Study on the Electrochemical Removal Mechanism of Oxytetracycline by a Ti/IrO2-Ta2O5 Plate
Abstract
1. Introduction
2. Materials and Methods
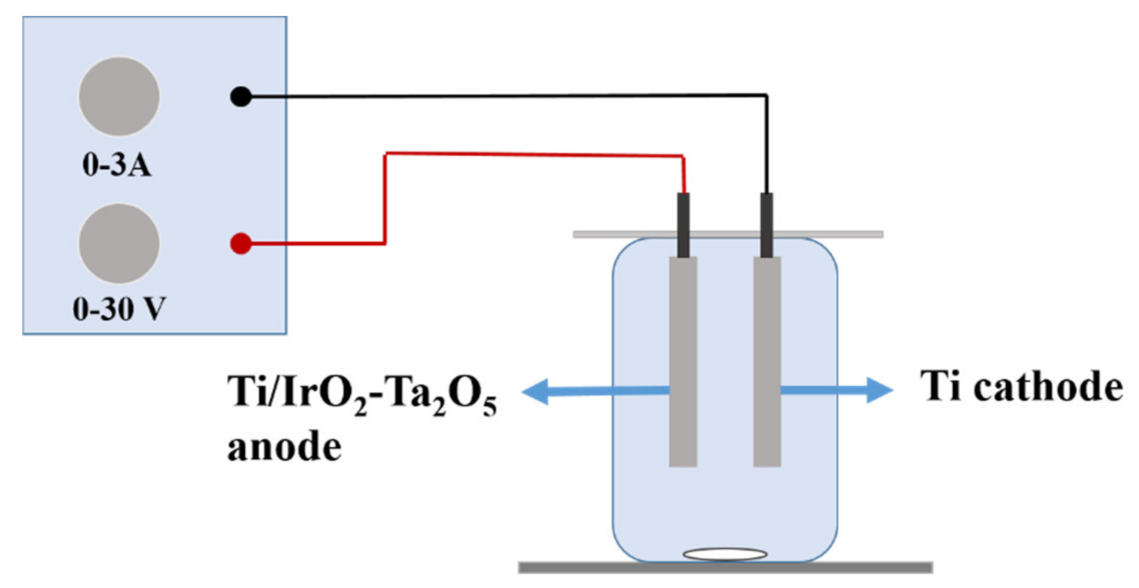
2.1. Experimental Equipment and Materials
2.2. Electrolytic Experiment Scheme
2.3. Preparation of Electrodes
2.4. Analysis Method
2.5. Electrolytic Data Analysis
3. Results and Discussion
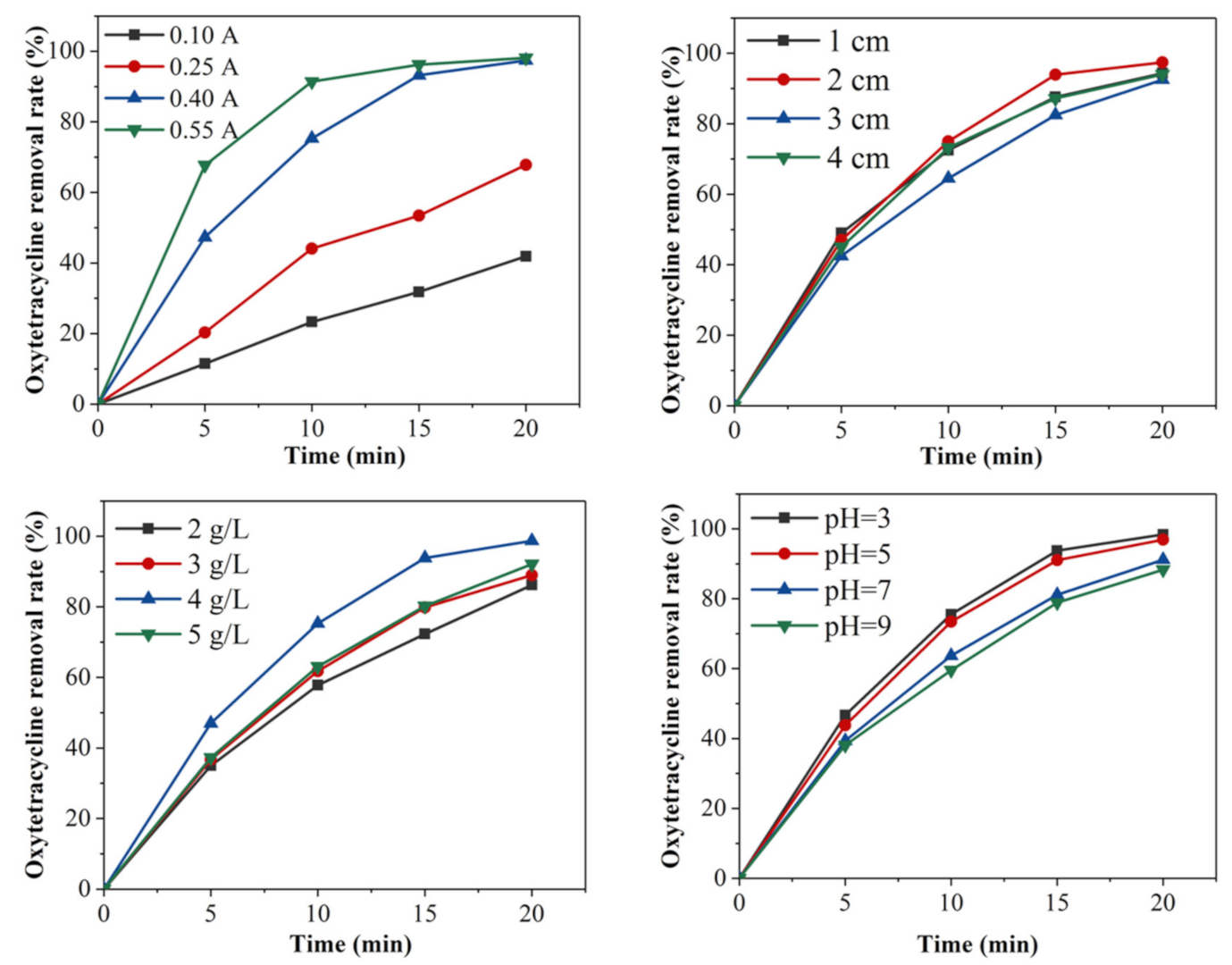
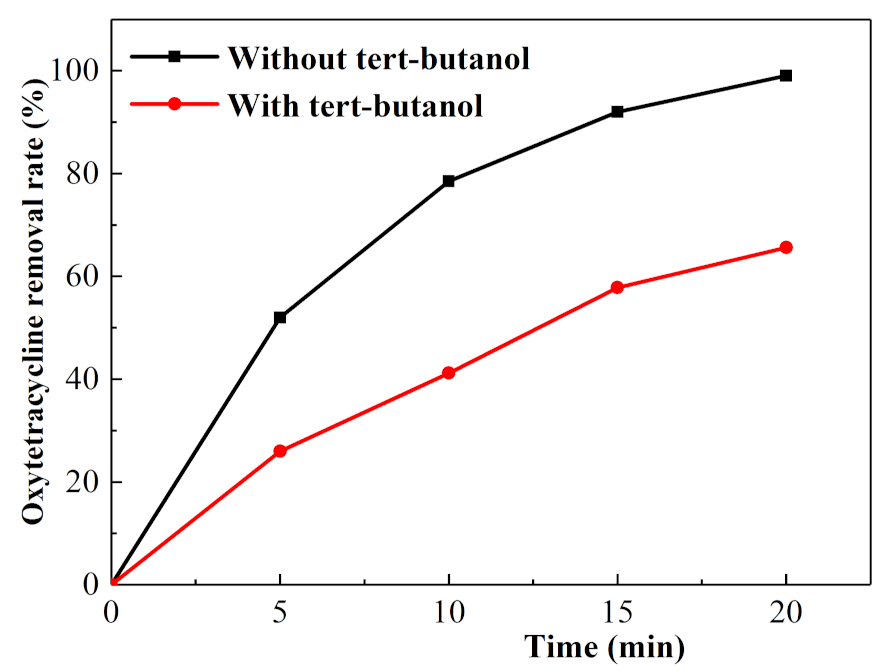
3.1. Factors Influencing the Degradation Effect of Oxytetracycline
3.2. Orthogonal Experiments for Determining Optimal Conditions
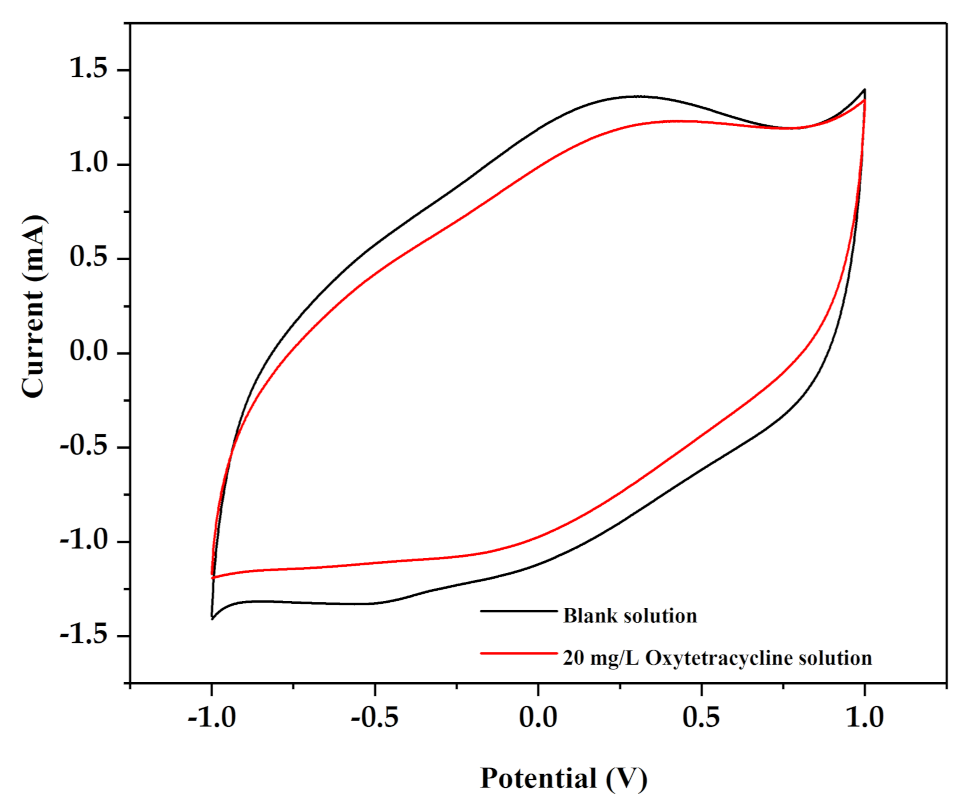
3.3. Characterization of the Ti/IrO2-Ta2O5 Anode
3.3.1. SEM Characterization
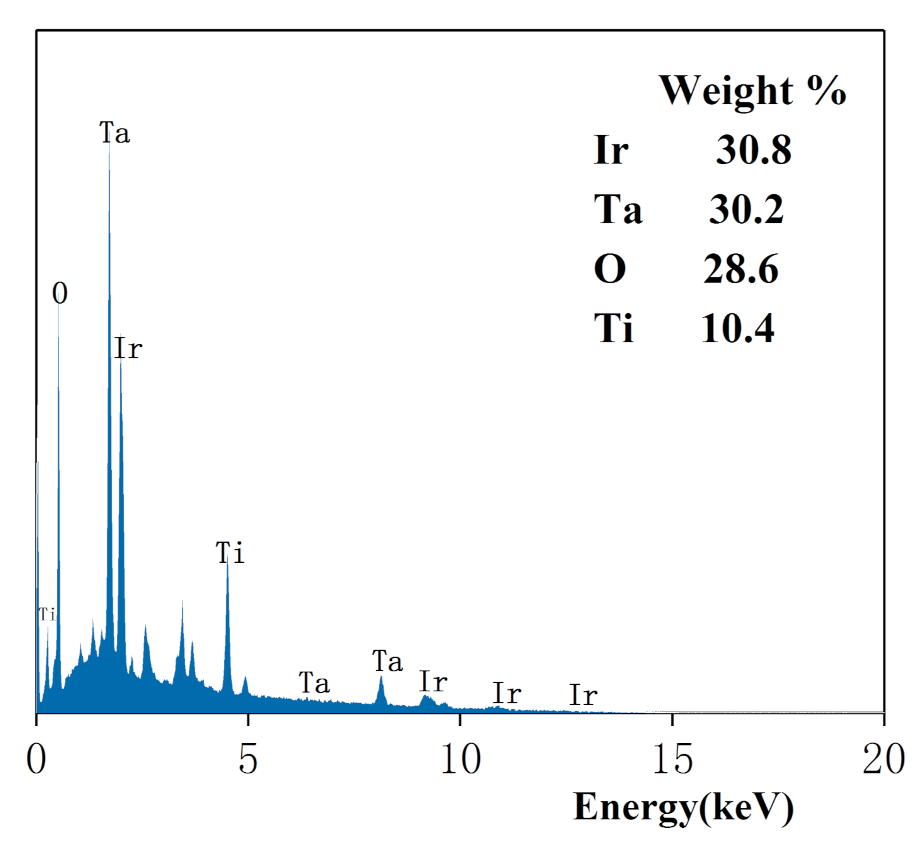
3.3.2. EDS Characterization
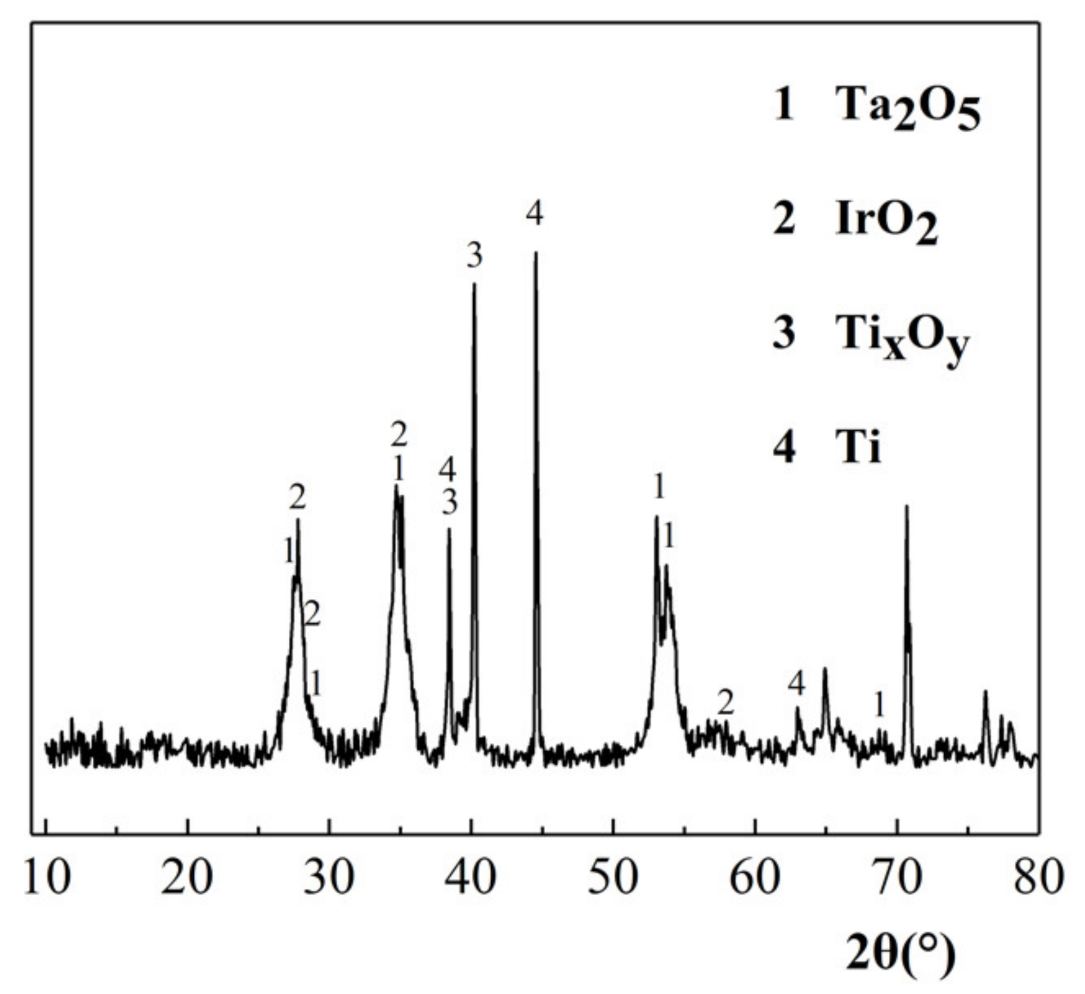
3.3.3. XRD Characterization
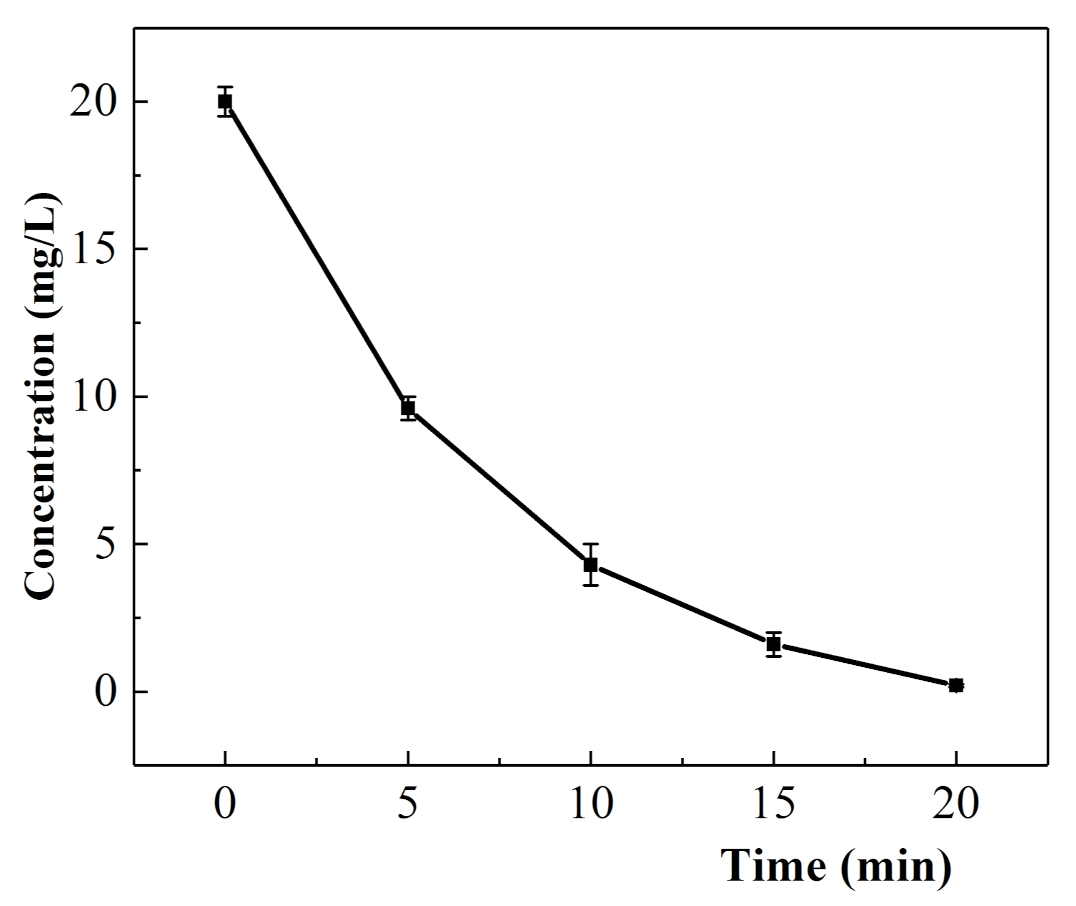
3.3.4. Kinetics of the Electrochemical Degradation of Oxytetracycline
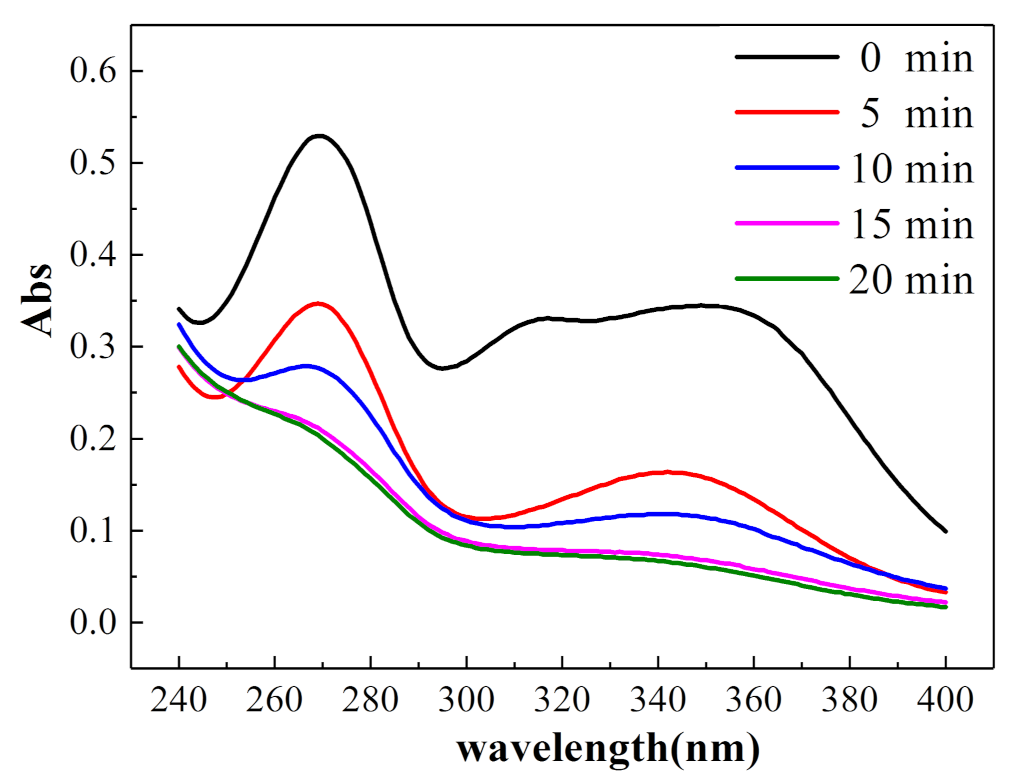
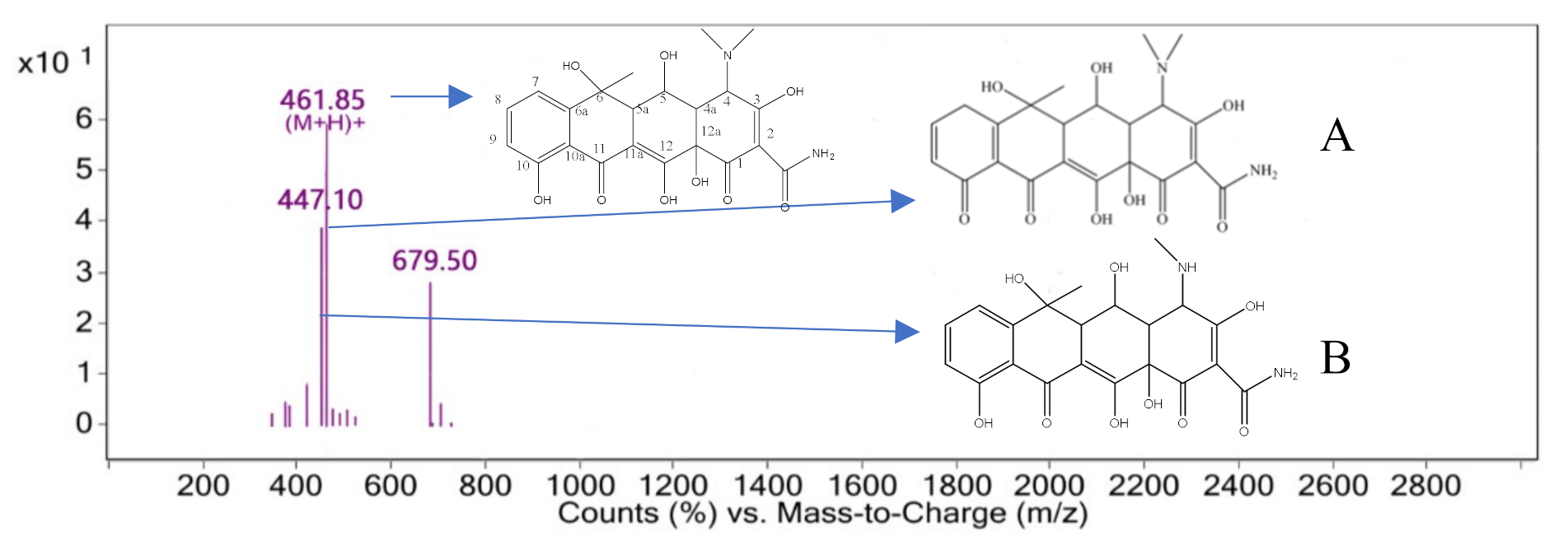
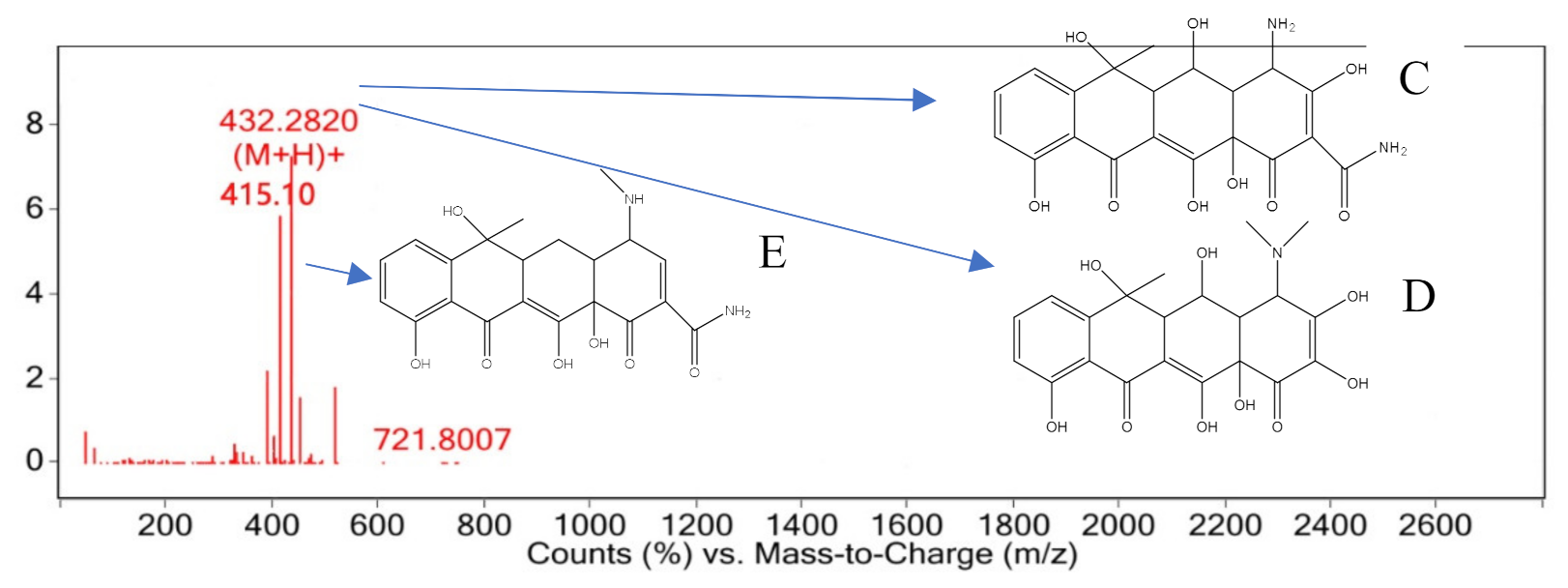
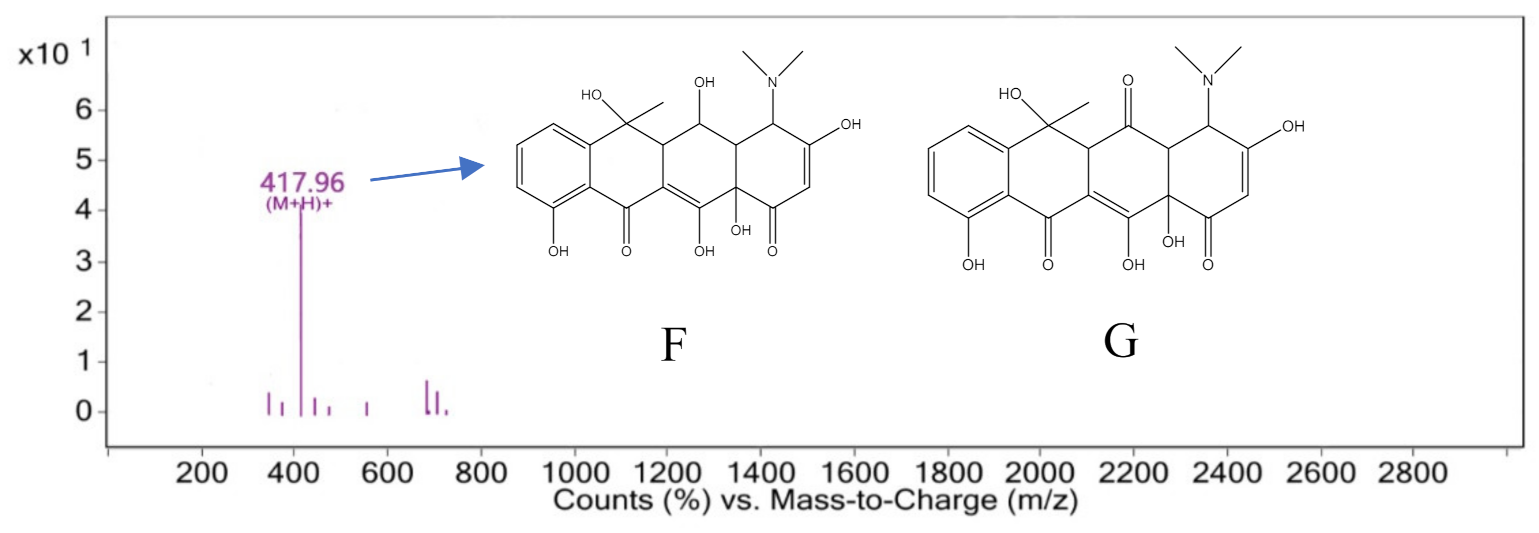
