Lactate Suppresses Growth of Esophageal Adenocarcinoma Patient-Derived Organoids through Alterations in Tumor NADH/NAD+ Redox State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Procurement
2.2. Generation and Passaging of Esophageal Adenocarcinoma Patient-Derived Organoids
2.3. Clinical/Pathologic Features of Tumor Samples
2.4. Formulation of Normal Glucose and Low-Glucose Human Gut Media
2.5. Lactate Treatment of PDOs
2.6. Quantification of PDO Size and Organoid Formation Rate
2.7. Extraction of PDOs for Immunohistochemistry, Immunofluorescence, and RNAseq
2.8. Paraffin Embedding of PDOs for Immunohistochemistry and Immunofluorescence
2.9. H&E, Immunofluorescence and Alcian Blue Staining of PDOs
2.10. Antibodies and Dilutions
2.11. Immunofluorescent Imaging of PDOs
2.12. Quantification of Ki67, CDX2 and Cytokeratin 8 Immunofluorescence
2.13. Histological Scoring of Esophageal Adenocarcinoma PDOs
2.14. NADH/NAD+ Quantification Assay
2.15. RNA Sequencing
3. Results
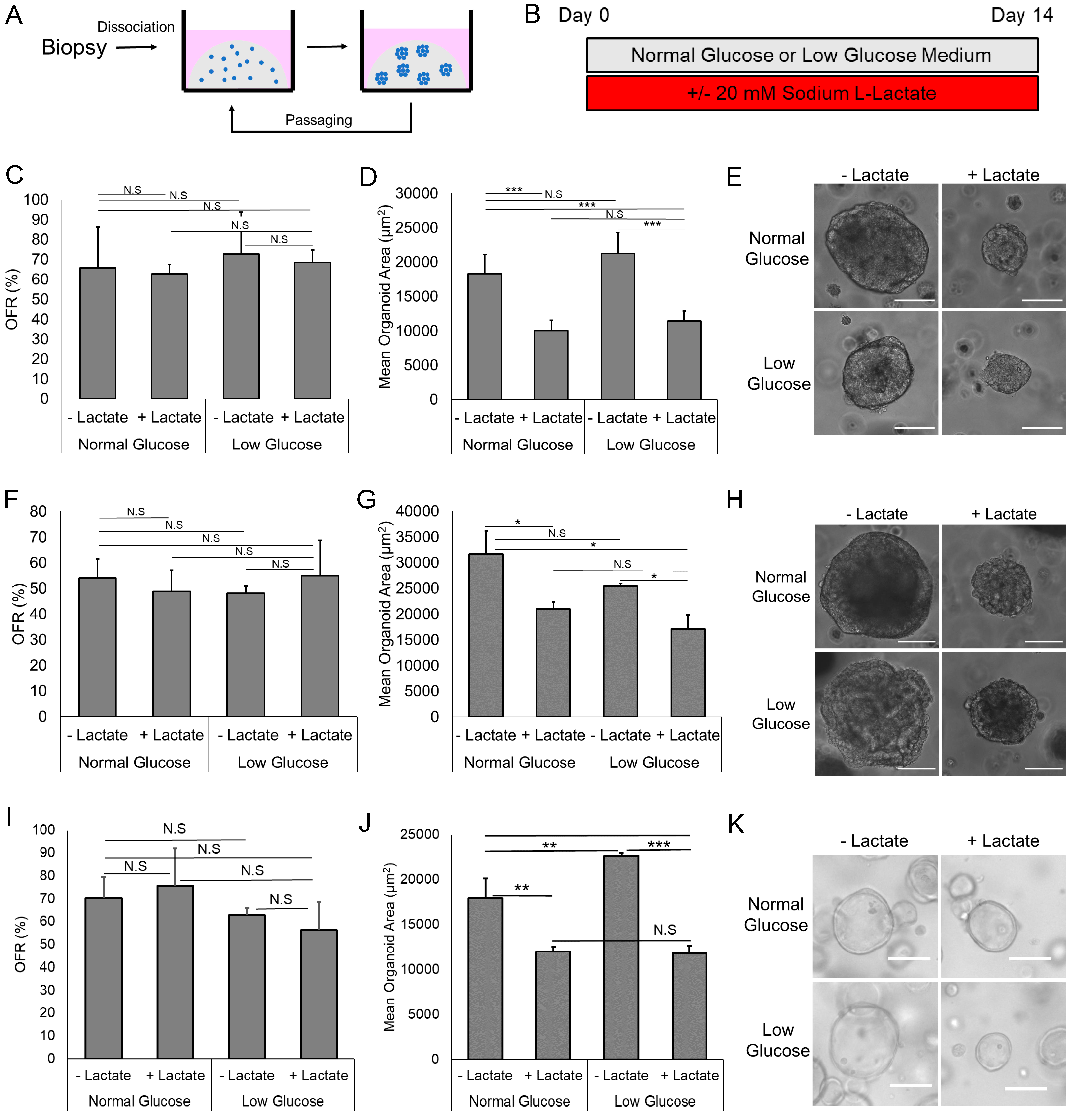
3.1. Lactate Suppresses EAC PDO Size but Not Formation Rate
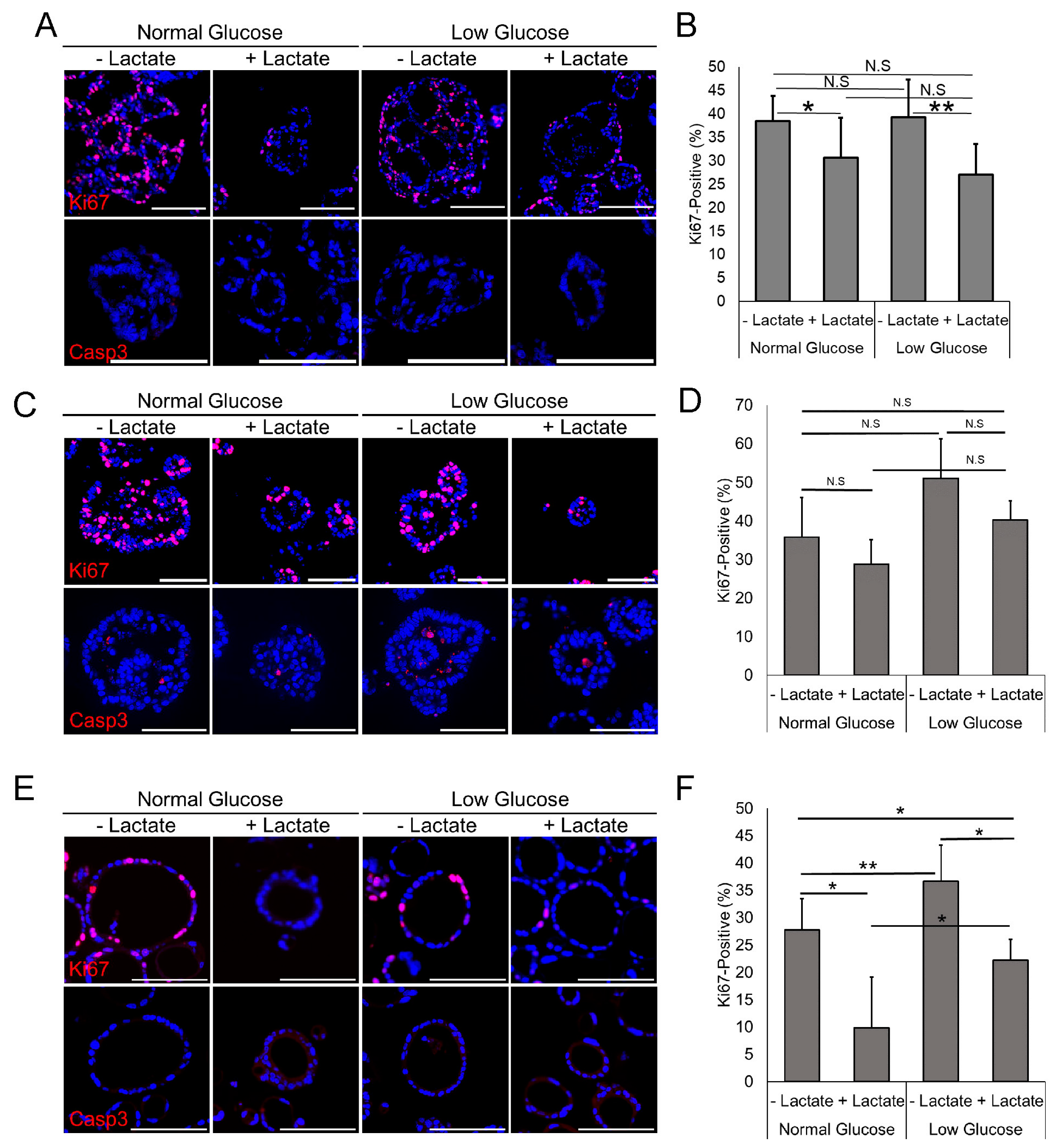
3.2. Lactate Reduces Proliferation but Does Not Increase Apoptosis
3.3. Lactate Has Variable Effect on the Cellular Atypia in EAC PDOs
3.4. Inhibition of Lactate Dehydrogenase Reverses the Growth Inhibition Caused by Lactate in EAC PDOs
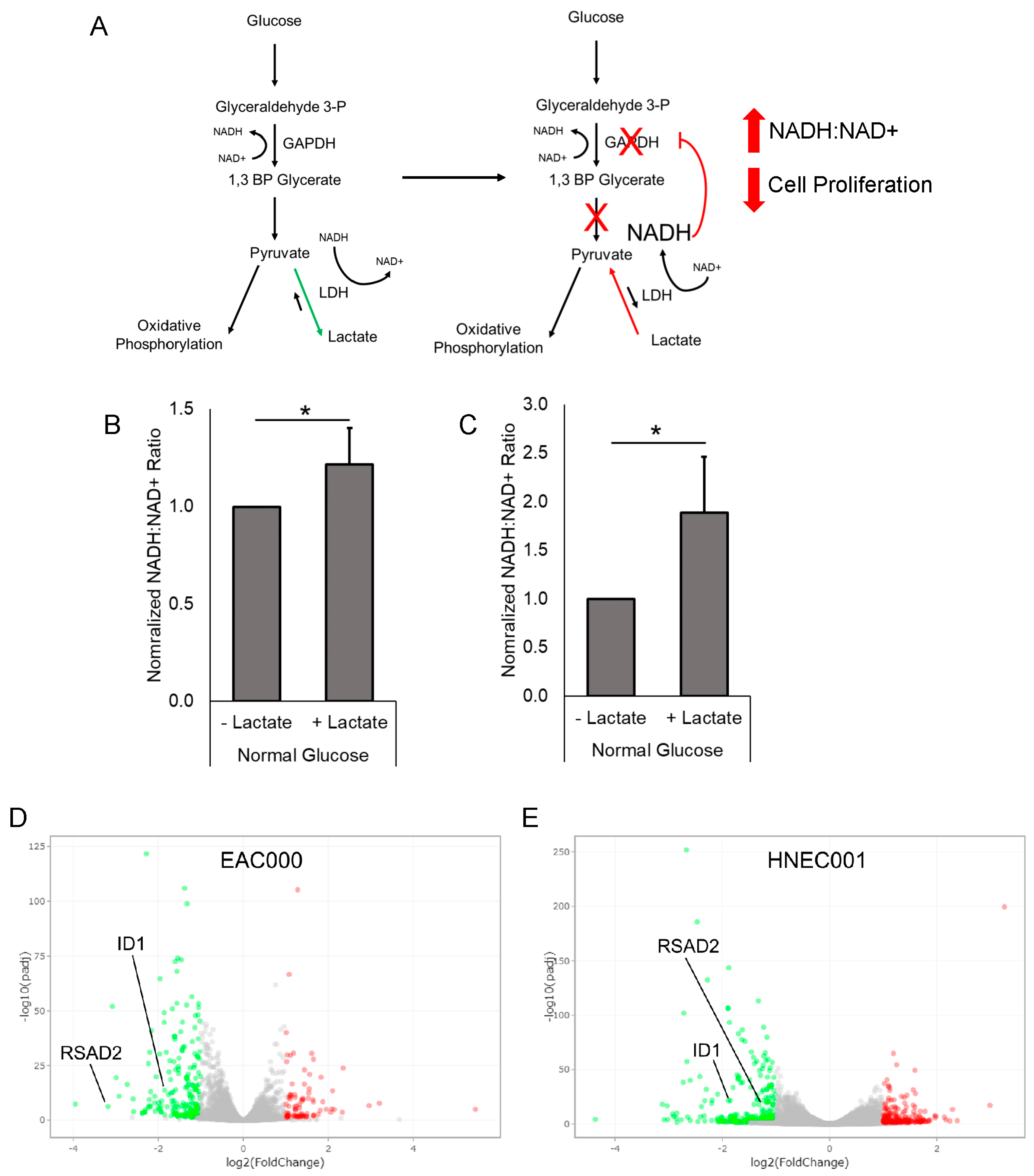
3.5. Treatment of EAC PDOs with Lactate Alters Tumor NADH/NAD+ Redox State
