A Novel Tandem Zinc Finger Protein in Gossypium hirsutum, GhTZF2, Interacts with GhMORF8 to Regulate Cotton Fiber Cell Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chromatin Immunoprecipitation
2.3. ChIP-Seq Library Building and Data Analysis
2.4. RNA Extraction and RT-qPCR Analysis
2.5. Screening of the Y2H Library and One-to-One Examination
2.6. LCI Assay
2.7. Sub-Cellular Localization via Transient Transfection into Tobacco Leaves
2.8. Co-IP Assay
2.9. mRNA Libraries Preparation and Transcriptome Analysis
3. Results
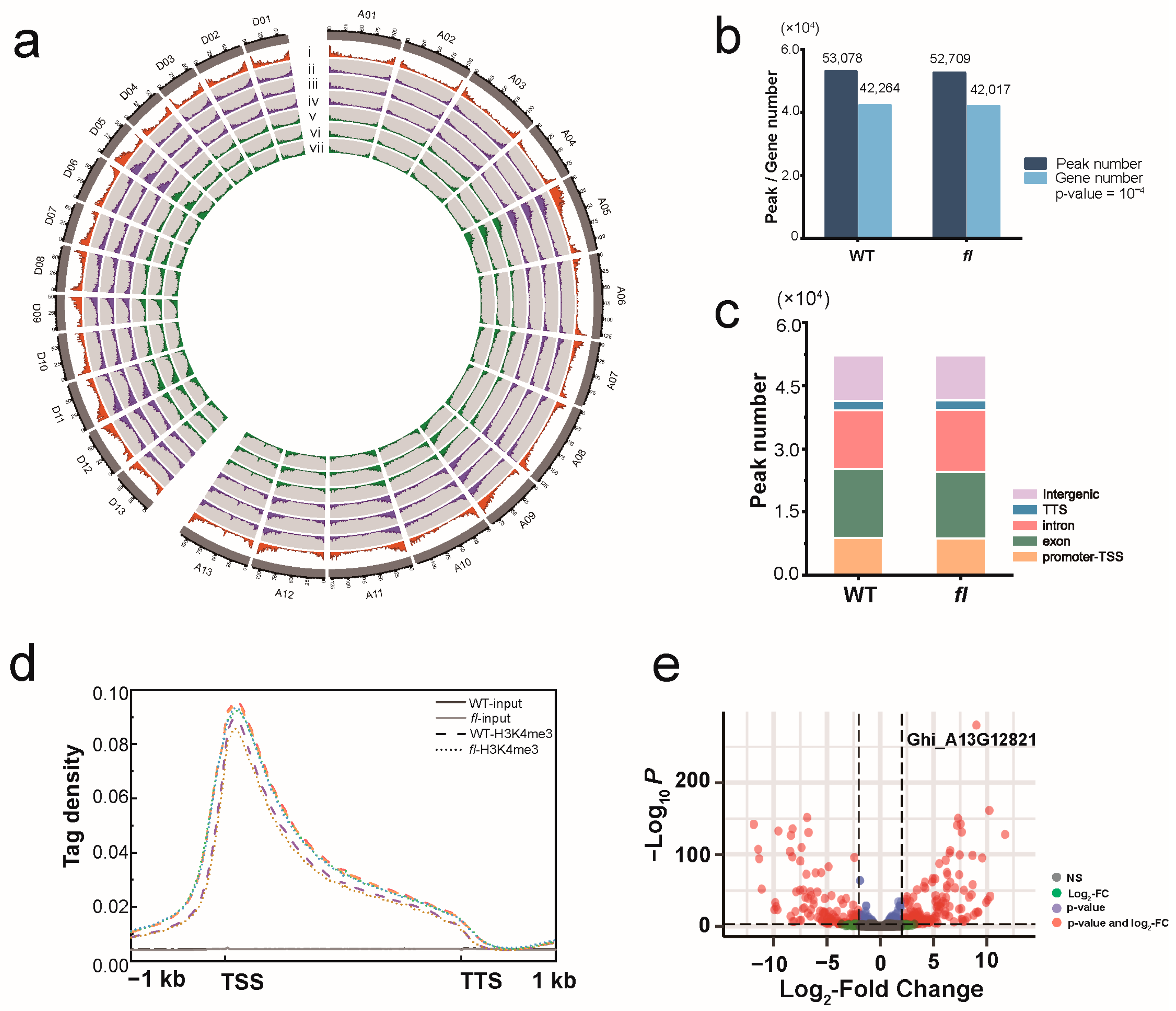
3.1. Visualizing Genome-Wide Enrichment Profiles of H3K4me3
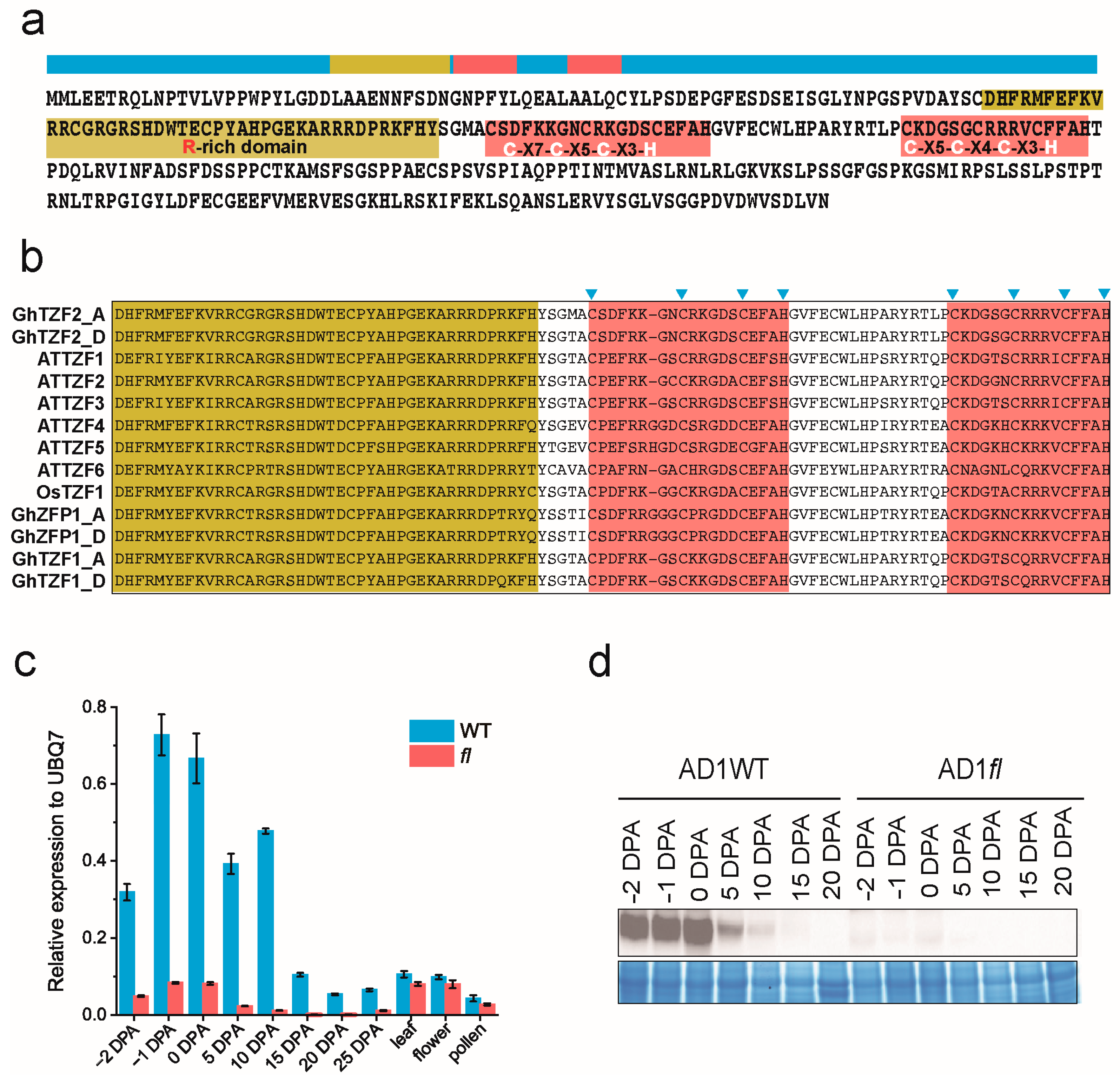
3.2. GhTZF2 Was Identified as a New RR-TZF Genes in Cotton
3.3. GhTZF2 Displayed Differential Expression Pattern in WT
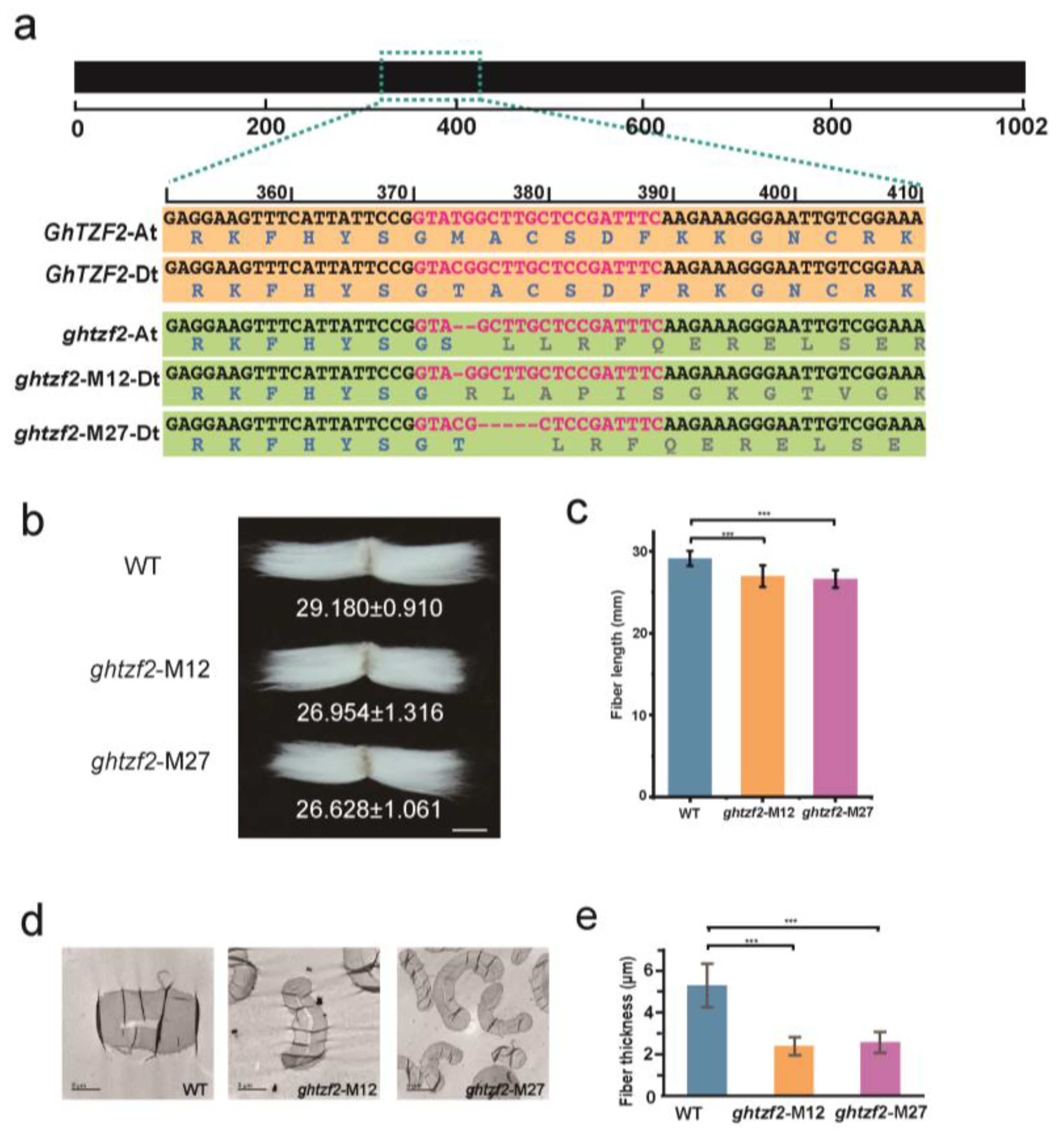
3.4. Morphological Alterations in GhTZF2-Knockout Lines
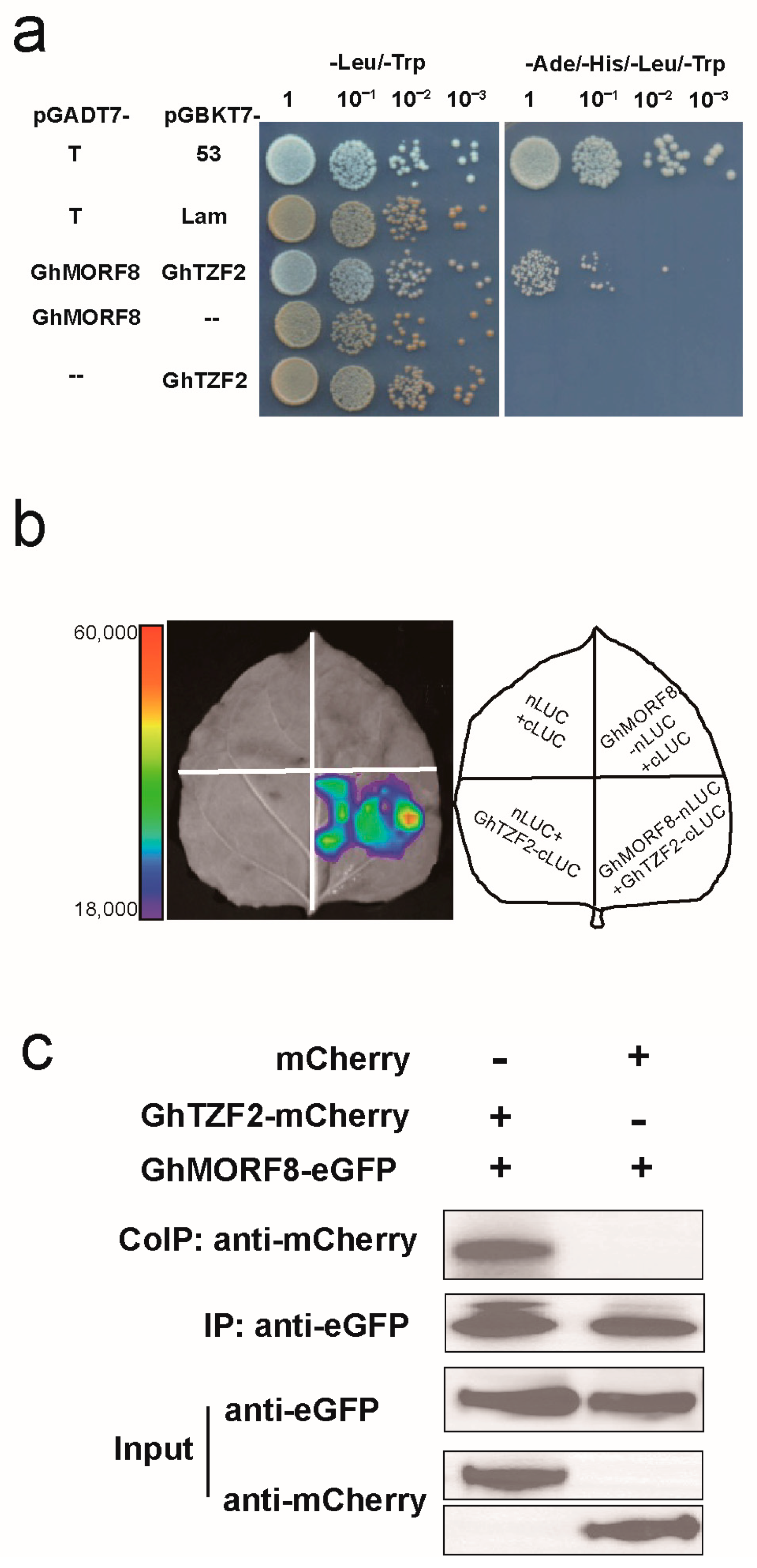
3.5. GhTZF2 Could Interact with GhMORF8
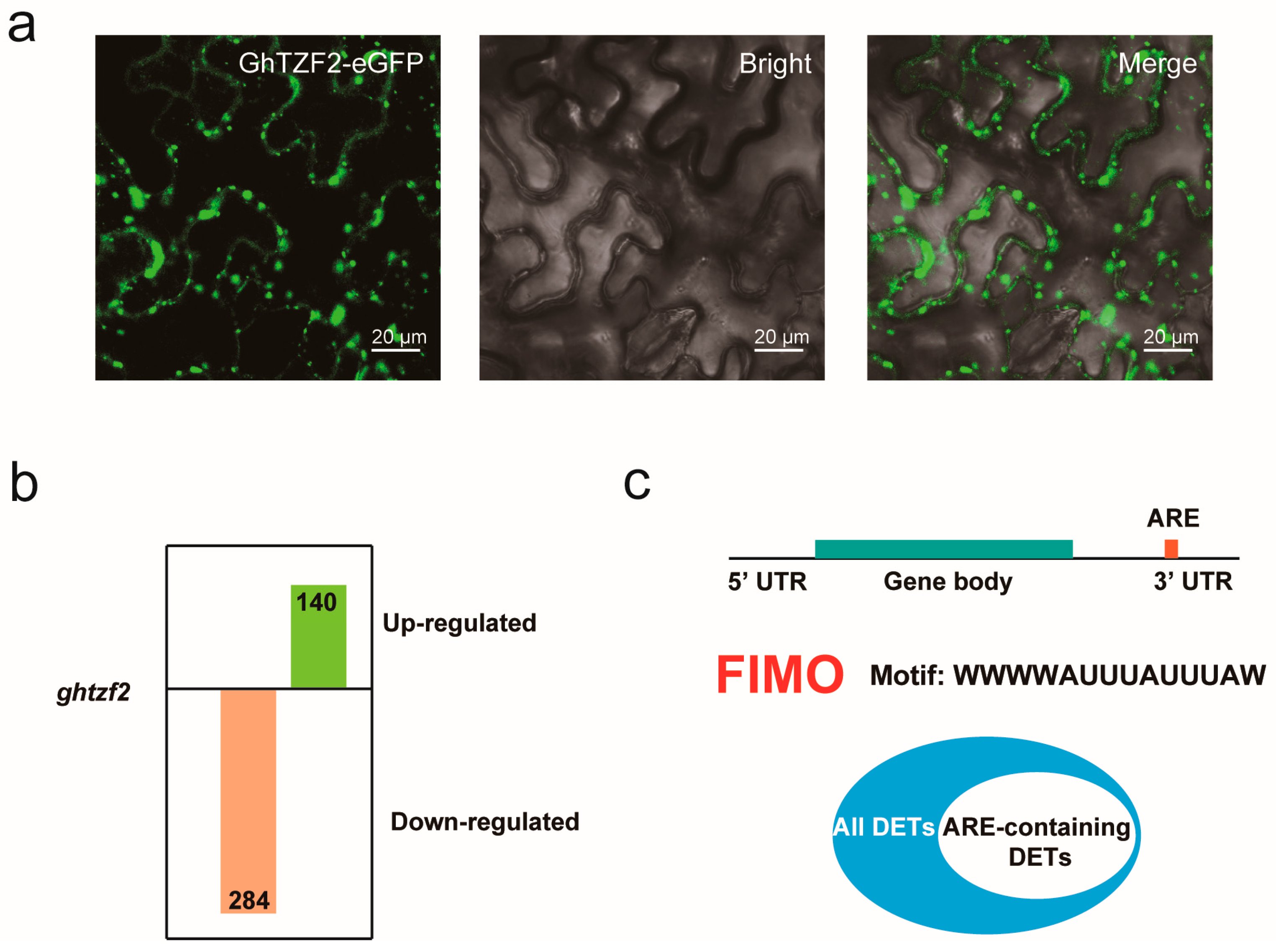
3.6. GhTZF2 Regulates mRNA Turnover by Forming Cytoplasmic Foci
4. Discussion
4.1. H3K4me3 Analysis Is an Efficient Strategy to Conduct Differentially Expressed Gene Analysis
4.2. GhTZF2 Functions to Determine the Fiber Diameter
4.3. GhTZF2 Might Be Involved in RNA Editing by Directly Interacting with GhMORF8
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Huang, J.Q.; Chen, X.Y.; Zhu, Y.X. Recent Advances and Future Perspectives in Cotton Research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Ucar, D.; Mahmoudi, S.; Karra, K.; Wong, E.D.; Devarajan, K.; Daugherty, A.C.; Kundaje, A.B.; Mancini, E.; et al. H3K4me3 Breadth Is Linked to Cell Identity and Transcriptional Consistency. Cell 2015, 163, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, H.; Wei, Q.; Zhao, X.; Wang, C.; Zhu, Q.; Yi, X.; Xu, W.; Liu, X.S.; Jin, W.; et al. Genome-wide analysis of histone modifications: H3K4me2, H3K4me3, H3K9ac, and H3K27ac in Oryza sativa L. Japonica. Mol. Plant 2013, 6, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Y.; Wu, C.; Yang, G.; Li, Y.; Zheng, C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008, 9, 44. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Y.; Cao, J.; Zhang, W.; Jiang, H.; Li, X.; Ma, Q.; Zhu, S.; Cheng, B. CCCH-type zinc finger family in maize: Genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS ONE 2012, 7, e40120. [Google Scholar] [CrossRef]
