Preoperative Short-Course Radiotherapy and Surgery versus Surgery Alone for Patients with Rectal Cancer: A Propensity Score-Matched Analysis at 18-Year Follow-Up
Abstract
:1. Introduction
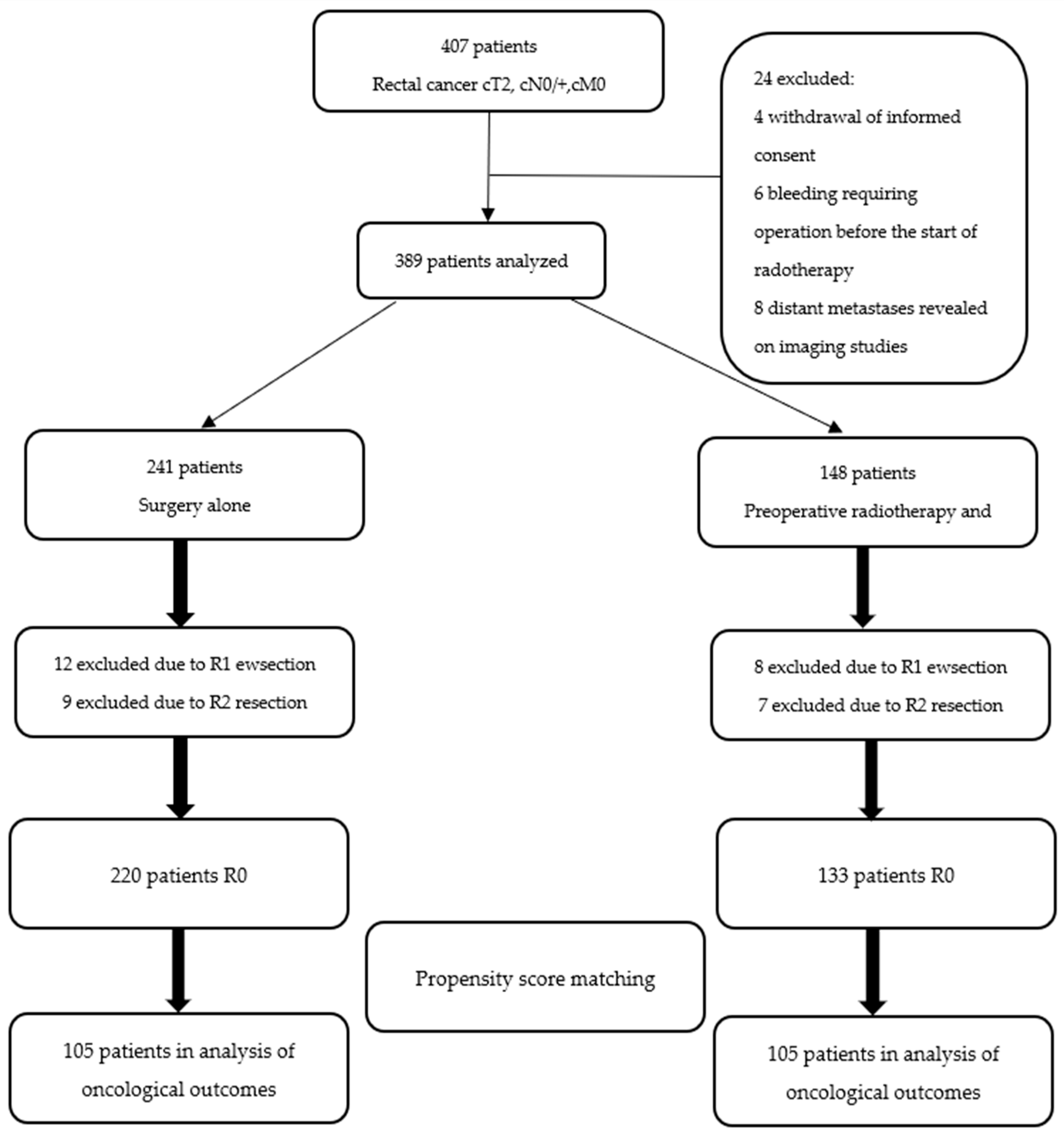
2. Materials and Methods
- -
- Primary rectal cancer cT2-cT4 cN0/+ (stage established by means of endorectal ultrasound and computed tomography)
- -
- No distant metastases (assessed by chest X-ray and abdominal computed tomography)
- -
- Tumor location below the level of S1/S2 with an inferior tumor margin 15 cm or less from the anal verge as measured during withdrawal of a rigid rectoscope.