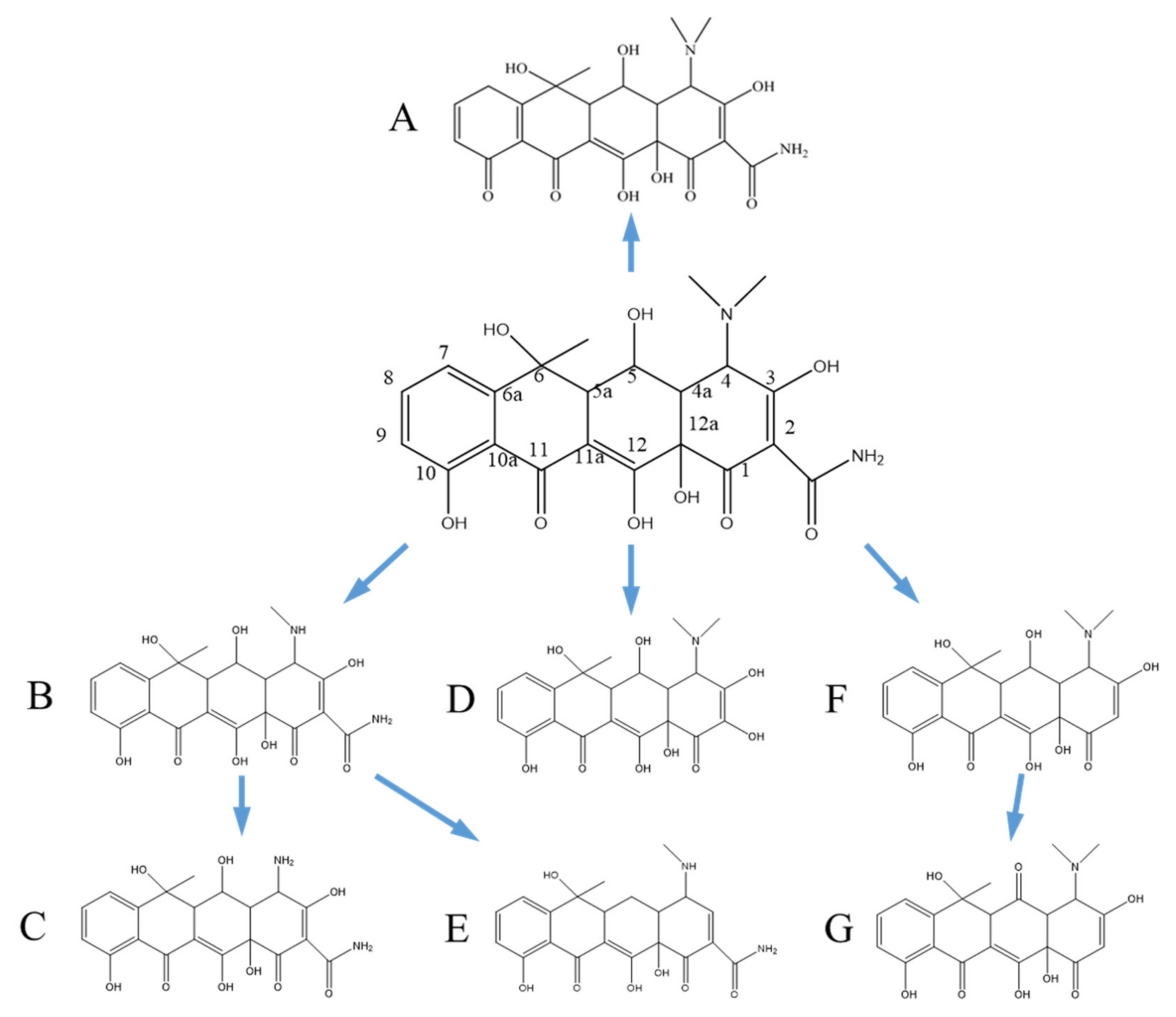
3.4. Degradation Pathway and Product Analysis of Oxytetracycline
3.5. Degradation Product Analysis of Oxytetracycline
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef]
- Huang, F.; An, Z.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009−2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q.; Zhang, J.; Dong, J.; Yan, H.; Chen, C.; Feng, R. Characterization and source identification of tetracycline antibiotics in the drinking water sources of the lower Yangtze River. J. Environ. Manag. 2019, 244, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Changanaqui, K.; Brillas, E.; Alarcón, H.; Sirés, I. ZnO/TiO2/Ag2Se nanostructures as photoelectrocatalysts for the degradation of oxytetracycline in water. Electrochim. Acta 2020, 331, 135194. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V. Antibiotic pollution in surface fresh waters: Occurrence and effects. J. Reiss Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Lenart-Boroń, A.; Prajsnar, J.; Guzik, M.; Boroń, P.; Chmiel, M. How much of antibiotics can enter surface water with treated wastewater and how it affects the resistance of waterborne bacteria: A case study of the Białka river sewage treatment plant. Environ. Res. 2020, 191, 110037. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Suitability of feathers as control matrix for antimicrobial treatments detection compared to muscle and liver of broilers. Food Control 2018, 91, 268–275. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, Q.; Tsang, D.C.W.; Zhu, L.Z.; Li, X.D. Antibiotics in the agricultural soils from the Yangtze River Delta, China. Chemosphere 2017, 189, 301–308. [Google Scholar] [CrossRef]
- Li, S.; Kuang, Y.; Hu, J.; You, M.; Guo, X.; Gao, Q.; Yang, X.; Chen, Q.; Sun, W.; Ni, J. Enrichment of antibiotics in an inland lake water. Environ. Res. 2020, 190, 110029. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Feng, M.; Tu, W.; Xiao, T.; Xiong, T.; Ang, H.; Yuan, X.; Chew, J.W. Visible-light-driven Removal of Tetracycline Antibiotics and Reclamation of Hydrogen Energy from Natural Water Matrices and Wastewater by Polymeric Carbon Nitride Foam. Water Res. 2018, 144, 215–225. [Google Scholar] [CrossRef]
- Yuan, A.; Lei, H.; Xi, F.; Liu, J.; Qin, L.; Chen, Z.; Dong, X. Graphene quantum dots decorated graphitic carbon nitride nanorods for photocatalytic removal of antibiotics. J. Colloid Interface Sci. 2019, 548, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, J.; Wan, H.; Guan, G. Boosting photocatalytic degradation of antibiotic wastewater by synergy effect of heterojunction and phosphorus doping. J. Colloid Interface Sci. 2021, 582, 961–968. [Google Scholar] [CrossRef]
- Yanwen, Q.; Quan, W.; Yingqun, M.; Chenchen, Y.; Zhichao, L. Antibiotics pollution in Gonghu Bay in the period of water diversion from Yangtze River to Taihu Lake. Environ. Earth Sci. 2018, 77, 419. [Google Scholar]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B Environ. 2013, 134–135, 83–92. [Google Scholar] [CrossRef]
- Arikan, O.; Mulbry, W.; Ingram, D.; Millner, P. Minimally managed composting of beef manure at the pilot scale: Effect of manure pile construction on pile temperature profiles and on the fate of oxytetracycline and chlortetracycline. Bioresour. Technol. 2009, 100, 4447–4453. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Uslu, M.