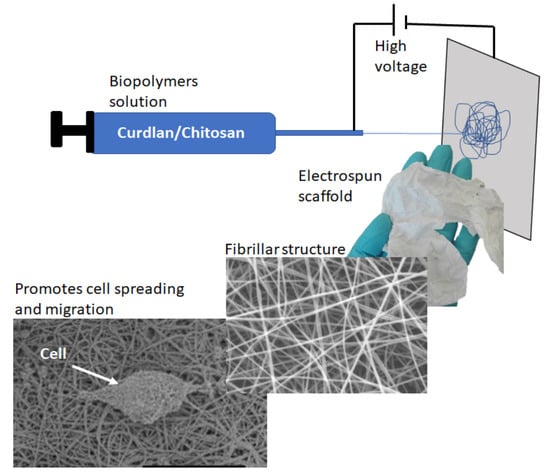
Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning of Chitosan and Curdlan–Chitosan into Fibrillar Scaffolds
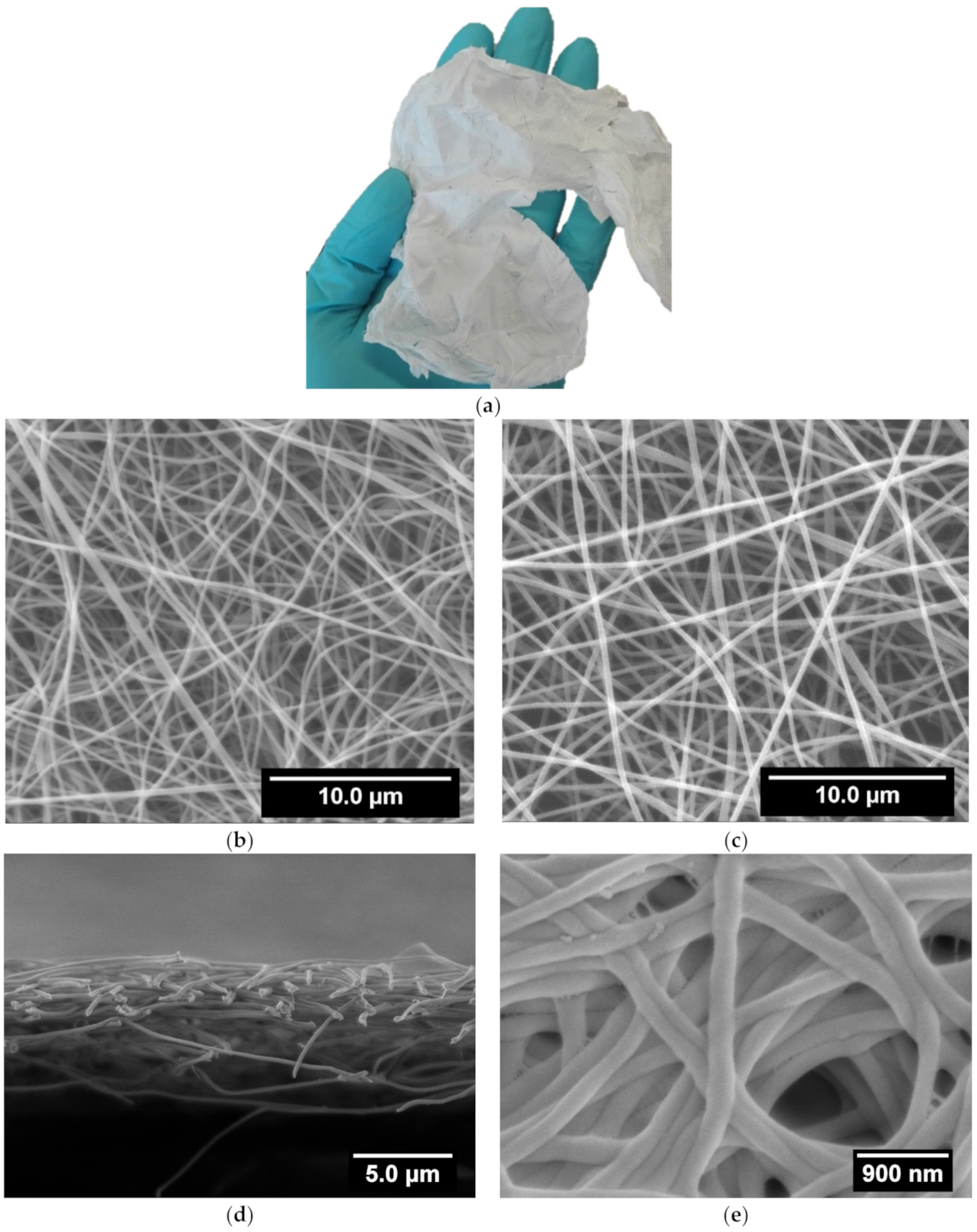
2.3. Characterization of the Fibrillar Scaffolds
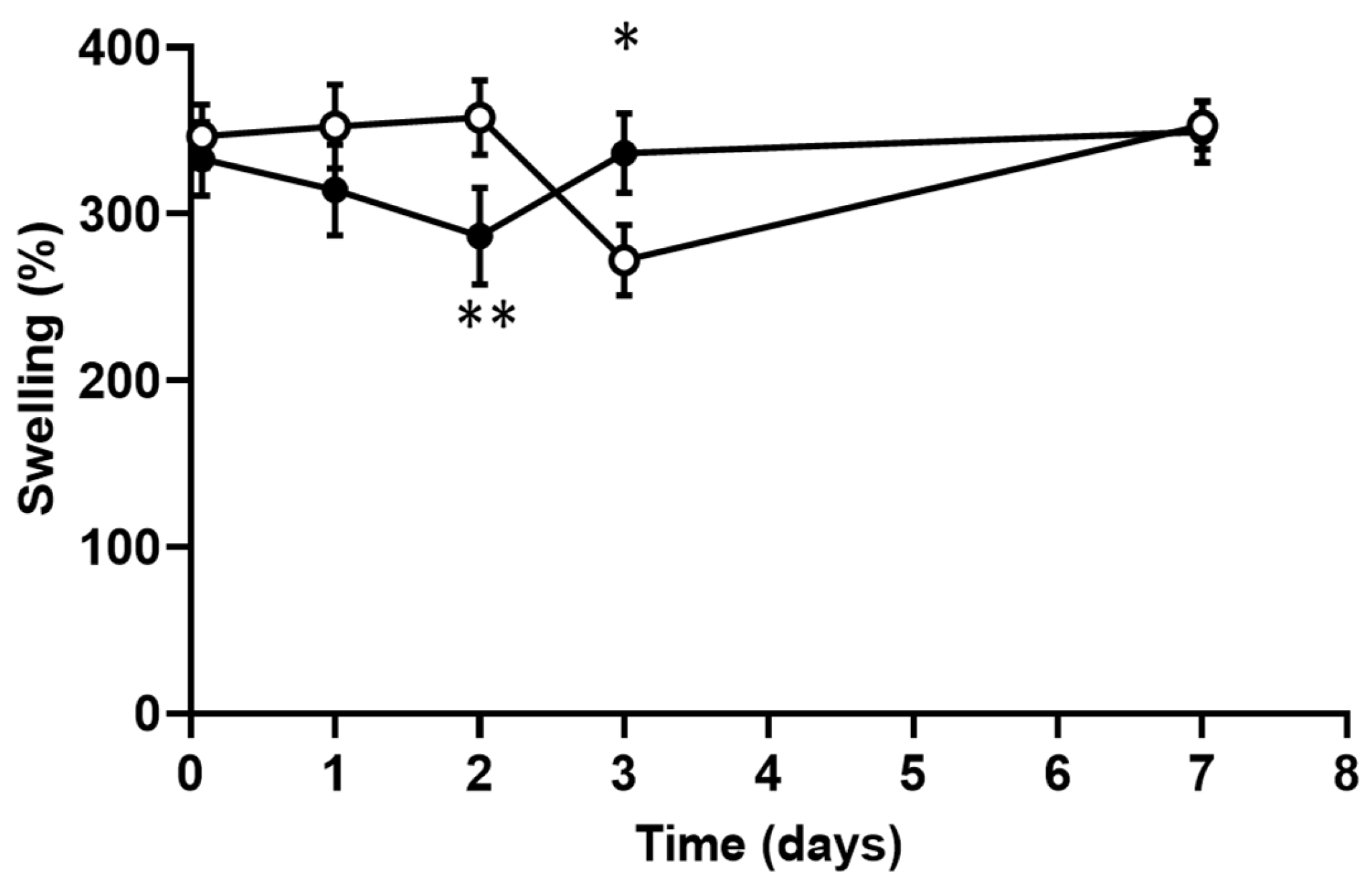
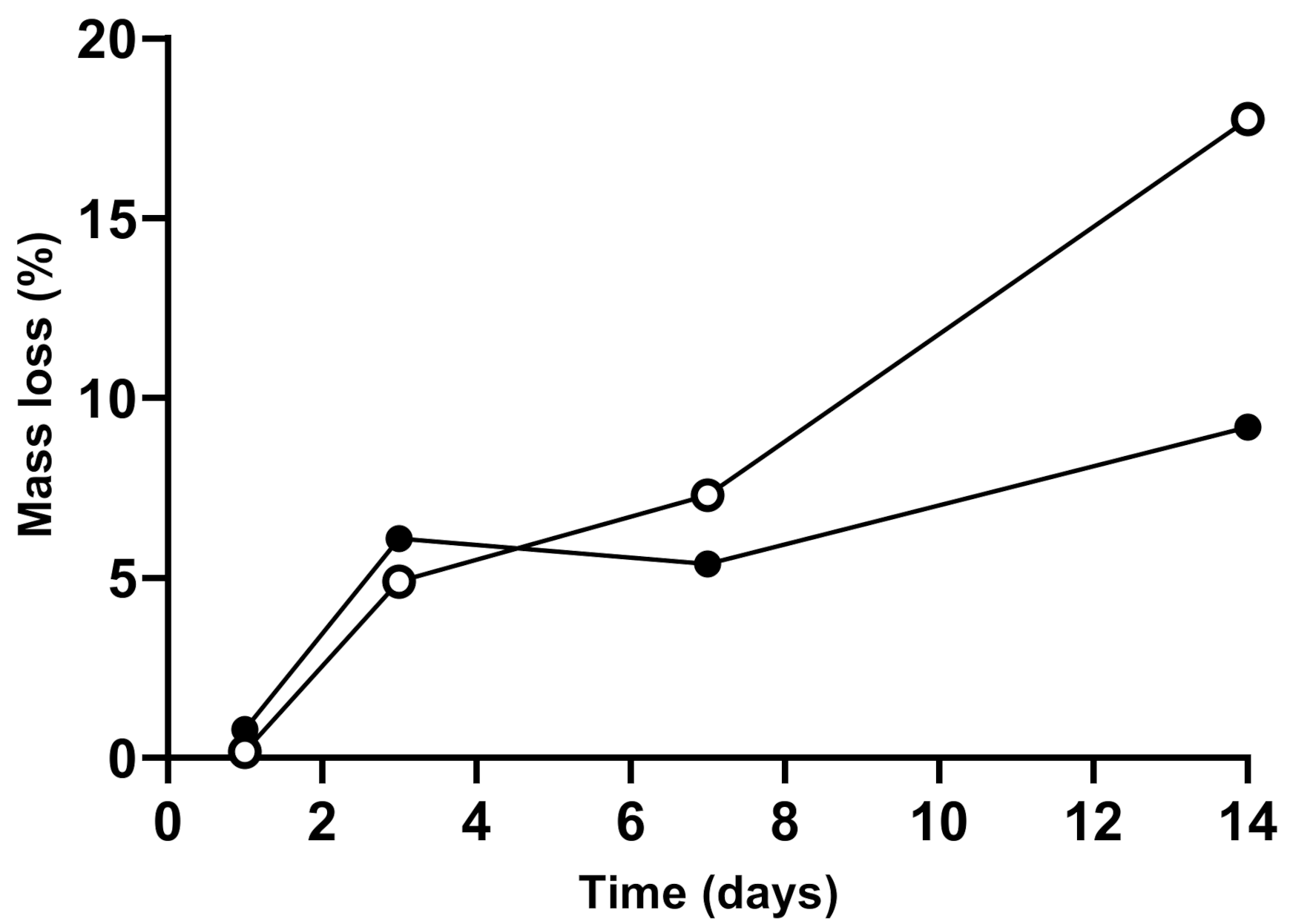
2.4. Swelling and Degradation Experiments
2.5. Cytotoxicity Test
2.6. Cell Spreading Adhesion Test
2.7. Scratch Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Scaffolds Preparation and Morphology
3.2. Scaffolds Chemical Composition
3.3. Scaffolds Swelling and Degradation
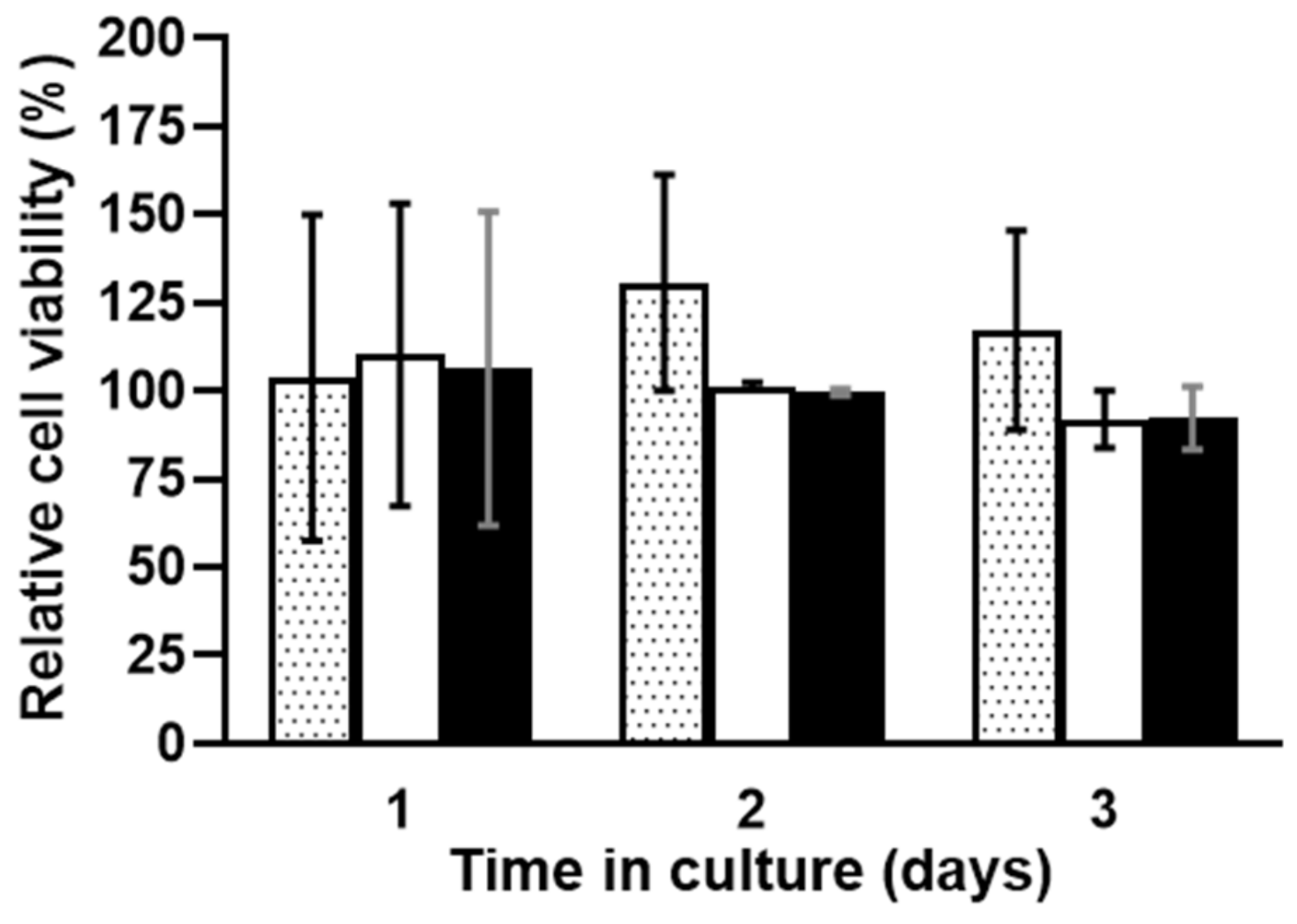
3.4. In Vitro Scaffold Cytotoxicity Assays
3.5. Cell Migration Study
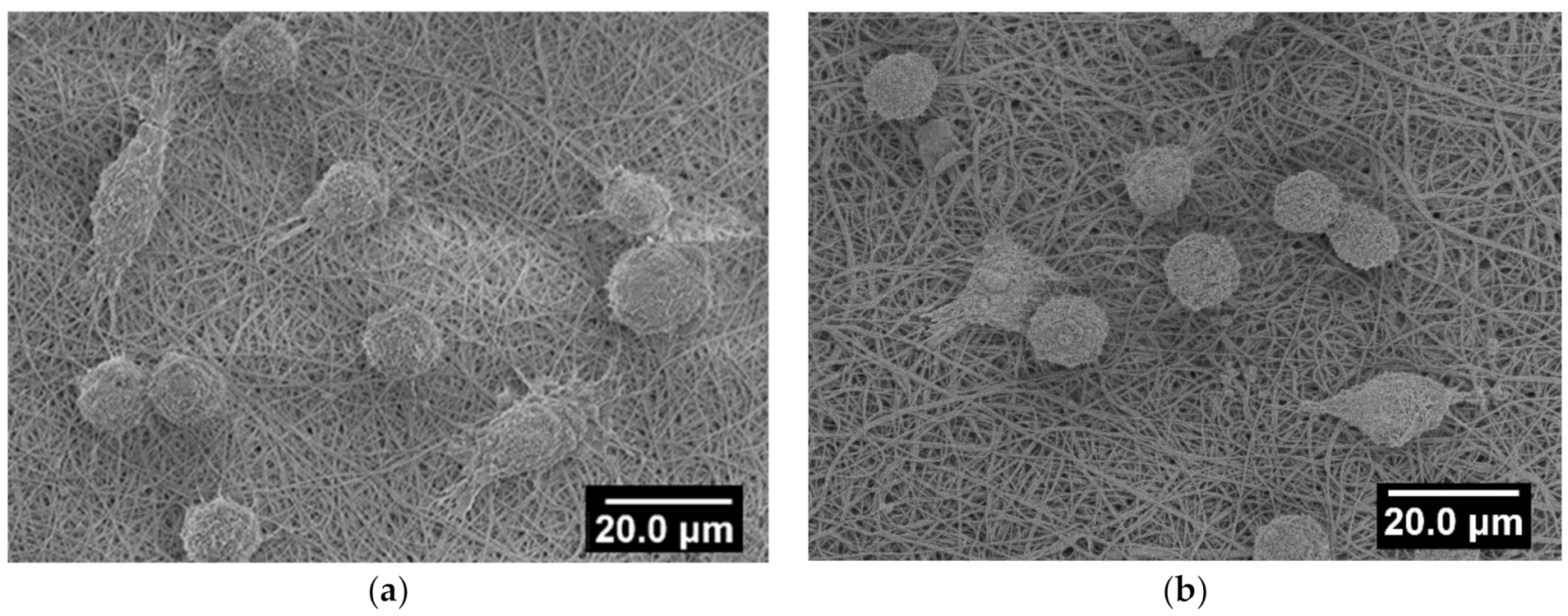
3.6. Cell Spreading Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shegarfi, H.; Reikeras, O. Bone transplantation and immune response. J. Orthop. Surg. 2009, 2, 206–211. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24, S36. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Donglu, S. Introduction to Biomaterials; Tsinghua University Press: Beijing, China, 2005. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progr. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Van de Vord, P.J.; Matthew, H.W.T.; De Silva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Khoulenjani, S.; Taghizadeh, S.M.; Mirzadeh, H. An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Rane, K.D.; Hoover, D.G. Production of Chitosan by fungi. Food Biotechnol. 1993, 7, 11–33. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in Nanostructured Thin Films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Leedy, M.R.; Martin, H.J.; Norowski, P.A.; Jennings, J.A.; Haggard, W.O.; Bumgardner, J.D. Use of Chitosan as a Bioactive Implant Coating for Bone-Implant Applications. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–165. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. In vitro evaluation of the risk of inflammatory response after chitosan/HA and chitosan/β-1,3-glucan/HA bone scaffold implantation. Mater. Sci. Eng. C 2016, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 131–137. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Bösiger, P.; Tegl, G.; Richard, I.M.T.; Le Gat, L.; Huber, L.; Stagl, V.; Mensah, A.; Guebitz, G.M.; Rossi, R.M.; Fortunato, G. Enzyme functionalized electrospun chitosan mats for antimicrobial treatment. Carbohydr. Polym. 2018, 181, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int. J. Pharm. 2012, 427, 379–384. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.-Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Ghadri, N.; Anderson, K.M.; Adatrow, P.; Stein, S.H.; Su, H.; Garcia-Godoy, F.; Karydis, A.; Bumgardner, J.D. Evaluation of Bone Regeneration of Simvastatin Loaded Chitosan Nanofiber Membranes in Rodent Calvarial Defects. J. Biomater. Nanobiotechnol. 2018, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Li, X.J.; Lin, M.; Li, Y.Y.; Dong, Y. Development of a novel absorbable nanofiber chitosan-collagen membrane by electrospinning and the preliminary study on guided bone regeneration. Zhonghua Kou Qiang Yi Xue Za Zhi 2018, 53, 85–91. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1→3)-β-d-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef]
- Mohsin, A.; Sun, J.; Khan, I.M.; Hang, H.; Tariq, M.; Tian, X.; Ahmed, W.; Niazi, S.; Zhuang, Y.; Chu, J.; et al. Sustainable biosynthesis of curdlan from orange waste by using Alcaligenes faecalis: A systematically modeled approach. Carbohydr. Polym. 2019, 205, 626–635. [Google Scholar] [CrossRef]
- Jezequel, V. Curdlan: A new functional β-glucan. Cereal Foods World 1998, 43, 361–364. [Google Scholar]
- Zhang, R.; Edgar, K. Properties, Chemistry, and Applications of the Bioactive Polysaccharide Curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Reynolds, J.; Law, W.-C.; Maponga, C.; Prasad, P.; Morse, G. Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. Nanomedicine 2014, 10, 831–838. [Google Scholar] [CrossRef]