- Chai, G.; Hu, R.; Zhang, D.; Qi, G.; Zuo, R.; Cao, Y.; Chen, P.; Kong, Y.; Zhou, G. Comprehensive analysis of CCCH zinc finger family in poplar (Populus trichocarpa). BMC Genom. 2012, 13, 253. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide analysis of the CCCH zinc finger gene family in Medicago truncatula. Plant Cell Rep. 2013, 32, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.L.; Xu, Z.S.; Zhao, G.Y.; Cui, X.Y.; Chen, M.; Li, L.C.; Ma, Y.Z. Genome-Wide Analysis of the C3H Zinc Finger Transcription Factor Family and Drought Responses of Members in Aegilops tauschii. Plant Mol. Biol. Rep. 2014, 32, 1241–1256. [Google Scholar] [CrossRef]
- Xu, R. Genome-wide analysis and identification of stress-responsive genes of the CCCH zinc finger family in Solanum lycopersicum. Mol. Genet. Genom. 2014, 289, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhong, Y.; Cheng, Z.M. Evolution and Expression Analysis of the CCCH Zinc Finger Gene Family in Vitis vinifera. Plant Genome 2014, 7, plantgenome2014-05. [Google Scholar] [CrossRef]
- Yuan, S.; Xu, B.; Zhang, J.; Xie, Z.; Cheng, Q.; Yang, Z.; Cai, Q.; Huang, B. Comprehensive analysis of CCCH-type zinc finger family genes facilitates functional gene discovery and reflects recent allopolyploidization event in tetraploid switchgrass. BMC Genom. 2015, 16, 129. [Google Scholar] [CrossRef]
- Pradhan, S.; Kant, C.; Verma, S.; Bhatia, S. Genome-wide analysis of the CCCH zinc finger family identifies tissue specific and stress responsive candidates in chickpea (Cicer arietinum L.). PLoS ONE 2017, 12, e0180469. [Google Scholar] [CrossRef]
- Pi, B.; He, X.; Ruan, Y.; Jang, J.C.; Huang, Y. Genome-wide analysis and stress-responsive expression of CCCH zinc finger family genes in Brassica rapa. BMC Plant Biol. 2018, 18, 373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cao, J.; Gao, C.; Gao, W.; Yan, S.; Yao, H.; Xu, K.; Liu, X.; Xu, D.; Pan, X.; et al. Identification of the wheat C3H gene family and expression analysis of candidates associated with seed dormancy and germination. Plant Physiol. Biochem. 2020, 156, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.L.; Wang, K.; Gao, Y.M.; Wu, M.; Xiang, Y. Identification of CCCH Zinc Finger Proteins Family in Moso Bamboo (Phyllostachys edulis), and PeC3H74 Confers Drought Tolerance to Transgenic Plants. Front. Plant Sci. 2020, 11, 579255. [Google Scholar] [CrossRef]