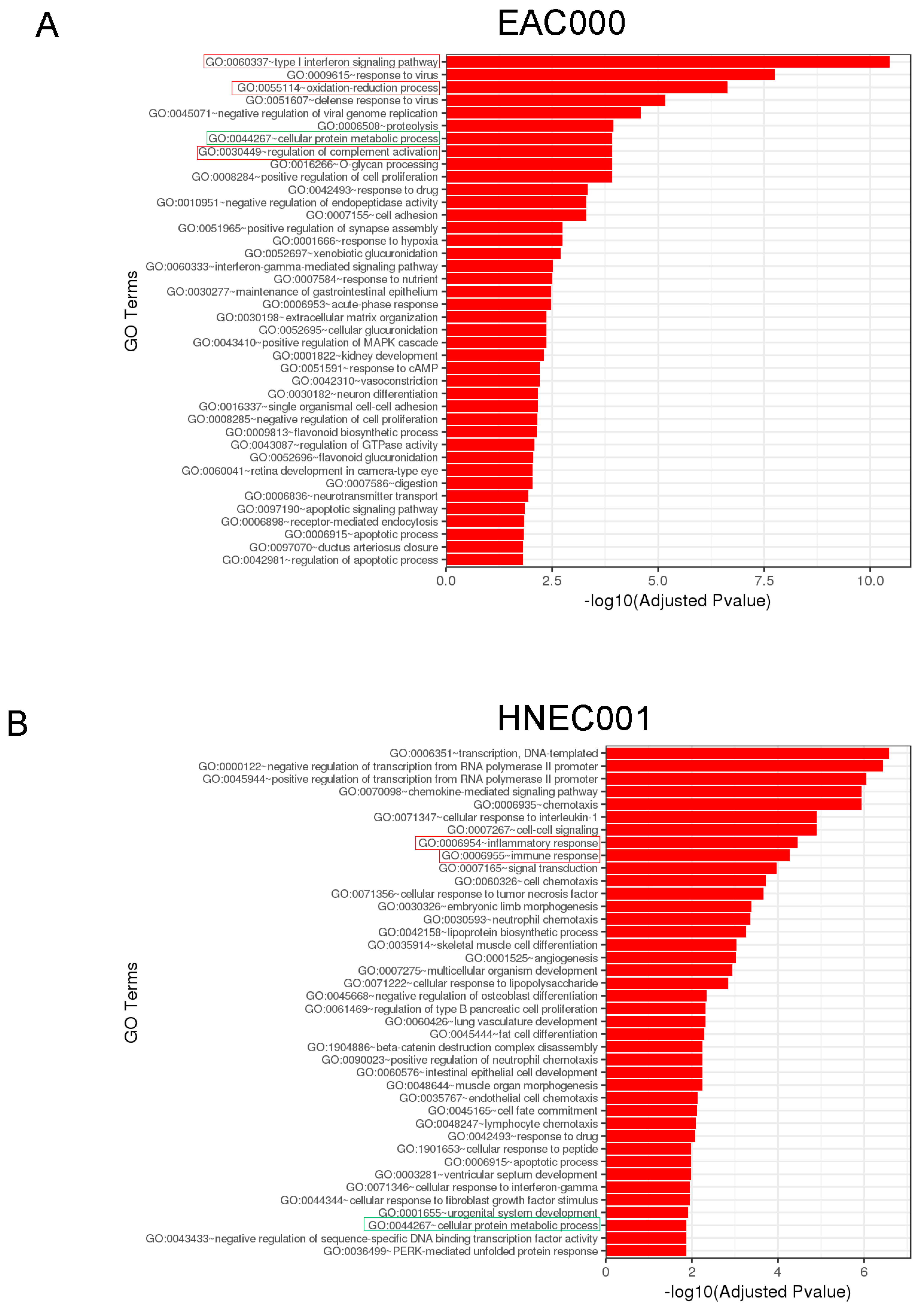
3.6. RNAseq and Gene Ontology Analyses Reveal Lactate-Mediated Downregulation of ID1 and RSAD2 along with Enrichment for Metabolic and Immunological Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hur, C.; Miller, M.; Kong, C.Y.; Dowling, E.C.; Nattinger, K.J.; Dunn, M.; Feuer, E.J. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013, 119, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- van Soest, E.M.; Dieleman, J.P.; Siersema, P.D.; Sturkenboom, M.C.J.M.; Kuipers, E.J. Increasing incidence of Barrett’s oesophagus in the general population. Gut 2005, 54, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.G.; Bhat, S.; Murray, L.J.; McManus, D.; Gavin, A.T.; Johnston, B.T. Increasing incidence of Barrett’s oesophagus: A population-based study. Eur. J. Epidemiol. 2011, 26, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, A.S.; Stabenau, K.A.; Altman, K.W.; Johnston, N. Cancer Risk in Barrett’s Esophagus: A Clinical Review. Int. J. Mol. Sci. 2023, 24, 6018. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Snider, E.J.; Compres, G.; Freedberg, D.E.; Giddins, M.J.; Khiabanian, H.; Lightdale, C.J.; Nobel, Y.R.; Toussaint, N.C.; Uhlemann, A.-C.; Abrams, J.A. Barrett’s esophagus is associated with a distinct oral microbiome. Clin. Transl. Gastroenterol. 2018, 9, e135. [Google Scholar] [CrossRef]
- Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Nobel, Y.R.; Stump, S.; Uhlemann, A.-C.; Lightdale, C.J.; Abrams, J.A. Alterations to the Esophageal Microbiome Associated with Progression from Barrett’s Esophagus to Esophageal Adenocarcinoma. Cancer Epidemiology Biomarkers Prev. 2019, 28, 1687–1693. [Google Scholar] [CrossRef]
- Solfisburg, Q.S.; Baldini, F.; Baldwin-Hunter, B.; Austin, G.I.; Lee, H.H.; Park, H.; Freedberg, D.E.; Lightdale, C.J.; Korem, T.; Abrams, J.A. The Salivary Microbiome and Predicted Metabolite Production Are Associated with Barrett’s Esophagus and High-Grade Dysplasia or Adenocarcinoma. Cancer Epidemiology Biomarkers Prev. 2023, 33, 371–380. [Google Scholar] [CrossRef]
- Zhou, J.; Shrestha, P.; Qiu, Z.; Harman, D.G.; Teoh, W.-C.; Al-Sohaily, S.; Liem, H.; Turner, I.; Ho, V. Distinct Microbiota Dysbiosis in Patients with Non-Erosive Reflux Disease and Esophageal Adenocarcinoma. J. Clin. Med. 2020, 9, 2162. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Liu, J.-J.; Zhao, H.-P.; Zhang, J.; Wang, J.-H. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2019, 25, 2149–2161. [Google Scholar] [CrossRef]
- Peter, S.; Pendergraft, A.; VanDerPol, W.; Wilcox, C.M.; Baig, K.R.K.K.; Morrow, C.; Izard, J.; Mannon, P.J. Mucosa-Associated Microbiota in Barrett’s Esophagus, Dysplasia, and Esophageal Adenocarcinoma Differ Similarly Compared With Healthy Controls. Clin. Transl. Gastroenterol. 2020, 11, e00199. [Google Scholar] [CrossRef] [PubMed]