- Hetland, G.; Sandven, P. β-1,3-Glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol. Med. Microbiol. 2002, 33, 41–45. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatanaka, K.; Toshiyuki, U.; Kaneko, Y.; Eiichiro, S.; Hiroshi, M.; Toru, M.; Osamu, Y.; Yamamoto, N. Synthesis and structural analysis of curdlan sulfate with a potent inhibitory effect in vitro of AIDS virus infection. Macromolecules 1990, 23, 3717–3722. [Google Scholar] [CrossRef]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshiga, K. Activation of Macrophages by Linear (1→3)-β-D-Glucans. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef] [Green Version]
- Przekora, A.; Benko, A.; Blazewicz, M.; Ginalska, G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomed. Mater. 2016, 11, 045001. [Google Scholar] [CrossRef]
- Yamasaki, T.; Ariyoshi, W.; Okinaga, T.; Adachi, Y.; Hosokawa, R.; Mochizuki, S.; Sakurai, K.; Nishihara, T. Dectin-1 Agonist, Curdlan, Regulates Osteoclastogenesis by Inhibiting Nuclear Factor of Activated T-cells Cytoplasmic 1 through Syk Kinase. J. Biol. Chem. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizawa, M.; Watanabe, K.; Tominari, T.; Matsumoto, C.