the detection of CNP, cGMP using ELISA.
2.3. Determination of the CNP/NPR-B Expression in Different Tissues of Rats by RT-PCR
RNA was extracted from heart, liver, spleen, lung, kidney, brain, testis, epididymis, prostate, and seminal vesicle glands of 10–12 w male SD rats. As previously described, two-step, real-time reverse transcription polymerase chain reaction (RT-PCR) was performed [13]. Briefly, each sample was transferred to a 1 mL Trizol reagent. One (1) µg total RNA was reverse-transcribed into first-strand cDNA using a First Strand cDNA Synthesis Kit (Thermo, Waltham, MA, USA). Then, cDNAs were used to perform the subsequent real-time fluorescence quantitative PCR with SYBR Green Master Mix (DBI, Freiberg, Germany). The relative expression of target genes was standardized by GADPH, and the final gene expression was calculated with 2−ΔΔCT. The primer information of all revolved genes can be seen in Table 1. The experiment was repeated three times to estimate expression stability.

Table 1.
Real-time RT-PCR primers.
2.4. Protein Concentration of CNP and cGMP in the Epididymal Fluid of Rats Using ELISA
Epididymal fluids at different segments in adult male SD rats were collected. The epididymal fluids containing epididymal tissue were centrifuged at 100× g for 2 min to remove tissue fragments. The supernatant was absorbed into a new centrifuge tube and centrifuged at 1000× g for 10 min to remove the sperm and unrelated cells. CNP and cGMP concentrations were quantified using a Rat Cyclic Guanosine Monophosphate (cGMP) Elisa Kit and CNP Elisa Kit (Elabscience Biotechnology Co., Wuhan, China) following the manufacturer’s instructions.
2.5. Immunohistochemistry
Immunohistochemistry was performed as previously described [19]. Briefly, male rats were sacrificed via neck dislocation. The epididymis was removed, fixed with 4% paraformaldehyde, and embedded in paraffin. The tissues were sliced, sectioned, dewaxed, rehydrated, and subjected to antigen retrieval in a microwave oven. Then the slices were incubated with primary rabbit polyclonal antibody against CNP (sc-20952) and goat polyclonal antibody against NPR-B (sc-16870) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Negative controls used rabbit or goat non-immune serum instead of primary antibodies. After several washings with PBS, the slices were hatched with secondary antibodies (Boster Biotechnology Co., Ltd., Wuhan, China). Finally, the slices were photographed under an Olympus BX-40 microscope (Olympus Corp., Melville, NY, USA).
2.6. Expression of NPR-B in the Spermatozoa by Immunofluorescence
Indirect immunofluorescence was applied to analyze the distribution of NPR-B in the different segments of epididymal spermatozoa. In short, the spermatozoa were dried, fixed, infiltrated, treated with serum, and tested with anti-human NPR-B polyclonal antibody (1:200) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Then, the spermatozoa were incubated with fluorescein isothiocyanate binding secondary antibody (1:100) (Servicebio Technology Co., Ltd., Wuhan, China). The negative control used 0.01 mol/L phosphate buffered saline (PBS; pH 7.4), replacing the primary antibody. Immunofluorescence results were evaluated using confocal laser scanning microscopy.
2.7. Mobility and Maturation of Epididymal Sperm Treated with CNP In Vitro
The SD rats were sacrificed via neck dislocation, and the epididymis was separated [15]. Then sperm were placed in HTF medium (Irvine Scientific, Santa Ana, CA, USA) with or without CNP (10−7 mol/L) at 37 °C. Ten (10) µL of sperm suspension was extracted to observe under the microscope; 200 sperm were counted and the percentage of forward motion (PR) and non-forward motion (NP) sperm were recorded according to reference [20]; then, sperm density was calculated by a blood cell counting plate. This process was repeated three times. Next, in order to detect sperm maturation-related factors, the sperm RNAs at the caput of epididymis were extracted to perform the RT-PCR after a 6-h incubation with CNP.
2.8. Detection of the Signaling Pathway of CNP in Epididymal Sperm Motility
To explore the signal pathway of CNP in rat epididymal sperm motility, different inhibitors were added to the HTF medium. The sperm suspensions were divided into six groups: control group (PBS medium only), CNP group (10−6 mol/L), 8-Br-cGMP group (Sigma, St. Louis, MO, USA) (10−4 mol/L), CNP with KT5823 group (10−6 mol/L CNP and 10−4 mol/L KT5823, 8-Br-cGMP with KT5823 group (10−4 mol/L 8-Br-cGMP and 10−4 mol/L KT5823) and CNP with NPR-B antagonist (P19) group (10−6 mol/L CNP and 10−6 mol/L P19). After 4 h of incubation, sperm motilities were detected. After a 6-h incubation, the gene expression of sperm maturation-related factors was detected.
2.9. Statistical Analyses
All data were statistically analyzed using SPSS software, Version 23.0 (IBM Corp., Armonk, NY, USA). The means ± standard deviations (SD) and 95% confidence intervals (CI) were reported for quantitative data, and percentages were reported for categorical data. The expression of CNP and NPR-B at different segments of epididymis and sperm motility inhibited by different inhibitors following stimulation by CNP were analyzed using general linear models—repeated measures ANOVA. The expression of maturation-related genes in the epididymal caput sperm was evaluated by one-way ANOVA. A level of p < 0.05 was considered statistically significant.