3. Results
3.1. Recurrences
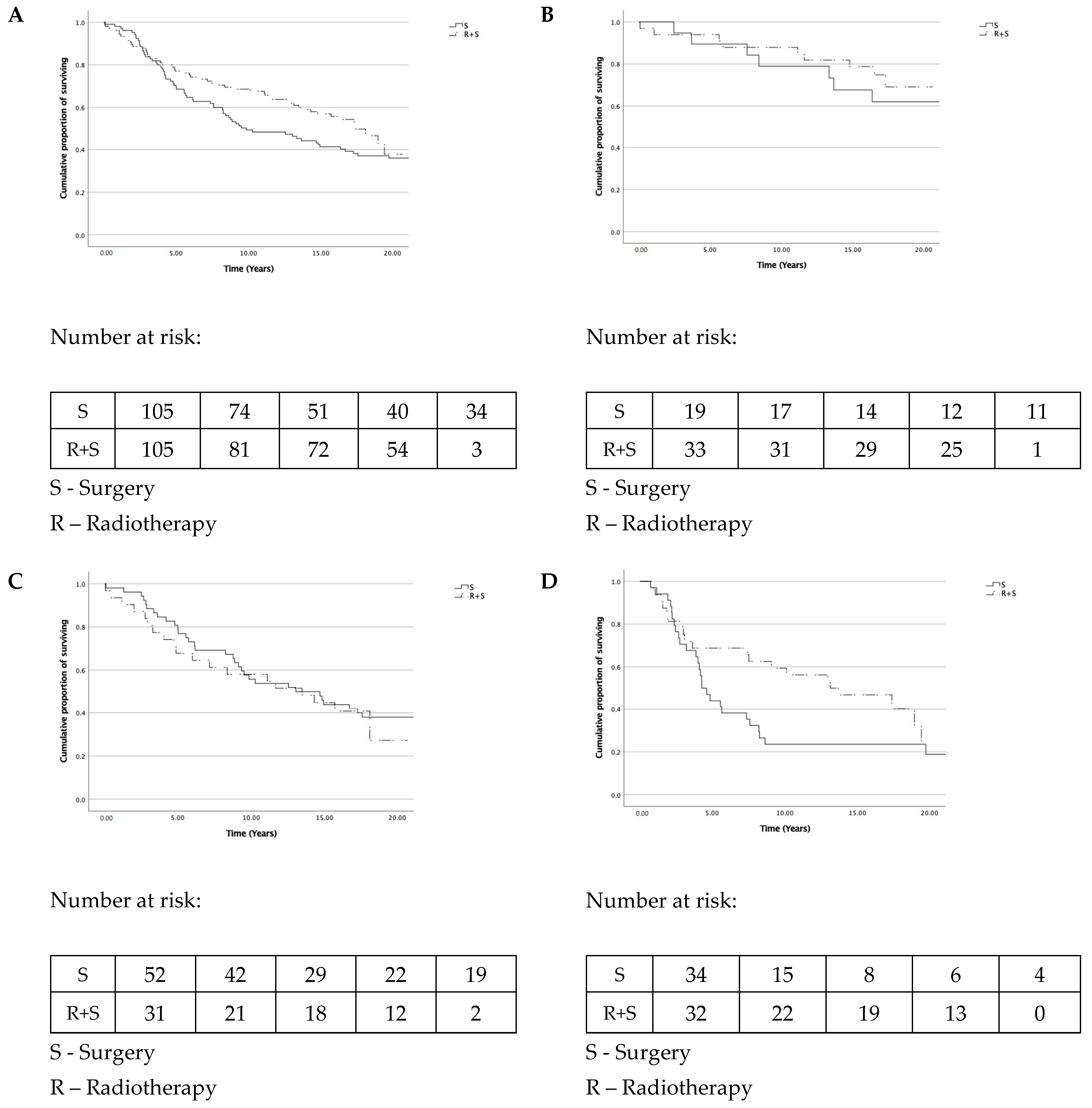
3.2. Overall Survival
3.3. Recurrence-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heald, R.J.; Husband, E.M.; Ryall, R.D.H. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? BJS 2005, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Dixon, M.; Durdey, P.; Williams, N. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Lancet 1986, 328, 996–999. [Google Scholar] [CrossRef]
- Abraha, I.; Aristei, C.; Palumbo, I.; Lupattelli, M.; Trastulli, S.; Cirocchi, R.; De Florio, R.; Valentini, V. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst. Rev. 2018, 10, CD002102. [Google Scholar] [CrossRef] [PubMed]
- Qiaoli, W.; Yongping, H.; Wei, X.; Guoqiang, X.; Yunhe, J.; Qiuyan, L.; Cheng, L.; Mengling, G.; Jiayi, L.; Wei, X.; et al. Preoperative short-course radiotherapy (5 × 5 Gy) with delayed surgery versus preoperative long-course radiotherapy for locally resectable rectal cancer: A meta-analysis. Int. J. Color. Dis. 2019, 34, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalez, A.B.; E Curtis, R.; Kry, S.F.; Gilbert, E.; Lamart, S.; Berg, C.D.; Stovall, M.; Ron, E. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet Oncol. 2011, 12, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B. Occurrence of Second Cancers in Patients Treated With Radiotherapy for Rectal Cancer. J. Clin. Oncol. 2005, 23, 6126–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiltink, L.; Nout, R.A.; Fiocco, M.; Kranenbarg, E.M.-K.; Juergenliemk-Schulz, I.M.; Jobsen, J.J.; Nagtegaal, I.; Rutten, H.J.T.; Van De Velde, C.J.H.; Creutzberg, C.; et al. No Increased Risk of Second Cancer After Radiotherapy in Patients Treated for Rectal or Endometrial Cancer in the Randomized TME, PORTEC-1, and PORTEC-2 Trials. J. Clin. Oncol. 2015, 33, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Yadav, V.; Singh, P.; Bhowmik, K.T. Radiation-Induced Malignancies Making Radiotherapy a “Two-Edged Sword”: A Review of Literature. World J. Oncol. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pach, R.; Kulig, J.; Richter, P.; Gach, T.; Szura, M.; Kowalska, T. Randomized clinical trial on preoperative radiotherapy 25 Gy in rectal cancer—treatment results at 5-year follow-up. Langenbeck’s Arch. Surg. 2012, 397, 801–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagtegaal, I.D.; Van De Velde, C.J.; Van Der Worp, E.; Kapiteijn, E.; Quirke, P.; Van Krieken, J.H.J.; the Pathology Review Committee for the Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic Evaluation of Rectal Cancer Resection Specimen: Clinical Significance of the Pathologist in Quality Control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish Rectal Cancer Trial: Long Lasting Benefits From Radiotherapy on Survival and Local Recurrence Rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.