Ö.; Balcıoğlu, I.A. Comparison of the ozonation and Fenton process performances for the treatment of antibiotic containing manure. Sci. Total Environ. 2009, 407, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Bo, G.; Wu, S.; Luo, L. Degradation of pollutants in polluted river water using Ti/IrO2-Ta2O5 coating electrode and evaluation of electrode characteristics. J. Clean. Prod. 2020, 273, 123019. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, C.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Anodic oxidation of oxytetracycline:Influence of the experimental conditions on the degradation rate and mechanism. J. Electrochem. Sci. Eng. 2014, 4, 203–213. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Cavalcanti, E.B.; Segura, S.G.; Centellas, F.; Brillas, E. Electrochemical incineration of omeprazole in neutral aqueous medium using a platinum or boron-doped diamond anode: Degradation kinetics and oxidation product. Water Res. 2013, 47, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LA under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Xu, L.; Xin, Y.; Wang, J. A comparative study on IrO2-Ta2O5 coated titanium electrodes prepared with different methods. Electrochim. Acta 2009, 54, 1820–1825. [Google Scholar] [CrossRef]
- Gonzalez, J.; Sun, K.; Huang, M.; Lambros, J.; Dillon, S.; Chasiotis, I. Three dimensional studies of particle failure in silicon based composite electrodes for lithium ion batteries. J. Power Sources 2014, 269, 334–343. [Google Scholar] [CrossRef]
- Bahramifar, S.; Haftbaradaran, H.; Mossaiby, F. Cohesive modeling of crack formation in two-phase planar electrodes subject to diffusion induced stresses using the distributed dislocation method. Int. J. Mech. Sci. 2020, 194, 106183. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhang, X.; Chen, T.; Dong, D.; Hua, X.; Guo, Z. Enhanced bioaccumulation of fluorinated antibiotics in crucian carp (Carassius carassius): Influence of fluorine substituent. Sci. Total Environ. 2020, 748, 141567. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Rajaram, N.; Mu, H.; Saad, N.K.; Nabil, A.; Ahmed, A.-O. Diameter-dependent electrochemical supercapacitive properties of anodized titanium oxide nanotubes. Scr. Mater. 2015, 104, 60–63. [Google Scholar]