3. Results
3.1. Expression of CNP/NPR-B in Different Rat Tissues and in Epididymis at Various Times after Birth
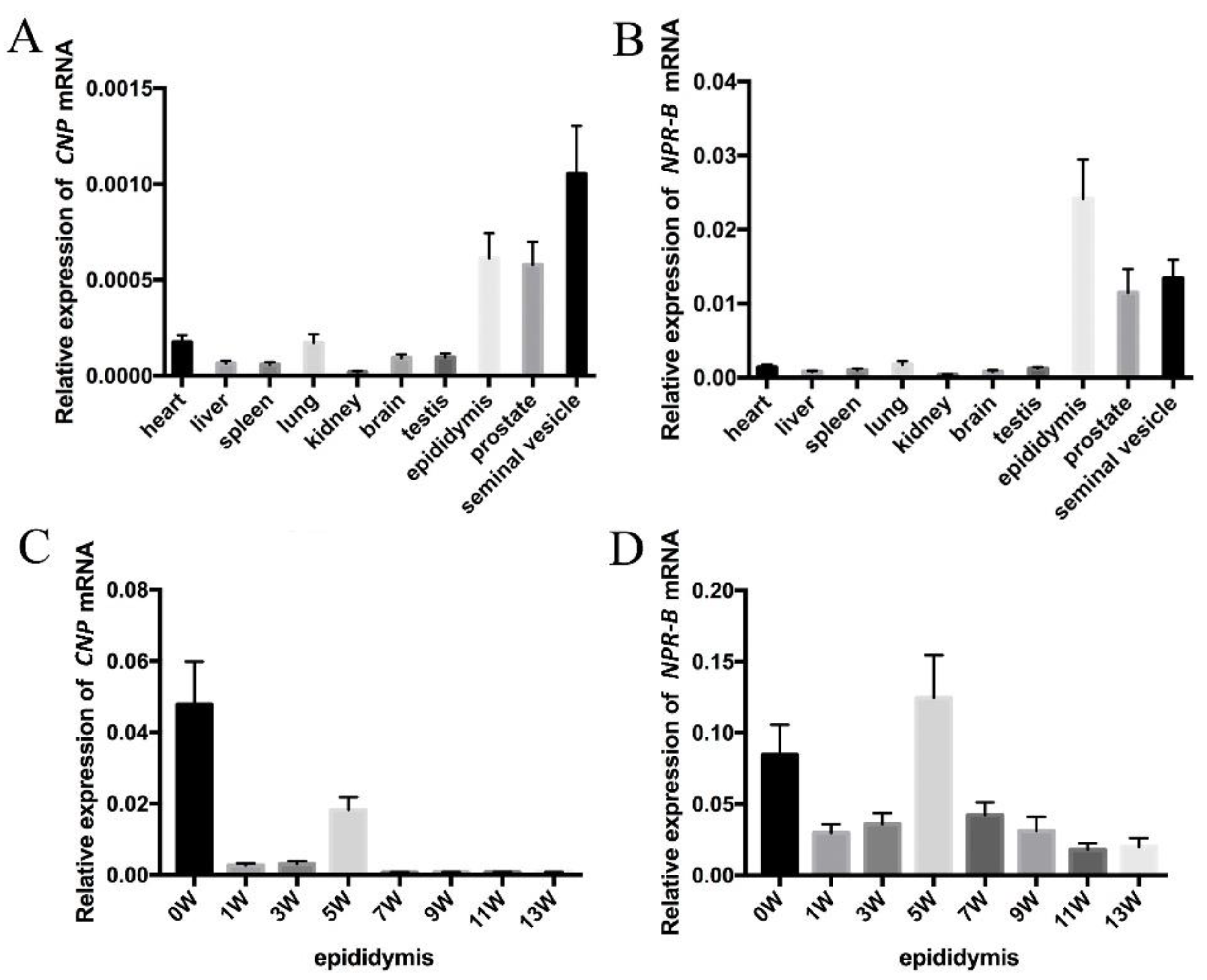
Based on PCR analyses on different rat tissues, CNP and NPR-B mRNA are expressed in all tissues and are more highly expressed in the male genital tract (seminal vesicle, epididymis and prostate) than in other tissues (such as heart, liver, spleen, lung, kidney, brain and testis) (Figure 1A,B). The expressions of CNP mRNA in epididymis was highest at birth (0 W), decreased dramatically at 1 W and 3 W, and then increased at 5 W with additional decreases at 7 W, 9 W, 11 W and 13 W. Similarly to CNP, the expression of NPR-B also showed two peaks—one at birth (0 W) and the other at the fifth week (5 W).
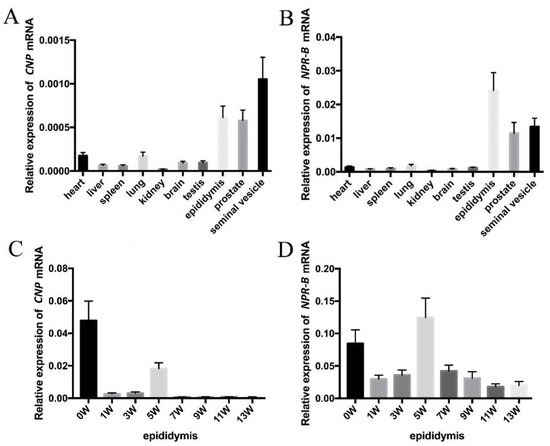
Figure 1.
The expression of CNP/NPR-B in rat. (A,B) The expression of CNP (A) and NPR-B (B) mRNA in different rat tissues. (C,D) The expression of CNP (C) and NPR-B (D) mRNA in epididymis at different stages after birth.