- Pi, B.; Pan, J.; Xiao, M.; Hu, X.; Zhang, L.; Chen, M.; Liu, B.; Ruan, Y.; Huang, Y. Systematic analysis of CCCH zinc finger family in Brassica napus showed that BnRR-TZFs are involved in stress resistance. BMC Plant Biol. 2021, 21, 555. [Google Scholar] [CrossRef]
- Ai, Q.; Pan, W.; Zeng, Y.; Li, Y.; Cui, L. CCCH Zinc finger genes in Barley: Genome-wide identification, evolution, expression and haplotype analysis. BMC Plant Biol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Bogamuwa, S.P.; Jang, J.C. Tandem CCCH zinc finger proteins in plant growth, development and stress response. Plant Cell Physiol. 2014, 55, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wells, M.L.; Lai, W.S.; Hicks, S.N.; Burkholder, A.B.; Perera, L.; Kimmel, A.R.; Blackshear, P.J. A post-transcriptional regulon controlled by TtpA, the single tristetraprolin family member expressed in Dictyostelium discoideum. Nucleic Acids Res. 2021, 49, 11920–11937. [Google Scholar] [CrossRef] [PubMed]
- D’Orso, F.; De Leonardis, A.M.; Salvi, S.; Gadaleta, A.; Ruberti, I.; Cattivelli, L.; Morelli, G.; Mastrangelo, A.M. Conservation of AtTZF1, AtTZF2, and AtTZF3 homolog gene regulation by salt stress in evolutionarily distant plant species. Front. Plant Sci. 2015, 6, 394. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.Y.; Shim, J.S.; Bang, S.W.; Kim, J.K. Overexpression of OsC3H10, a CCCH-Zinc Finger, Improves Drought Tolerance in Rice by Regulating Stress-Related Genes. Plants 2020, 9, 1298. [Google Scholar] [CrossRef]
- Maldonado-Bonilla, L.D.; Eschen-Lippold, L.; Gago-Zachert, S.; Tabassum, N.; Bauer, N.; Scheel, D.; Lee, J. The Arabidopsis tandem zinc finger 9 protein binds RNA and mediates pathogen-associated molecular pattern-triggered immune responses. Plant Cell Physiol. 2014, 55, 412–425. [Google Scholar] [CrossRef]
- Pomeranz, M.; Lin, P.C.; Finer, J.; Jang, J.C. AtTZF gene family localizes to cytoplasmic foci. Plant Signal. Behav. 2010, 5, 190–192. [Google Scholar] [CrossRef]
- Pomeranz, M.; Finer, J.; Jang, J.C. Putative molecular mechanisms underlying tandem CCCH zinc finger protein mediated plant growth, stress, and gene expression responses. Plant Signal. Behav. 2011, 6, 647–651. [Google Scholar] [CrossRef]
- Kim, W.C.; Kim, J.Y.; Ko, J.H.; Kang, H.; Kim, J.; Han, K.H. AtC3H14, a plant-specific tandem CCCH zinc-finger protein, binds to its target mRNAs in a sequence-specific manner and affects cell elongation in Arabidopsis thaliana. Plant J. 2014, 80, 772–784. [Google Scholar] [CrossRef]