- Ben, W.; Qiang, Z.; Pan, X.; Chen, M. Removal of veterinary antibiotics from sequencing batch reactor (SBR) pretreated swine wastewater by Fenton’s reagent. Water Res. 2009, 43, 4392–4402. [Google Scholar] [CrossRef] [PubMed]

| Factors | Level | ||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |
| A: Current intensity (A) | 0.300 | 0.400 | 0.500 |
| B: Electrolyte concentration (mg/L) | 3 | 4 | 5 |
| C: Solution pH | 2 | 3 | 4 |
| Serial Number | A | B | C | Degradation Rate (%) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 87.38 |
| 2 | 1 | 2 | 2 | 97.03 |
| 3 | 1 | 3 | 3 | 83.57 |
| 4 | 2 | 1 | 2 | 93.85 |
| 5 | 2 | 2 | 3 | 97.51 |
| 6 | 2 | 3 | 1 | 98.80 |
| 7 | 3 | 1 | 3 | 98.04 |
| 8 | 3 | 2 | 1 | 98.85 |
| 9 | 3 | 3 | 2 | 98.23 |
| K1 | 89.327 | 93.090 | 95.010 | / |
| K2 | 96.720 | 97.797 | 96.370 | / |
| K3 | 98.373 | 93.533 | 93.040 | / |
| R | 9.046 | 4.707 | 3.330 | / |
| Reaction Order | Kinetic Equation | Fitting Curve | R2 |
|---|---|---|---|