3.2. Expression of CNP/NPR-B in Different Segments of Rat Epididymis
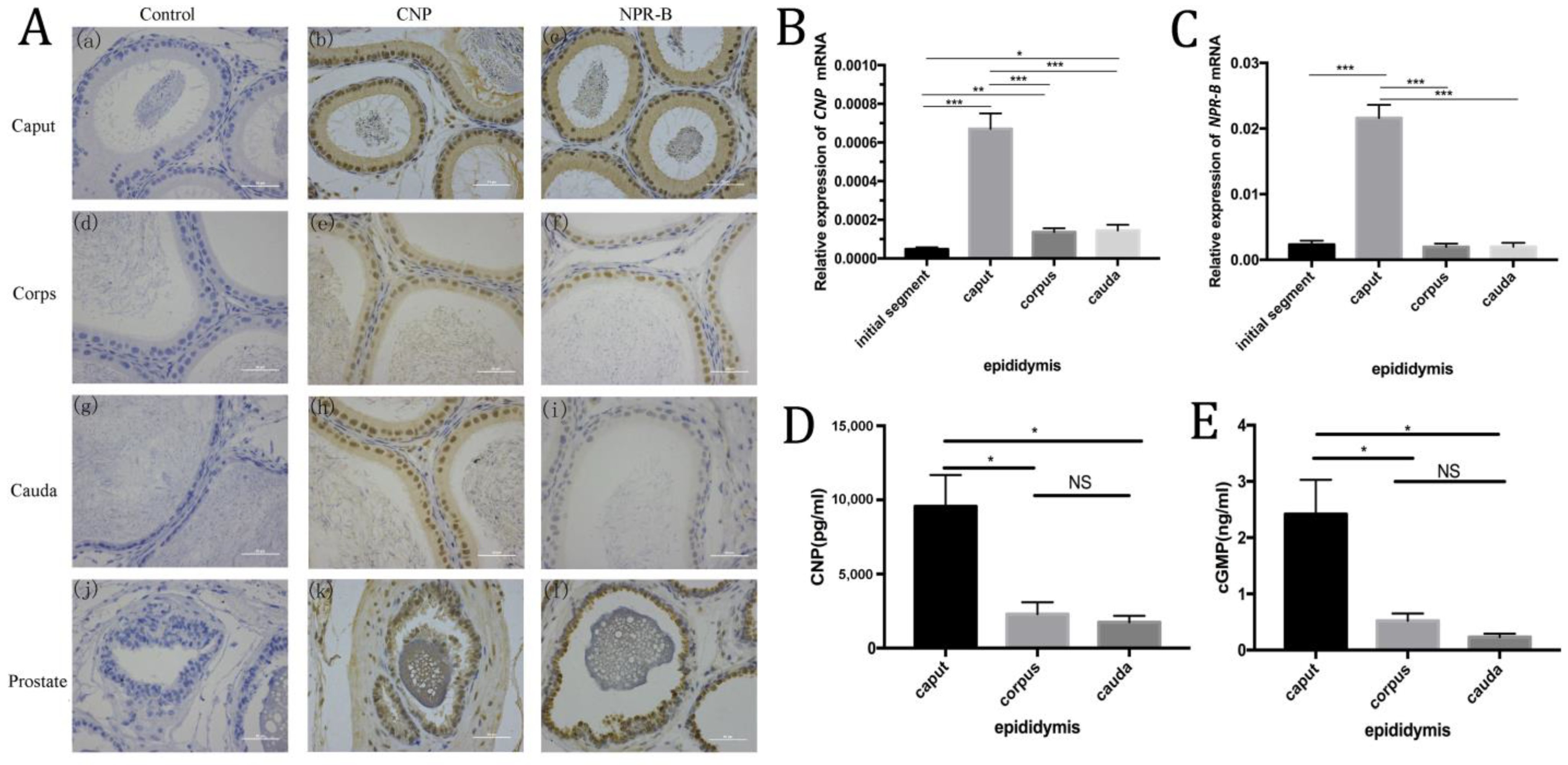
Immunohistochemistry showed CNP and NPR-B were mainly located in rat epididymal epithelial cells (such as principal cells), and the expression of CNP and NPR-B in the caput were higher than those in the corps and the cauda (Figure 2A). RT-PCR showed similar trends, i.e., that the level of CNP and NPR-B mRNA in the caput were much higher than in other segments (such as the initial segment, corps and cauda) (p < 0.01) (Figure 2B,C). In rat epididymal fluid, ELISA results demonstrated that the contents of CNP and cGMP in the caput were significantly higher than those in the corps and the cauda (p < 0.05) (Figure 2D,E).
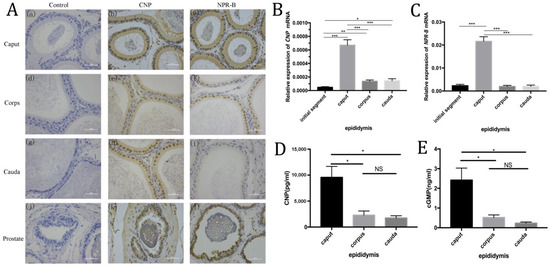
Figure 2.
The expression of CNP/NPR-B in the different segments of epididymis. (A) Immunohistochemistry shows the localization of CNP and NPR-B in different segments of the epididymis and prostate. a–c: caput of epididymis; d–f: corpse of epididymis; g–i: cauda of epididymis; j–l: prostate. (B,C) The level of CNP (B) and NPR-B (C) mRNA in different segments of epididymis detected by RT- PCR. (D,E) The content of CNP (D) and cGMP (E) in different segments of epididymal fluid by ELISA. (* p < 0.05, ** p < 0.01, *** p < 0.01, NS: not significant).
3.3. Expression of CNP, NPR-B in the Castrated Rat Model
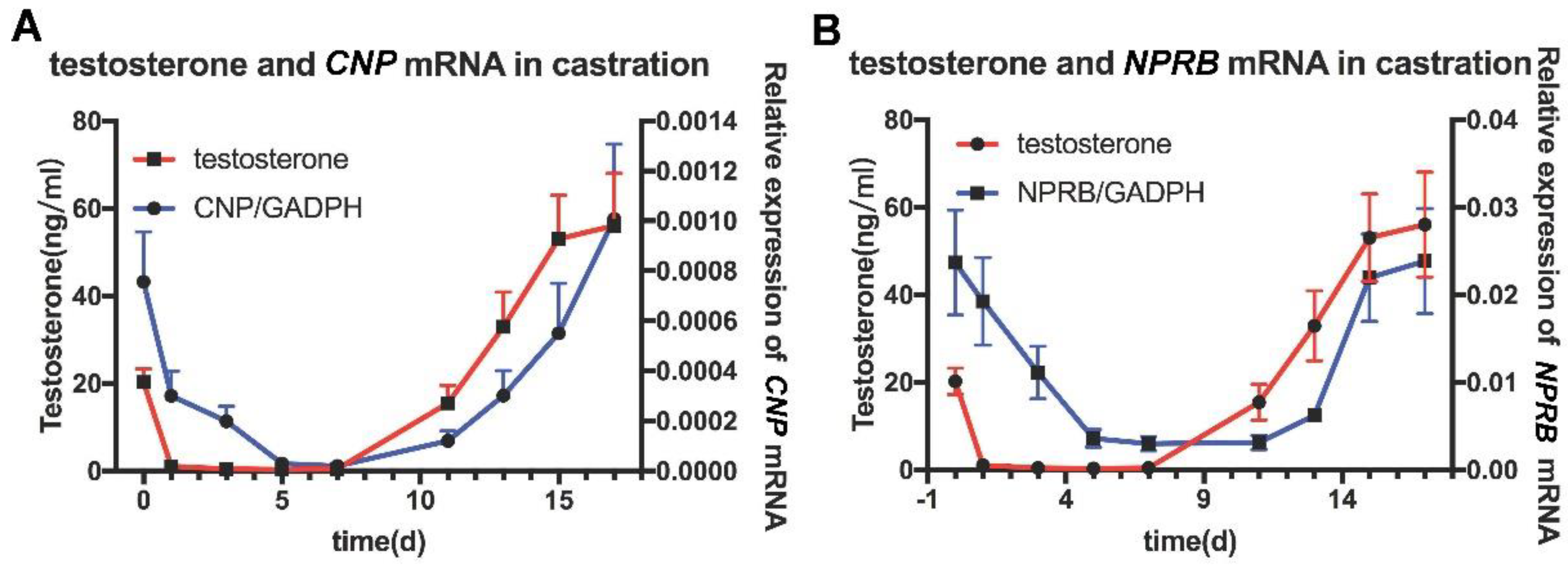
In order to investigate whether CNP/NPR-B is regulated by testosterone, castration rat models were established. The expression of CNP/NPR-B mRNA in the epididymis decreased significantly on the first day (D1) after castration and almost disappeared on D5 (Figure 3). After testosterone was supplemented at D10, the expression of CNP/NPR-B mRNA was immediately restored and increased with the level of serum testosterone (Figure 3). The changes of CNP and NPR-B followed that of testosterone and showed the similar trends.