- DE LA Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Hall, S. Probiotics, D–Lactic acidosis, oxidative stress and strain specificity. Gut Microbes 2017, 8, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Karakasheva, T.A.; Gabre, J.T.; Sachdeva, U.M.; Cruz-Acuña, R.; Lin, E.W.; DeMarshall, M.; Falk, G.W.; Ginsberg, G.G.; Yang, Z.; Kim, M.M.; et al. Patient-derived organoids as a platform for modeling a patient’s response to chemoradiotherapy in esophageal cancer. Sci. Rep. 2021, 11, 21304. [Google Scholar] [CrossRef]
- Brizel, D.M.; Prosnitz, R.G.; Hunter, S.; Fisher, S.R.; Clough, R.L.; Downey, M.A.; Scher, R.L. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int. J. Radiat. Oncol. 2004, 58, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Whelan, K.A.; Kagawa, S.; Tanaka, K.; Giroux, V.; Chandramouleeswaran, P.M.; Long, A.; Sahu, V.; Darling, D.S.; Que, J.; et al. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Sachdeva, U.M.; Shimonosono, M.; Flashner, S.; Cruz-Acuña, R.; Gabre, J.T.; Nakagawa, H. Understanding the cellular origin and progression of esophageal cancer using esophageal organoids. Cancer Lett. 2021, 509, 39–52. [Google Scholar] [CrossRef]
- Shimonosono, M.; Tanaka, K.; Flashner, S.; Takada, S.; Matsuura, N.; Tomita, Y.; Sachdeva, U.M.; Noguchi, E.; Sangwan, V.; Ferri, L.; et al. Alcohol Metabolism Enriches Squamous Cell Carcinoma Cancer Stem Cells That Survive Oxidative Stress via Autophagy. Biomolecules 2021, 11, 1479. [Google Scholar] [CrossRef]