| First-order reaction | 0.92669 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, W.; Dong, H.; Hu, X.; Fang, B.; Gao, G.; Zhao, R. Study on the Electrochemical Removal Mechanism of Oxytetracycline by a Ti/IrO2-Ta2O5 Plate. Int. J. Environ. Res. Public Health 2021, 18, 1708. https://doi.org/10.3390/ijerph18041708
Zhang Y, Jiang W, Dong H, Hu X, Fang B, Gao G, Zhao R. Study on the Electrochemical Removal Mechanism of Oxytetracycline by a Ti/IrO2-Ta2O5 Plate. International Journal of Environmental Research and Public Health. 2021; 18(4):1708. https://doi.org/10.3390/ijerph18041708
Chicago/Turabian StyleZhang, Yinghao, Wenqiang Jiang, Hao Dong, Xuyang Hu, Baihui Fang, Guangfei Gao, and Rui Zhao. 2021. "Study on the Electrochemical Removal Mechanism of Oxytetracycline by a Ti/IrO2-Ta2O5 Plate" International Journal of Environmental Research and Public Health 18, no. 4: 1708. https://doi.org/10.3390/ijerph18041708
APA StyleZhang, Y., Jiang, W., Dong, H., Hu, X., Fang, B., Gao, G., & Zhao, R. (2021). Study on the Electrochemical Removal Mechanism of Oxytetracycline by a Ti/IrO2-Ta2O5 Plate. International Journal of Environmental Research and Public Health, 18(4), 1708. https://doi.org/10.3390/ijerph18041708