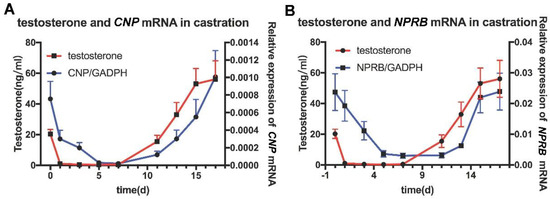
Figure 3.
The expression of CNP/NPR−B in the castrated rat model. The red line represents the content of testosterone in the serum, while the blue line represents the expression of CNP (A) or NPR−B (B) mRNA in the epididymis.
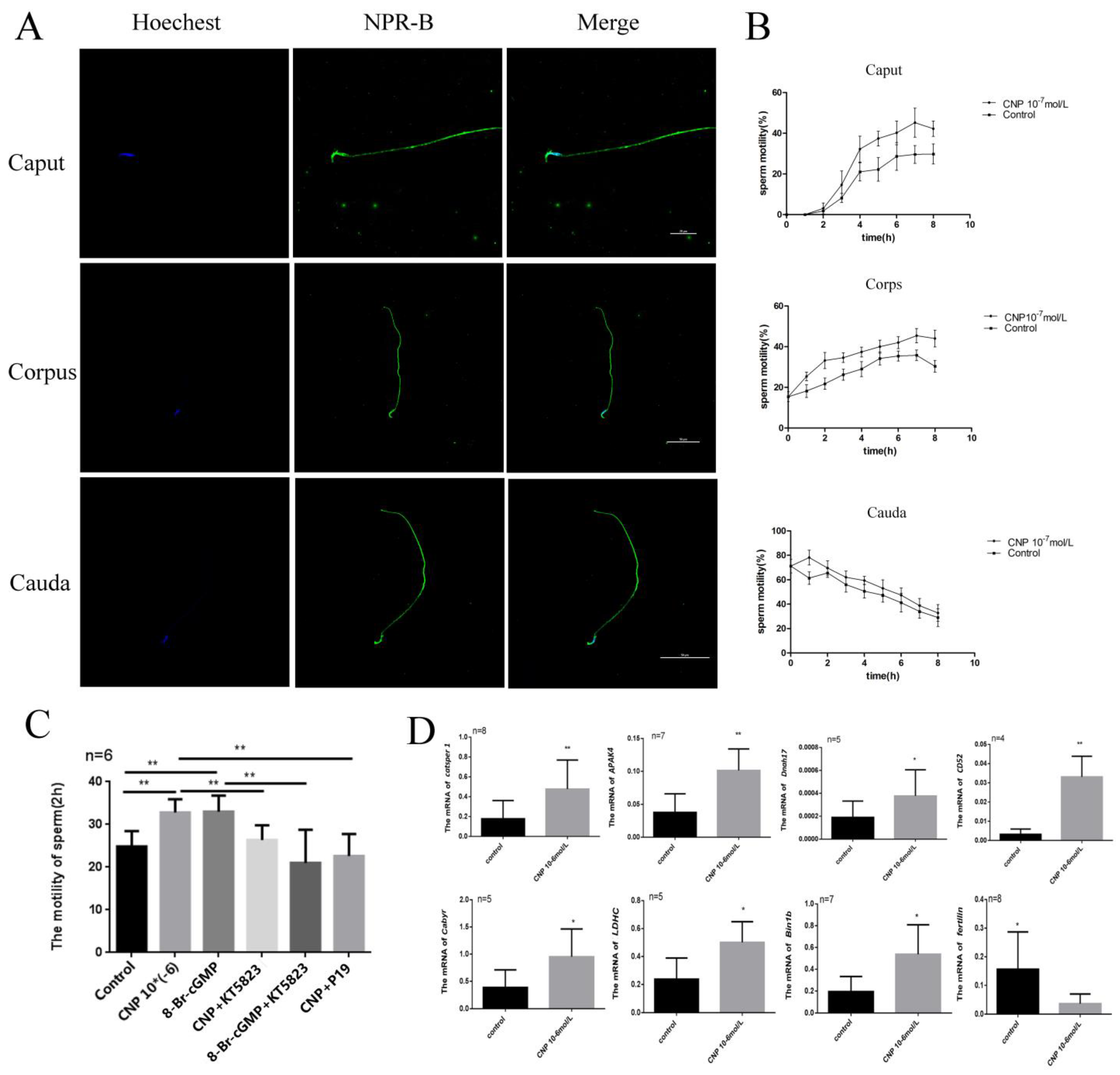
3.4. Effect of CNP on Motility of the Epididymis Sperm In Vitro
Immunofluorescence showed that NPR-B is mainly located in the head and middle tail of spermatozoa at different segments of epididymis (Figure 4A). After incubation with CNP, the sperm motility in the caput, corps, and cauda of the epididymis all increased (Figure 4B). Interestingly, CNP could promote the acquisition of sperm motility in the caput of the epididymis (Figure 4C). To explore the signal pathway of CNP in epididymal sperm, cGMP analogues (8-Br-cGMP), a PKG inhibitor (KT5823), and an NPR-B antagonist (P19) were added (Figure 4C). The results exhibited that 8-Br-cGMP, similarly to CNP, could increase the sperm motility (p < 0.01) (Figure 4C). On the contrary, P19 and KT5823 could inhibit the effect of CNP on epididymal sperm motility after 2 h in vitro culture (p < 0.01) (Figure 4C).