- Ždralević, M.; Brand, A.; Di Ianni, L.; Dettmer, K.; Reinders, J.; Singer, K.; Peter, K.; Schnell, A.; Bruss, C.; Decking, S.-M.; et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J. Biol. Chem. 2018, 293, 15947–15961. [Google Scholar] [CrossRef]
- Becker, L.; Huang, Q.; Mashimo, H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett’s esophagus and esophageal adenocarcinoma. Dis. Esophagus 2010, 23, 168–174. [Google Scholar] [CrossRef]
- Neyaz, A.; Odze, R.D.; Rickelt, S.; Nieman, L.T.; Bledsoe, J.R.; Mahadevan, K.K.; Arora, K.; Jeck, W.R.; Taylor, M.S.; Gala, M.; et al. LGR5 in Barrett’s Esophagus and its Utility in Predicting Patients at Increased Risk of Advanced Neoplasia. Clin. Transl. Gastroenterol. 2020, 12, e00272. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Freeman, A.K.; Botelho, N.K.; Tran, D.; Levert-Mignon, A.J.M.; Lord, R.V.N. Expression of the putative stem cell marker Musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis. Esophagus 2010, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Quinn, W.J.; Jiao, J.; TeSlaa, T.; Stadanlick, J.; Wang, Z.; Wang, L.; Akimova, T.; Angelin, A.; Schäfer, P.M.; Cully, M.D.; et al. Lactate Limits T Cell Proliferation via the NAD(H) Redox State. Cell Rep. 2020, 33, 108500. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.H.; Vowles, J.; Browne, C.; McCullagh, J.; James, W.S. ddhCTP produced by the radical-SAM activity of RSAD2 (viperin) inhibits the NAD+-dependent activity of enzymes to modulate metabolism. FEBS Lett. 2020, 594, 1631–1644. [Google Scholar] [CrossRef]