- Qu, J.; Kang, S.G.; Wang, W.; Musier-Forsyth, K.; Jang, J.C. The Arabidopsis thaliana tandem zinc finger 1 (AtTZF1) protein in RNA binding and decay. Plant J. 2014, 78, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Lei, Y.; Yang, S.; Wu, J.; Li, J.; Bao, B.; Cai, Y.; Wang, S.; Lin, J.; Wang, Y.; et al. CaC3H14 encoding a tandem CCCH zinc finger protein is directly targeted by CaWRKY40 and positively regulates the response of pepper to inoculation by Ralstonia solanacearum. Mol. Plant Pathol. 2018, 19, 2221–2235. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Zhi, Y.; Li, X.; Zhang, Q.; Niu, J.; Wang, J.; Zhai, H.; Zhao, N.; Li, J.; et al. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019, 223, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Jan, A.; Ishizaki, T.; Valencia, M.; Dedicova, B.; Maruyama, K.; Ogata, T.; Todaka, D.; Yamaguchi-Shinozaki, K.; Nakashima, K.; et al. Expression of the CCCH-tandem zinc finger protein gene OsTZF5 under a stress-inducible promoter mitigates the effect of drought stress on rice grain yield under field conditions. Plant Biotechnol. J. 2020, 18, 1711–1721. [Google Scholar] [CrossRef]
- Tang, X.F.; Wang, D.; Liu, Y.; Lu, M.Z.; Zhuang, Y.M.; Xie, Z.; Wang, C.P.; Wang, S.M.; Kong, Y.Z.; Chai, G.H.; et al. Dual regulation of xylem formation by an auxin-mediated PaC3H17-PaMYB199 module in Populus. New Phytol. 2020, 225, 1545–1561. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, X.; Liu, W.; Liu, T.; Yu, Y.; Cao, J. BcMF30a and BcMF30c, Two Novel Non-Tandem CCCH Zinc-Finger Proteins, Function in Pollen Development and Pollen Germination in Brassica campestris ssp. chinensis. Int. J. Mol. Sci. 2020, 21, 6428. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, G.; Lei, S.; Zhang, C.; Bin, X.; Huang, B. CCCH protein-PvCCCH69 acted as a repressor for leaf senescence through suppressing ABA-signaling pathway. Hortic. Res. 2021, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Qi, G.; Wang, D.; Zhuang, Y.; Xu, H.; Bai, Z.; Bai, M.Y.; Hu, R.; Wang, Z.Y.; Zhou, G.; et al. The CCCH Zinc Finger Protein C3H15 Negatively Regulates Cell Elongation by Inhibiting Brassinosteroid Signaling. Plant Physiol. 2022, 189, 285–300. [Google Scholar] [CrossRef]