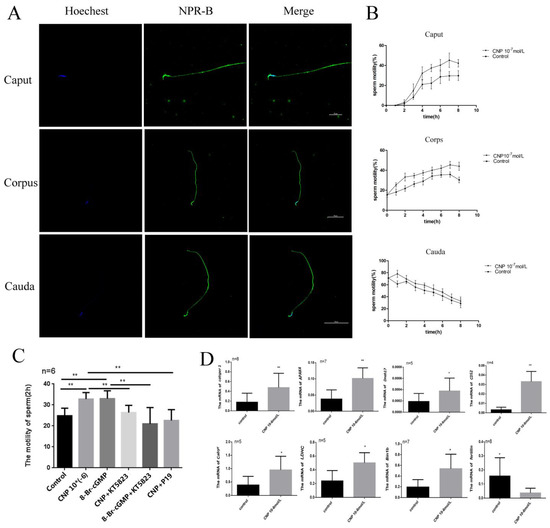
Figure 4.
The effect of CNP on maturation of the epididymis sperm in vitro. (A) Spermatozoa immunofluorescence in the different segments of the epididymis detected by laser confocal photography (600×). (B) Sperm motilities at different segments of epididymis detected at 0, 2, 4, 6 and 8 h after incubation with CNP. (C) Sperm motility at the caput of the epididymis after incubation with either CNP, 8-Br-cGMP, KT5823, or NPR-B antagonist. (D) Expression of maturation-related genes in the epididymal caput sperm after incubation with CNP for 6 h. (* p < 0.05, ** p < 0.01).
3.5. Effect of CNP on Maturation-Related Factors of the Epididymis Sperm In Vitro
After incubation with CNP 10−6 nmol/L for 6 h, the gene expression of sperm maturation-related factors was detected. The results showed CNP can significantly increase the expression of Catsper 1, A-kinase anchor protein 4 (AKAP4), Cluster of Differentiation 52 (CD52), lactate dehydrogenase (LDCH), dynein axonemal heavy chain 17 (Dnah17) and Bin1b (* p < 0.05, ** p < 0.01, Figure 4D), and decrease the expression of Fertilin ((p < 0.05) (Figure 4D).
4. Discussion
This study first explored the role of CNP in epididymal sperm maturation. Results showed that CNP/NPR-B was extremely highly expressed in the male reproductive duct (epididymis, seminal vesicles, and prostate gland). In the epididymis, CNP/NPR-B were located mainly in the caput, and their expression was regulated by testosterone. CNP could not only initiate epididymal sperm motility through the cGMP pathway but also regulate the expression of sperm motility and mature-related genes (such as Bin1b, Catsper 1, Dnah17, and Fertilin), which indicated that CNP could regulate the process of sperm maturation in the epididymis.
The epididymis has many tissue-specific or predominant genes. For example, Lipocalin 5 (Lcn5) and Lipocalin 8 (Lcn8) are epididymis-specific lipocalin genes [21]. Robertson M et al. analyzed publicly available human and mouse RNA-seq datasets and found a plethora of novel testis- and epididymis-specific genes (Spint3, Spint4, Spint5, and Ces5a) [22]. Oh J et al. identified and characterized 32 novel epididymis-specific or predominant genes by an integrative approach and found that six of the 32 novel genes were identified as β-defensins and eight contained a protease inhibitor domain [23]. In our studies, we found CNP/NPR-B also were epididymis predominant genes, which were highly expressed in the epididymis. This result was similar to other study reports [8,9,10].
The epididymis is immature at birth, and epithelial cells acquire their fully differentiated phenotype during an extended postnatal period [24]. Moreover, the process of epithelial cell differentiation needs luminal testicular factors [25]. First, mature spermatids release from rat testes around postnatal D44 [26]. Then, the sperm undergo the maturation process in the epididymis [27]. Bin1b, which is exclusively expressed in the caput region of the rat epididymis, starts to express on the 30th day after birth and reaches its peak in the 9th month after birth, which is responsible for sperm maturation, storage, and protection [28]. Mehmet Özbek et al. showed NPPC staining was observed intensely in all epithelial cells in the rat epididymis during the postnatal period [29]. In our experiments, we detected the expression levels of CNP and NPR-B in the rat epididymis at different postnatal periods and found that the expression levels of CNP/NPR-B in the epididymis peaked at 0 W and 5 W after birth. We presumed that high expression of CNP/NPR-B at birth may be attributed to fetal epididymis development, while the peak expression at postnatal 5 W was involved in preparing proteins for the initial coming wave of sperm.
The epididymis Is mainly divided ”nto ’hree parts: caput, corps, and cauda. The caput mainly contributes to sperm maturation, while the cauda plays a role in sperm storage [30]. Each part has different gene expression profiles, which ensure different epididymis functions necessary for different stages of sperm maturation [31]. Zhao W et al. examined region-specific gene expressions in yak epididymis by RNA-seq analysis and showed that the caput segment relatively highly expressed Sal1, LCN6, PTDS, DEFB109, DEFB 119, DEFB 123, SPAG11, PROC, CST3, ADAM28, KCNJ12, and SLC13A2, while the cauda epididymis highly expressed MCT7, PAG4, OAS1, TGM3, and PRSS45 [32]. Kim SZ et al. demonstrated that specific natriuretic peptides receptors are localized in surrounding smooth muscle cells of the duct of the epididymis of the freshwater turtle, which suggests involvement in the control of the transport of sperm [33]. Mietens A et al. showed that atrial natriuretic peptide (ANP) and nitric oxide (NO) promote relaxation of smooth muscle cells in the epididymal duct to ensure that immotile spermatozoa acquire their fertilizing capacity [34]. Thong A et al. found that NPR-B was located in epididymal epithelial cells, while neutral endopeptidase (NEP) lay exclusively in apical parts of epithelial cells and that NEP inhibitors could raise cGMP by decreasing CNP degradation while being involved in the regulation of epididymal function [35]. In this experiment, the expression of CNP/NPR-B in different segments of the epididymis were examined, and the results showed that both CNP and NPR-B were highly expressed in the caput of epididymis and located in the epididymal epithelial cells, which indicated CNP/NPR-B might be involved in sperm maturation in the epididymis.