- Ebrahimi, K.H.; Gilbert-Jaramillo, J.; James, W.S.; McCullagh, J.S. Interferon-stimulated gene products as regulators of central carbon metabolism. FEBS J. 2021, 288, 3715–3726. [Google Scholar] [CrossRef]
- Sharma, B.K.; Kolhe, R.; Black, S.M.; Keller, J.R.; Mivechi, N.F.; Satyanarayana, A. Inhibitor of differentiation 1 transcription factor promotes metabolic reprogramming in hepatocellular carcinoma cells. FASEB J. 2015, 30, 262–275. [Google Scholar] [CrossRef]
- Petrova, B.; Warren, A.; Vital, N.Y.; Culhane, A.J.; Maynard, A.G.; Wong, A.; Kanarek, N. Redox Metabolism Measurement in Mammalian Cells and Tissues by LC-MS. Metabolites 2021, 11, 313. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Gao, Y.; Pang, Y.; Xie, C.; Jiang, C. An Integrated LC-MS/MS Strategy for Quantifying the Oxidative-Redox Metabolome in Multiple Biological Samples. Anal. Chem. 2020, 92, 8810–8818. [Google Scholar] [CrossRef] [PubMed]
- Namin, B.M.; Daryani, N.E.; Mirshafiey, A.; Yazdi, M.K.S.; Dallal, M.M.S. Effect of probiotics on the expression of Barrett’s oesophagus biomarkers. J. Med Microbiol. 2015, 64, 348–354. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Mahieu, N.G.; Huang, X.; Singh, M.; A Crawford, P.; Johnson, S.L.; Gross, R.W.; Schaefer, J.; Patti, G.J. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 2016, 12, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Huang, X.; Yang, M.; Zhou, S.; Li, Z.; Fang, Z.; Tang, Y.; Chen, Q.; Hou, H.; et al. Lactate in the tumor microenvironment: A rising star for targeted tumor therapy. Front. Nutr. 2023, 10, 1113739. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.H.; Mitani, Y.; Li, T.; Sachdeva, U.; Flashner, S.; Klein-Szanto, A.; Dunbar, K.J.; Abrams, J.; Nakagawa, H.; Gabre, J. Lactate Suppresses Growth of Esophageal Adenocarcinoma Patient-Derived Organoids through Alterations in Tumor NADH/NAD+ Redox State. Biomolecules 2024, 14, 1195. https://doi.org/10.3390/biom14091195
Su SH, Mitani Y, Li T, Sachdeva U, Flashner S, Klein-Szanto A, Dunbar KJ, Abrams J, Nakagawa H, Gabre J. Lactate Suppresses Growth of Esophageal Adenocarcinoma Patient-Derived Organoids through Alterations in Tumor NADH/NAD+ Redox State. Biomolecules. 2024; 14(9):1195. https://doi.org/10.3390/biom14091195
Chicago/Turabian StyleSu, Steven H., Yosuke Mitani, Tianxia Li, Uma Sachdeva, Samuel Flashner, Andres Klein-Szanto, Karen J. Dunbar, Julian Abrams, Hiroshi Nakagawa, and Joel Gabre. 2024. "Lactate Suppresses Growth of Esophageal Adenocarcinoma Patient-Derived Organoids through Alterations in Tumor NADH/NAD+ Redox State" Biomolecules 14, no. 9: 1195. https://doi.org/10.3390/biom14091195
APA StyleSu, S. H., Mitani, Y., Li, T., Sachdeva, U., Flashner, S., Klein-Szanto, A., Dunbar, K. J., Abrams, J., Nakagawa, H., & Gabre, J. (2024). Lactate Suppresses Growth of Esophageal Adenocarcinoma Patient-Derived Organoids through Alterations in Tumor NADH/NAD+ Redox State. Biomolecules, 14(9), 1195. https://doi.org/10.3390/biom14091195