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Low Molecular-Weight Curdlan, (1→3)-β-Glucan Suppresses TLR2-Induced RANKL-Dependent Bone Resorption. Biol. Pharm. Bull. 2018, 41, 1282–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przekora, A.; Palka, K.; Ginalska, G. Chitosan/β-1,3-glucan/calcium phosphate ceramics composites—Novel cell scaffolds for bone tissue engineering application. J. Biotechnol. 2014, 182–183, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Tu, L.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vepari, C.; Jin, H.-J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Sayyari, N. Electrospun triazole-based chitosan nanofibers as a novel scaffolds for bone tissue repair and regeneration. Carbohydr. Polym. 2020, 230, 115707. [Google Scholar] [CrossRef]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filee, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noel, A.; Jerome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.Y.; Kumar, T.S.S.; Selvaraj, R.; Doble, M. Silver Loaded Nanofibrous Curdlan Mat for Diabetic Wound Healing: An In Vitro and In Vivo Study. Macromol. Mater. Eng. 2018, 303, 1800234. [Google Scholar] [CrossRef]
- Basha, R.Y.; Kumar, T.S.S.; Doble, M. Electrospun Nanofibers of Curdlan (β-1,3 Glucan) Blend as a Potential Skin Scaffold Material. Macromol. Mater. Eng. 2017, 302, 1600417. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Min, B.-M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Schoenmaker, B.; Kalaoglu, Ö.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Wade, R.J.; Burdick, J.A. Engineering ECM signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Grandpierre, C.; Janssen, H.-G.; Laroche, C.; Michaud, P.; Warrand, J. Enzymatic and chemical degradation of curdlan targeting the production of β-(1→3) oligoglucans. Carbohydr. Polym. 2008, 71, 277–286. [Google Scholar] [CrossRef]
- Berdal, M.; Appelbom, H.I.; Eikrem, J.H.; Lund, Å.; Zykova, S.; Busund, L.-T.; Seljelid, R.; Jenssen, T. Aminated β-1,3-d-glucan improves wound healing in diabetic db/db mice. Wound Repair Regen. 2007, 15, 825–832. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Williams, D.L.; Browder, I.W. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen. 2002, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.F.; Cordeiro, A.L.; Sampaio, P.; Barbosa, M.A. Attachment, spreading and short-term proliferation of human osteoblastic cells cultured on chitosan films with different degrees of acetylation, Journal of Biomaterials Science. Polym. Ed. 2007, 18, 469–485. [Google Scholar] [CrossRef]