Androgen is essential for the maturation of spermatozoa in the epididymis [36]. Half of the epididymis-specific or -predominant genes are regulated by androgens, and most of the androgen-regulated genes were located in the caput region [23,37]. SPAG11A, a member of the beta defensin protein family, was expressed exclusively in the principal cells of the mouse caput epididymis and regulated by androgen—typical of genes that are involved in creating a suitable microenvironment for sperm maturation [38]. The gene expression of RNase9, a potential regulator of sperm maturation in the rat epididymis caput, decreased dramatically after castration and was restored with androgen replacement, which exhibited an androgen-dependent expression pattern [39]. Fernandez CD et al. [40] found that DES accelerated sperm transit time in the epididymis and consequently decreased sperm density and diminished sperm motility; testosterone supplementation was able to restore the transit time to values close to normality and improved the sperm motility. Khurana ML. et al. [41] showed that different natriuretic peptides (including CNP) could stimulate steroidogenesis in purified mouse Leydig cells. Dehydroepiandrosterone (DHEA) treatment caused high expression of CNP and NPR-B in granulosa cells, which is involved in the oocyte meiotic arrest and very low ovulation rate in the polycystic ovary syndrome [42]. The amino-terminal propeptide of CNP levels increases markedly during testosterone treatment in children of short stature due to GH deficiency, known as idiopathic short stature [43]. In this study, CNP/NPR-B mRNA expression in the rat epididymis dramatically decreased after castration and was restored quickly after added testosterone in vivo, suggesting that CNP/NPR-B expression was regulated by testosterone. Epididymal sperm maturation mainly includes two aspects—the acquisition of motility and the gain of fertilization ability. This process happens mainly in the caput of the epididymis. As the spermatozoa moved through the corpus epididymis, motility increased sharply, and continued to improve through the cauda epididymis and vas deferens [44,45]. Björkgren et al. demonstrated that mouse beta-defensin 41 (DEFB41) specifically expressed in the caput of the epididymis and could initiate sperm motility [46]. Bin1b participates in the initiation of epididymal sperm motility along with antibacterial activity [28]. Deficient human Bin1b will lead to sperm vitality decline and genital tract infection [47]. Sperm motility initiation in the epididymis can be induced by changes in metabolism, cAMP (cyclic adenosine mono-phosphate), calcium and pH, and some protein kinases and phosphatases (such as sperm specific protein phosphatase PP1γ2, glycogen synthase kinase 3, and the calcium-regulated phosphatase calcineurin) are involved in epididymal sperm maturation [48]. This experiment showed CNP could initiate epididymal sperm motility, and its mechanism was enacted via stimulating the cGMP pathway by binding NPR-B in sperm. These findings are in line with those of our previous study [16].
The acquisition of fertilization ability in epididymal sperm is mainly manifested in the changes of lipid and protein on the sperm surface [49,50]. This process is controlled by epididymal epithelial cells that would respond to their surrounding environment and communicate with spermatozoa [50]. Zhao et al. demonstrated that the rat epididymis-specific β-defensin 15 (Defb15) can bind to the acrosomal region of caput sperm and that inhibiting the function of Defb15 resulted in a significant decline in sperm motility and embryonic development failure [51]. Fertilin is a kind of sperm plasma membrane protein that can mediate sperm-egg membrane interactions [52]. Moreover, fertilin will migrate from the acrosomal region to the acrosomal ridge during sperm maturation [53]. Our studies showed that CNP, mainly located in the caput of the epididymis, could regulate the expression of maturation-related genes (such as LDCH, Dnah17, Bin1b, Catsper 1, APAK4, and CD52) in the epididymal sperm.
5. Conclusions
In summary, we detected a high expression of CNP/NPR-B in rat epididymis, especially in the caput, and found that the expression was regulated by testosterone. CNP not only promote the acquisition of epididymal sperm mobility via the NPR-B/cGMP signal pathway, but also regulate sperm mature-related genes (such as Bin1b, Catsper 1, Dnah17, and Fertilin), thus playing a role in sperm maturation in the epididymis (Figure 5). Our study highlighted the important role of CNP in epididymal sperm maturation, including promoting the acquirement of sperm motility, which may provide a potential therapy for patients with abnormal sperm maturation.
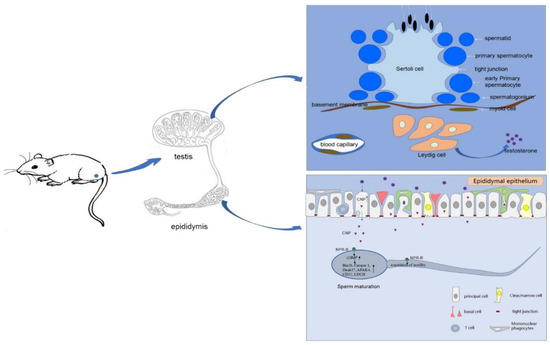
Figure 5.
The role of CNP in sperm maturation. Testosterone secreted by Leydig cells could promote the expression of CNP/NPR-B in rat epididymal epithelium, especially in the caput. CNP not only promotes the acquisition of epididymal sperm mobility via the NPR-B/cGMP signal pathway but also stimulates sperm mature-related genes (such as Bin1b, Catsper 1, Dnah17), thus playing a role in sperm maturation in epididymis.
Author Contributions
H.Z. and Y.Y. performed experiments, and completed, analyzed data compilation, and wrote the article. C.M., T.Z., Y.K. and N.L. performed the necessary literature searches and rectified. D.H. designed the study and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the open project of the National Natural Science Foundation of China (No. 81771575) and the Guangdong Basic and Applied Basic Research Foundation (NO. 2021A1515220178).