- Guo, C.; Chen, L.; Cui, Y.; Tang, M.; Guo, Y.; Yi, Y.; Li, Y.; Liu, L.; Chen, L. RNA Binding Protein OsTZF7 Traffics Between the Nucleus and Processing Bodies/Stress Granules and Positively Regulates Drought Stress in Rice. Front. Plant Sci. 2022, 13, 802337. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Pak, S.; Zeng, M.; Sun, J.; Yu, S.; He, Y.; Li, C. PuC3H35 confers drought tolerance by enhancing lignin and proanthocyanidin biosynthesis in the roots of Populus ussuriensis. New Phytol. 2022, 233, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Yu, Y.P.; Wang, D.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zheng, C.C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, X.; Wang, L.; Xu, J.; Zhang, X. GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol. Biol. 2014, 85, 163–177. [Google Scholar] [CrossRef]
- Gendrel, A.V.; Lippman, Z.; Martienssen, R.; Colot, V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2005, 2, 213–218. [Google Scholar] [CrossRef]
- Huang, G.; Wu, Z.G.; Percy, R.G.; Bai, M.Z.; Li, Y.; Frelichowski, J.E.; Hu, J.; Wang, K.; Yu, J.Z.; Zhu, Y.X. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat. Genet. 2020, 52, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Ramirez, F.; Dundar, F.; Diehl, S.; Gruning, B.A.; Manke, T. deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014, 42, W187–W191. [Google Scholar] [CrossRef]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Howe, F.S.; Fischl, H.; Murray, S.C.; Mellor, J. Is H3K4me3 instructive for transcription activation. Bioessays 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Liu, W.; Li, J.; Li, C.; Kou, X.; Chen, J.; Zhao, Y.; Gao, H.; Wang, H.; et al. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 2016, 537, 558–562. [Google Scholar] [CrossRef]
- Tao, X.; Feng, S.; Zhao, T.; Guan, X. Efficient chromatin profiling of H3K4me3 modification in cotton using CUT&Tag. Plant Methods 2020, 16, 120. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Crevillen, P.; Yang, H.C.; Cui, X.; Greeff, C.; Trick, M.; Qiu, Q.; Cao, X.F.; Dean, C. Epigenetic reprogramming that prevents transgenerational inheritance of the vernalized state. Nature 2014, 515, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.C.; Wei, Y.Y.; Shi, Y.N.; Deng, X.J.; Gao, J.J.; Feng, Y.L.; Zheng, D.Y.; Cheng, X.J.; Li, Z.G.; Wang, T.; et al. Profiling of H3K4me3 and H3K27me3 and Their Roles in Gene Subfunctionalization in Allotetraploid Cotton. Front. Plant Sci. 2021, 12, 761059. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Lin, M.; Lin, Z.; Wang, P.; Yang, Q.; Ye, Z.; Shen, C.; Li, J.; Zhang, L.; et al. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nat. Genet. 2017, 49, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lopez-Arredondo, D.; Yu, G.; Wang, Y.; Wang, B.; Wall, S.B.; Zhang, X.; Fang, H.; Barragan-Rosillo, A.C.; Pan, X.; et al. Genome-wide chromatin accessibility analysis unveils open chromatin convergent evolution during polyploidization in cotton. Proc. Natl. Acad. Sci. USA 2022, 119, e2209743119. [Google Scholar] [CrossRef]
- You, Q.; Yi, X.; Zhang, K.; Wang, C.; Ma, X.; Zhang, X.; Xu, W.; Li, F.; Su, Z. Genome-wide comparative analysis of H3K4me3 profiles between diploid and allotetraploid cotton to refine genome annotation. Sci. Rep. 2017, 7, 9098. [Google Scholar] [CrossRef]
- Qin, Y.M.; Zhu, Y.X. How cotton fibers elongate: A tale of linear cell-growth mode. Curr. Opin. Plant Biol. 2011, 14, 106–111. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Nowak, J.; Wang, G.; Han, L.; Feng, Z.; Mendrinna, A.; Ma, Y.; Wang, H.; Zhang, X.; et al. Live-cell imaging of the cytoskeleton in elongating cotton fibres. Nat. Plants 2019, 5, 498–504. [Google Scholar] [CrossRef]
- Graham, B.P.; Haigler, C.H. Microtubules exert early, partial, and variable control of cotton fiber diameter. Planta 2021, 253, 47. [Google Scholar] [CrossRef]
- Stiff, M.R.; Haigler, C.H. Cotton fiber tips have diverse morphologies and show evidence of apical cell wall synthesis. Sci. Rep. 2016, 6, 27883. [Google Scholar] [CrossRef]
- Pu, L.; Li, Q.; Fan, X.P.; Yang, W.C.; Xue, Y.B. The R2R3 MYB Transcription Factor GhMYB109 Is Required for Cotton Fiber Development. Genetics 2008, 180, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.F.; Liang, X.O.; Pu, L.; Zhang, Y.S.; Xue, Y.B. Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.). Bba-Gene Struct. Expr. 2003, 1630, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, X.; Tu, L.; Zhu, L.; Zhu, S.; Ge, Z.; Zhang, X. GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. Plant J. 2016, 88, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Wu, Y.R.; Yang, Y.M.; Llewellyn, D.J.; Dennis, E.S. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009, 59, 52–62. [Google Scholar] [CrossRef]
- Shan, C.M.; Shangguan, X.X.; Zhao, B.; Zhang, X.F.; Chao, L.M.; Yang, C.Q.; Wang, L.J.; Zhu, H.Y.; Zeng, Y.D.; Guo, W.Z.; et al. Control of cotton fibre elongation by a homeodomain transcription factor GhHOX3. Nat. Commun. 2014, 5, 5519. [Google Scholar] [CrossRef]