- Asghari Sana, F.; Çapkın Yurtsever, M.; Kaynak Bayrak, G.; Tunçay, E.Ö.; Kiremitçi, A.S.; Gümüşderelioğlu, M. Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin. Cytotechnology 2017, 69, 617–630. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toullec, C.; Le Bideau, J.; Geoffroy, V.; Halgand, B.; Buchtova, N.; Molina-Peña, R.; Garcion, E.; Avril, S.; Sindji, L.; Dube, A.; et al. Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration. Polymers 2021, 13, 526. https://doi.org/10.3390/polym13040526
Toullec C, Le Bideau J, Geoffroy V, Halgand B, Buchtova N, Molina-Peña R, Garcion E, Avril S, Sindji L, Dube A, et al. Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration. Polymers. 2021; 13(4):526. https://doi.org/10.3390/polym13040526
Chicago/Turabian StyleToullec, Clément, Jean Le Bideau, Valerie Geoffroy, Boris Halgand, Nela Buchtova, Rodolfo Molina-Peña, Emmanuel Garcion, Sylvie Avril, Laurence Sindji, Admire Dube, and et al. 2021. "Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration" Polymers 13, no. 4: 526. https://doi.org/10.3390/polym13040526
APA StyleToullec, C., Le Bideau, J., Geoffroy, V., Halgand, B., Buchtova, N., Molina-Peña, R., Garcion, E., Avril, S., Sindji, L., Dube, A., Boury, F., & Jérôme, C. (2021). Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration. Polymers, 13(4), 526. https://doi.org/10.3390/polym13040526