Institutional Review Board Statement
This study was approved by the Ethical Committee of Tongji Medical College, Huazhong University of Science and Technology (No. S1188).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dun, M.D.; Aitken, R.J.; Nixon, B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update 2012, 18, 420–435. [Google Scholar] [CrossRef]
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Update 2016, 22, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Sipilä, P. The impact of epididymal proteins on sperm function. Reproduction 2019, 158, R155–R167. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Biswas, S.; Mukhopadhyay, P.K. Molecular perspective concerning fluoride and arsenic mediated disorders on epididymal maturation of spermatozoa: A concise review. Hum. Exp. Toxicol. 2021, 40, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Ozkocer, S.E.; Konac, E. The current perspective on genetic and epigenetic factors in sperm maturation in the epididymis. Andrologia 2021, 53, e13989. [Google Scholar] [CrossRef]
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide (cnp): A new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 168, 863–870. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand nppc and its receptor npr2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Chrisman, T.D.; Schulz, S.; Potter, L.R.; Garbers, D.L. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J. Biol. Chem. 1993, 268, 3698–3703. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Gøtze, J.P.; Jensen, H.L.; Rehfeld, J.F. ProCNP and CNP are expressed primarily in male genital organs. Regul. Pept. 2008, 146, 204–212. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Rehfeld, J.F.; Pedersen, F.; Kastrup, J.; Videbaek, R.; Goetze, J.P. Measurement of Pro-C-Type Natriuretic Peptide in Plasma. Clin. Chem. 2005, 51, 2173–2176. [Google Scholar] [CrossRef][Green Version]
- Sogawa, C.; Fujiwara, Y.; Tsukamoto, S.; Ishida, Y.; Yoshii, Y.; Furukawa, T.; Kunieda, T.; Saga, T. Mutant phenotype analysis suggests potential roles for C-type natriuretic peptide receptor (NPR-B) in male mouse fertility. Reprod. Biol. Endocrinol. 2014, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, L.; Zhao, Q.; Gong, Y.; Guan, G.; Huang, S. C-Type Natriuretic Peptide/Natriuretic Peptide Receptor 2 Is Involved in Cell Proliferation and Testosterone Production in Mouse Leydig Cells. World J. Mens. Health. 2019, 37, 186–198. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Y.; Mei, C.; Li, N.; Wu, K.; Huang, D. C-type natriuretic peptide stimulates function of the murine Sertoli cells via activation of the NPR-B/cGMP/PKG signaling pathway. Acta Biochim. Pol. 2021, 68, 603–609. [Google Scholar] [CrossRef]
- Kong, N.; Xu, X.; Zhang, Y.; Wang, Y.; Hao, X.; Zhao, Y.; Qiao, J.; Xia, G.; Zhang, M. Natriuretic peptide type C induces sperm attraction for fertilization in mouse. Sci. Rep. 2017, 7, 39711. [Google Scholar] [CrossRef]
- Xia, H.; Chen, Y.; Wu, K.J.; Zhao, H.; Xiong, C.L.; Huang, D.H. Role of C-type natriuretic peptide in the function of normal human sperm. Asian J. Androl. 2016, 18, 80–84. [Google Scholar]
- Wu, K.; Mei, C.; Chen, Y.; Guo, L.; Yu, Y.; Huang, D. C-type natriuretic peptide regulates sperm capacitation by the cGMP/PKG signalling pathway via Ca2+ influx and tyrosine phosphorylation. Reprod. Biomed. Online 2019, 38, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Mosli, H.H.; Esmat, A.; Atawia, R.T.; Shoieb, S.M.; Mosli, H.A.; Abdel-Naim, A.B. Metformin Attenuates Testosterone-Induced Prostatic Hyperplasia in Rats: A Pharmacological Perspective. Sci. Rep. 2015, 5, 15639. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Dong, X.; Fu, S.; Wang, X.; Li, H.; Song, G.; Huang, D. C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia. Int. J. Mol. Sci. 2022, 23, 10370. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Suzuki, K.; Yu, X.; Chaurand, P.; Araki, Y.; Lareyre, J.J.; Caprioli, R.M.; Orgebin-Crist, M.C.; Matusik, R.J. Epididymis-specific lipocalin promoters. Asian J. Androl. 2007, 9, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Kent, K.; Tharp, N.; Nozawa, K.; Dean, L.; Mathew, M.; Grimm, S.L.; Yu, Z.; Légaré, C.; Fujihara, Y.; et al. Large-scale discovery of male reproductive tract-specific genes through analysis of RNA-seq datasets. BMC Biol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.; Woo, J.M.; Choi, E.; Park, I.; Han, C.; Baek, N.; Lee, H.; Kim, D.H.; Cho, C. Systematic identification and integrative analysis of novel genes expressed specifically or predominantly in mouse epididymis. BMC Genom. 2006, 7, 314. [Google Scholar] [CrossRef]
- Breton, S.; Ruan, Y.C.; Park, Y.J.; Kim, B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J. Androl. 2016, 18, 3–9. [Google Scholar] [CrossRef]
- Xu, B.; Abdel-Fattah, R.; Yang, L.; Crenshaw, S.A.; Black, M.B.; Hinton, B.T. Testicular Lumicrine Factors Regulate ERK, STAT, and NFKB Pathways in the Initial Segment of the Rat Epididymis to Prevent Apoptosis1. Biol. Reprod. 2011, 84, 1282–1291. [Google Scholar] [CrossRef][Green Version]