- Pei, Y. The homeodomain-containing transcription factor, GhHOX3, is a key regulator of cotton fiber elongation. Sci. China Life Sci. 2015, 58, 309–310. [Google Scholar] [CrossRef]
- Walford, S.A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. Plant J. 2012, 71, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Walford, S.A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. GhMYB25-like: A key factor in early cotton fibre development. Plant J. 2011, 65, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, M.; Li, W.; Xu, M.; Shao, L.; Liu, Y.; Zhao, G.; Liu, Z.; Xu, Z.; You, J.; et al. Single-cell RNA-seq reveals fate determination control of an individual fibre cell initiation in cotton (Gossypium hirsutum). Plant Biotechnol. J. 2022, 20, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Xiao, G.; Liu, H.; Zhang, L.; Zhao, L.; Tang, M.; Huang, S.; An, Y.; Yu, J. Two pivotal RNA editing sites in the mitochondrial atp1mRNA are required for ATP synthase to produce sufficient ATP for cotton fiber cell elongation. New Phytol. 2018, 218, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, Q.; Yin, P. RNA editing machinery in plant organelles. Sci. China Life Sci. 2018, 61, 162–169. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, W.; Hedtke, B.; Zhong, L.; Liu, L.; Peng, L.; Lu, C.; Grimm, B.; Lin, R. Tetrapyrrole biosynthetic enzyme protoporphyrinogen IX oxidase 1 is required for plastid RNA editing. Proc. Natl. Acad. Sci. USA 2014, 111, 2023–2028. [Google Scholar] [CrossRef]
- Sun, T.; Shi, X.; Friso, G.; Van Wijk, K.; Bentolila, S.; Hanson, M.R. A zinc finger motif-containing protein is essential for chloroplast RNA editing. PLoS Genet. 2015, 11, e1005028. [Google Scholar] [CrossRef]
- Sun, T. Expanded Function of the P-Type Pentatricopeptide Repeat Protein ATP4 in RNA Editing. Plant Physiol. 2020, 184, 1625–1626. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Q.; Guan, Z.; Wang, Q.; Li, L.; Ruan, F.; Lin, R.; Zou, T.; Yin, P. MORF9 increases the RNA-binding activity of PLS-type pentatricopeptide repeat protein in plastid RNA editing. Nat. Plants 2017, 3, 17037. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.B.; Shi, X.W.; Kobylarz, A.T.; Lucas, M.K.; Wessendorf, R.L.; Hines, K.M.; Bentolila, S.; Hanson, M.R.; Lu, Y. An Organelle RNA Recognition Motif Protein Is Required for Photosystem II Subunit psbF Transcript Editing. Plant Physiol. 2017, 173, 2278–2293. [Google Scholar] [CrossRef]