-A.; et al. Randomized Trial of Short-Course Radiotherapy Versus Long-Course Chemoradiation Comparing Rates of Local Recurrence in Patients With T3 Rectal Cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, T.E.; Endreseth, B.H.; Romundstad, P.R.; Wibe, A. Circumferential resection margin as a prognostic factor in rectal cancer. BJS 2009, 96, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Lutz, M.P.; Zalcberg, J.R.; Glynne-Jones, R.; Ruers, T.; Ducreux, M.; Arnold, D.; Aust, D.; Brown, G.; Bujko, K.; Cunningham, C.; et al. Second St. Gallen European Organisation for Research and Treatment of Cancer Gastrointestinal Cancer Conference: Consensus recommendations on controversial issues in the primary treatment of rectal cancer. Eur. J. Cancer 2016, 63, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-H.; Liu, C.-J.; Chao, T.-F.; Chen, T.-J.; Hu, Y.-W. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J. Gastroenterol. 2018, 24, 4586–4595. [Google Scholar] [CrossRef] [PubMed]
- Martling, A.; Smedby, K.E.; Birgisson, H.; Olsson, H.; Granath, F.; Ekbom, A.; Glimelius, B. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. BJS 2017, 104, 278–287. [Google Scholar] [CrossRef] [PubMed]

| Surgery Alone | Preoperative Radiotherapy and Surgery | p Value | |
|---|---|---|---|
| n = 220 | n = 133 | ||
| Sex | 0.644 * | ||
| Men | 123 (56%) | 71 (53%) | |
| Women | 97 (44%) | 62 (47%) | |
| Age (years, median, minimum-maximum) | 65 (36–86) | 62 (26–92) | 0.163 † |
| Pretreatment tumor stage (T) | 0.815 * | ||
| cT2 | 46 (21%) | 26 (20%) | |
| cT3 | 161 (73%) | 97 (73%) | |
| cT4 | 13 (6%) | 10 (8%) | |
| Pretreatment nodal status (n) | 0.0005 * | ||
| cN0 | 136 (62%) | 106 (80%) | |
| cN+ | 84 (38%) | 27 (20%) | |
| Height from anal verge | 0.055 * | ||
| <5 cm | 46 (21%) | 38 (29%) | |
| 5–10 cm | 128 (58%) | 79 (59%) | |
| >10 cm | 46 (21%) | 16 (12%) | |
| Chemotherapy received | 0.836 * | ||
| Yes | 143 (65%) | 85 (64%) | |
| No | 77 (35%) | 48 (36%) | |
| Lymph node yield (median, minimum-maximum) | 16 (0–68) | 16 (0–79) | 0.406 † |
| Surgery Alone | Preoperative Radiotherapy and Surgery n = 105 | p Value | |
|---|---|---|---|
| n = 105 | |||
| Sex | 1.000 * | ||
| Men | 61 (58%) | 61 (58%) | |
| Women | 44 (42%) | 44 (42%) | |
| Age (years, median, minimum-maximum) | 64 (36–80) | 61 (26–78) | 0.222 † |
| Pretreatment tumor stage (T) | 0.549 * | ||
| cT2 | 25 (24%) | 22 (21%) | |
| cT3 | 74 (70%) | 73 (70%) | |
| cT4 | 6 (6%) | 10 (9%) | |
| Pretreatment nodal status (n) | 0.287 * | ||
| cN0 | 71 (68%) | 78 (74%) | |
| cN+ | 34 (32%) | 27 (26%) | |