- Jahnukainen, K.; Chrysis, D.; Hou, M.; Parvinen, M.; Eksborg, S.; Soder, O. Increased apoptosis occurring during the first wave of spermatogenesis is stage-specific and primarily affects midpachytene spermatocytes in the rat testis. Biol. Reprod. 2004, 70, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Li, P.; Chan, H.C.; He, B.; So, S.C.; Chung, Y.W.; Shang, Q.; Zhang, Y.D.; Zhang, Y.L. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001, 291, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Özbek, M.; Hitit, M.; Öztop, M.; Beyaz, F.; Ergün, E.; Ergün, L. Spatiotemporal expression patterns of natriuretic peptides in rat testis and epididymis during postnatal development. Andrologia 2019, 51, e13387. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fok, K.L.; Dai, P.; Qiao, F.; Zhang, M.; Liu, H.; Sang, M.; Ye, M.; Liu, Y.; Zhou, Y.; et al. Spatio-temporal landscape of mouse epididymal cells and specific mitochondria-rich segments defined by large-scale single-cell RNA-seq. Cell Discov. 2021, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.A.; Yang, R.; Leir, S.H.; Eggener, S.E.; Harris, A. Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions. Mol. Hum. Reprod. 2016, 22, 69–82. [Google Scholar] [CrossRef]
- Zhao, W.; Quansah, E.; Yuan, M.; Gou, Q.; Mengal, K.; Li, P.; Wu, S.; Xu, C.; Yi, C.; Cai, X. Region-specific gene expression in the epididymis of Yak. Theriogenology 2019, 139, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Z.; Kang, S.Y.; Lee, S.J.; Cho, K.W. Localization of receptors for natriuretic peptide and endothelin in the duct of the epididymis of the freshwater turtle. Gen. Comp. Endocrinol. 2000, 118, 26–38. [Google Scholar] [CrossRef]
- Mietens, A.; Tasch, S.; Feuerstacke, C.; Eichner, G.; Volkmann, J.; Schermuly, R.T.; Grimminger, F.; Müller, D.; Middendorff, R. Phosphodiesterase 5 (PDE5) inhibition, ANP and NO rapidly reduce epididymal duct contractions, but long-term PDE5 inhibition in vivo does not. Mol. Cell Endocrinol. 2012, 349, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Thong, A.; Müller, D.; Feuerstacke, C.; Mietens, A.; Stammler, A.; Middendorff, R. Neutral endopeptidase (CD10) is abundantly expressed in the epididymis and localized to a distinct population of epithelial cells—Its relevance for CNP degradation. Mol. Cell Endocrinol. 2014, 382, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Hardy, M.P.; Inigo, I.V.; Huhtaniemi, I.; Bardin, C.W.; Moo-Young, A.J. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: A quantitative immunohistochemical study. Biol. Reprod. 2000, 63, 368–376. [Google Scholar] [CrossRef]
- Vreeburg, J.T.; Holland, M.K.; Cornwall, G.A.; Orgebin-Crist, M.C. Secretion and transport of mouse epididymal proteins after injection of 35S-methionine. Biol. Reprod. 1990, 43, 113–120. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Loanda, E.; Sari, P.; Midoen, Y.H.; Soeharso, P. Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod. Biol. Endocrinol. 2013, 11, 59. [Google Scholar] [CrossRef]
- Zhu, C.F.; Liu, Q.; Zhang, L.; Yuan, H.X.; Zhen, W.; Zhang, J.S.; Chen, Z.J.; Hall, S.H.; French, F.S.; Zhang, Y.L. RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol. Reprod. 2007, 76, 63–73. [Google Scholar] [CrossRef]
- Fernandez, C.D.; Porto, E.M.; Arena, A.C.; Kempinas Wde, G. Effects of altered epididymal sperm transit time on sperm quality. Int. J. Androl. 2008, 31, 427–437. [Google Scholar] [CrossRef]
- Khurana, M.L.; Pandey, K.N. Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: Activation of cholesterol side-chain cleavage enzyme. Endocrinology 1993, 133, 2141–2149. [Google Scholar] [CrossRef]
- Reis, A.M.; Honorato-Sampaio, K. C-type natriuretic peptide: A link between hyperandrogenism and anovulation in a mouse model of polycystic ovary syndrome. Clin. Sci. 2018, 132, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Olney, R.C.; Prickett, T.C.; Yandle, T.G.; Espiner, E.A.; Han, J.C.; Mauras, N. Amino-terminal propeptide of C-type natriuretic peptide and linear growth in children: Effects of puberty, testosterone, and growth hormone. J. Clin. Endocrinol. Metab. 2007, 92, 4294–5298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Der Horst, G.; Seier, J.V.; Spinks, A.C.; Hendricks, S. The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey, Cercopithecus aethiops. Int. J. Androl. 1999, 22, 197–207. [Google Scholar] [CrossRef]
- Guyonnet, B.; Dacheux, F.; Dacheux, J.L.; Gatti, J.L. The epididymal transcriptome and proteome provide some insights into new epididymal regulations. J. Androl. 2011, 32, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Alvarez, L.; Blank, N.; Balbach, M.; Turunen, H.; Laajala, T.D.; Toivanen, J.; Krutskikh, A.; Wahlberg, N.; Huhtaniemi, I.; et al. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol. Cell Endocrinol. 2016, 427, 143–154. [Google Scholar] [CrossRef]
- Diao, R.; Fok, K.L.; Chen, H.; Yu, M.K.; Duan, Y.; Chung, C.M.; Li, Z.; Wu, H.; Li, Z.; Zhang, H.; et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014, 6, 249ra108. [Google Scholar] [CrossRef]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Cohen, D.J.; Rochwerger, L.; Ellerman, D.A.; Morgenfeld, M.M.; Busso, D.; Cuasnicú, P.S. Relationship between the association of rat epididymal protein “DE” with spermatozoa and the behavior and function of the protein. Mol. Reprod. Dev. 2000, 56, 180–188. [Google Scholar] [CrossRef]
- Chen, H.; Alves, M.B.R.; Belleannée, C. Contribution of epididymal epithelial cell functions to sperm epigenetic changes and the health of progeny. Hum. Reprod. Update 2021, 28, 51–66. [Google Scholar] [CrossRef]
- Zhao, Y.; Diao, H.; Ni, Z.; Hu, S.; Yu, H.; Zhang, Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol. Life Sci. 2011, 68, 697–708. [Google Scholar] [CrossRef]
- Gundogan, G.I.; Irez, T.; Bozkurt, H.H. Is there a relationship between infertility and fertilin β protein distribution? Rev. Int. Androl. 2022, 20, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Guyonnet, B.; Dacheux, J.L.; Gatti, J.L.; Puigmulé, M.; Bonet, S.; Pinart, E. Expression, immunolocalization and processing of fertilins ADAM-1 and ADAM-2 in the boar (Sus domesticus) spermatozoa during epididymal maturation. Reprod. Biol. Endocrinol. 2011, 9, 96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).