- Hartel, B.; Zehrmann, A.; Verbitskiy, D.; van der Merwe, J.A.; Brennicke, A.; Takenaka, M. MEF10 is required for RNA editing at nad2-842 in mitochondria of Arabidopsis thaliana and interacts with MORF8. Plant Mol. Biol. 2013, 81, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Brehme, N.; Bayer-Csaszar, E.; Glass, F.; Takenaka, M. The DYW Subgroup PPR Protein MEF35 Targets RNA Editing Sites in the Mitochondrial rpl16, nad4 and cob mRNAs in Arabidopsis thaliana. PLoS ONE 2015, 10, e0140680. [Google Scholar] [CrossRef] [PubMed]
- Glass, F.; Hartel, B.; Zehrmann, A.; Verbitskiy, D.; Takenaka, M. MEF13 Requires MORF3 and MORF8 for RNA Editing at Eight Targets in Mitochondrial mRNAs in Arabidopsis thaliana. Mol. Plant 2015, 8, 1466–1477. [Google Scholar] [CrossRef]
- Li, M.; Xia, L.; Zhang, Y.; Niu, G.; Li, M.; Wang, P.; Zhang, Y.; Sang, J.; Zou, D.; Hu, S.; et al. Plant editosome database: A curated database of RNA editosome in plants. Nucleic Acids Res. 2019, 47, D170–D174. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yu, J.; Zhang, Y.; Xie, Y.; Wu, B.; Miao, Y. MORF9 Functions in Plastid RNA Editing with Tissue Specificity. Int. J. Mol. Sci. 2019, 20, 4635. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xi, W.; Hao, J.; Zhang, L.; Wen, X.; Wu, Z.; Zhu, Y. A Novel Tandem Zinc Finger Protein in Gossypium hirsutum, GhTZF2, Interacts with GhMORF8 to Regulate Cotton Fiber Cell Development. Agronomy 2023, 13, 519. https://doi.org/10.3390/agronomy13020519
Li Y, Xi W, Hao J, Zhang L, Wen X, Wu Z, Zhu Y. A Novel Tandem Zinc Finger Protein in Gossypium hirsutum, GhTZF2, Interacts with GhMORF8 to Regulate Cotton Fiber Cell Development. Agronomy. 2023; 13(2):519. https://doi.org/10.3390/agronomy13020519
Chicago/Turabian StyleLi, Yang, Wei Xi, Jianfeng Hao, Li Zhang, Xingpeng Wen, Zhiguo Wu, and Yuxian Zhu. 2023. "A Novel Tandem Zinc Finger Protein in Gossypium hirsutum, GhTZF2, Interacts with GhMORF8 to Regulate Cotton Fiber Cell Development" Agronomy 13, no. 2: 519. https://doi.org/10.3390/agronomy13020519
APA StyleLi, Y., Xi, W., Hao, J., Zhang, L., Wen, X., Wu, Z., & Zhu, Y. (2023). A Novel Tandem Zinc Finger Protein in Gossypium hirsutum, GhTZF2, Interacts with GhMORF8 to Regulate Cotton Fiber Cell Development. Agronomy, 13(2), 519. https://doi.org/10.3390/agronomy13020519