| Pretreatment stage (cUICC) | 0.537 * | ||
| I | 19 (18%) | 19 (18%) | |
| II | 52 (50%) | 59 (56%) | |
| III | 34 (32%) | 27 (26%) | |
| Height from anal verge | 0.897 * | ||
| <5 cm | 25 (24%) | 23 (22%) | |
| 5–10 cm | 66 (63%) | 66 (63%) | |
| >10 cm | 14 (13%) | 16 (15%) | |
| Chemotherapy received | 0.558 * | ||
| Yes | 37 (35%) | 33 (31%) | |
| No | 68 (65%) | 72 (69%) | |
| Lymph node yield (median, minimum-maximum) | 17 (0–68) | 16 (0–79) | 0.481 † |
| Surgery | Radiotherapy + Surgery | p Value | |
|---|---|---|---|
| n = 105 | n = 105 | ||
| Local recurrence | |||
| HR (95% CI) | 1.00 (ref) | 0.278 (0.119–0.652) | 0.003 |
| Overall survival | |||
| HR (95% CI) | 1.00 (ref) | 0.740 (0.513–1.068) | 0.108 |
| Recurrence-free survival | |||
| HR (95% CI) | 1.00 (ref) | 0.395 (0.230–0.677) | 0.001 |
| Variable | Description | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p * | OR (95% CI) | p * | ||
| Sex | Male | 1 | 0.455 | ||
| Female | 1.350 (0.615–2.963) | ||||
| Age | ≤65 years | 1 | 0.707 | ||
| >65 years | 0.854 (0.375–1.944) | ||||
| ypT stage | 0–2 | 1 | 0.200 | ||
| 3–4 | 1.804 (0.731–4.449) | ||||
| ypN stage | 0 | 1 | 0.001 | 1 | 0.726 |
| 1 | 8.225 (3.828–17.672) | 1.450 (0.417–5.044) | |||
| 2 | 3.804 (1.863–7.769) | 1.436 (0.450–4.578) | |||
| Number of harvested LNs | <12 | 1 | 0.001 | 1 | 0.604 |
| ≥12 | 0.242 (0.129–0.453) | 0.766 (0.280–2.098) | |||
| Radiotherapy | No | 1 | 0.001 | 1 | 0.001 |
| Yes | 0.071 (0.033–0.154) | 0.212 (0.088–0.512) | |||
| Tumour location | <5 cm | 1 | 0.001 | 1 | 0.001 |
| 5–10 cm | 0.158 (0.096–0.260) | 0.299 (0.143–0.625) | |||
| >10 cm | 0.034 (0.005–0.253) | 0.085 (0.011–0.674) | |||
| Chemotherapy | No | 1 | 0.161 | ||
| Yes | 0.566 (0.255–1.254) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pach, R.; Richter, P.; Sierzega, M.; Papp, N.; Szczepanik, A. Preoperative Short-Course Radiotherapy and Surgery versus Surgery Alone for Patients with Rectal Cancer: A Propensity Score-Matched Analysis at 18-Year Follow-Up. Biomedicines 2021, 9, 725. https://doi.org/10.3390/biomedicines9070725
Pach R, Richter P, Sierzega M, Papp N, Szczepanik A. Preoperative Short-Course Radiotherapy and Surgery versus Surgery Alone for Patients with Rectal Cancer: A Propensity Score-Matched Analysis at 18-Year Follow-Up. Biomedicines. 2021; 9(7):725. https://doi.org/10.3390/biomedicines9070725
Chicago/Turabian StylePach, Radoslaw, Piotr Richter, Marek Sierzega, Natalia Papp, and Antoni Szczepanik. 2021. "Preoperative Short-Course Radiotherapy and Surgery versus Surgery Alone for Patients with Rectal Cancer: A Propensity Score-Matched Analysis at 18-Year Follow-Up" Biomedicines 9, no. 7: 725. https://doi.org/10.3390/biomedicines9070725







