Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin
Abstract
1. Introduction
2. Results
2.1. Effects of ABA and NAA on Berry Development and Ripening
2.1.1. Evolution of Sugar and Acidity
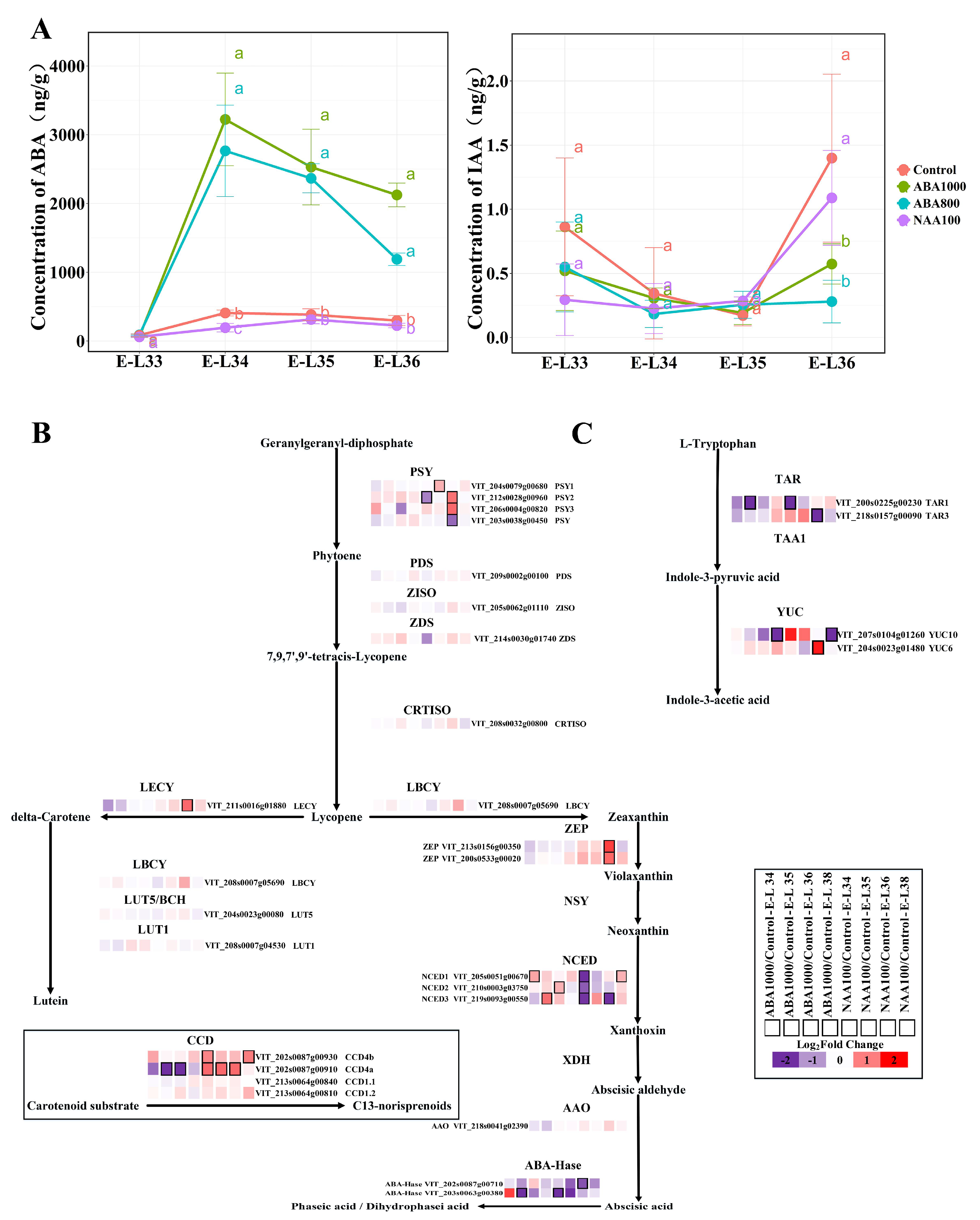
2.1.2. Changes of Endogenous ABA and Auxin Biosynthesis and Signaling Pathway
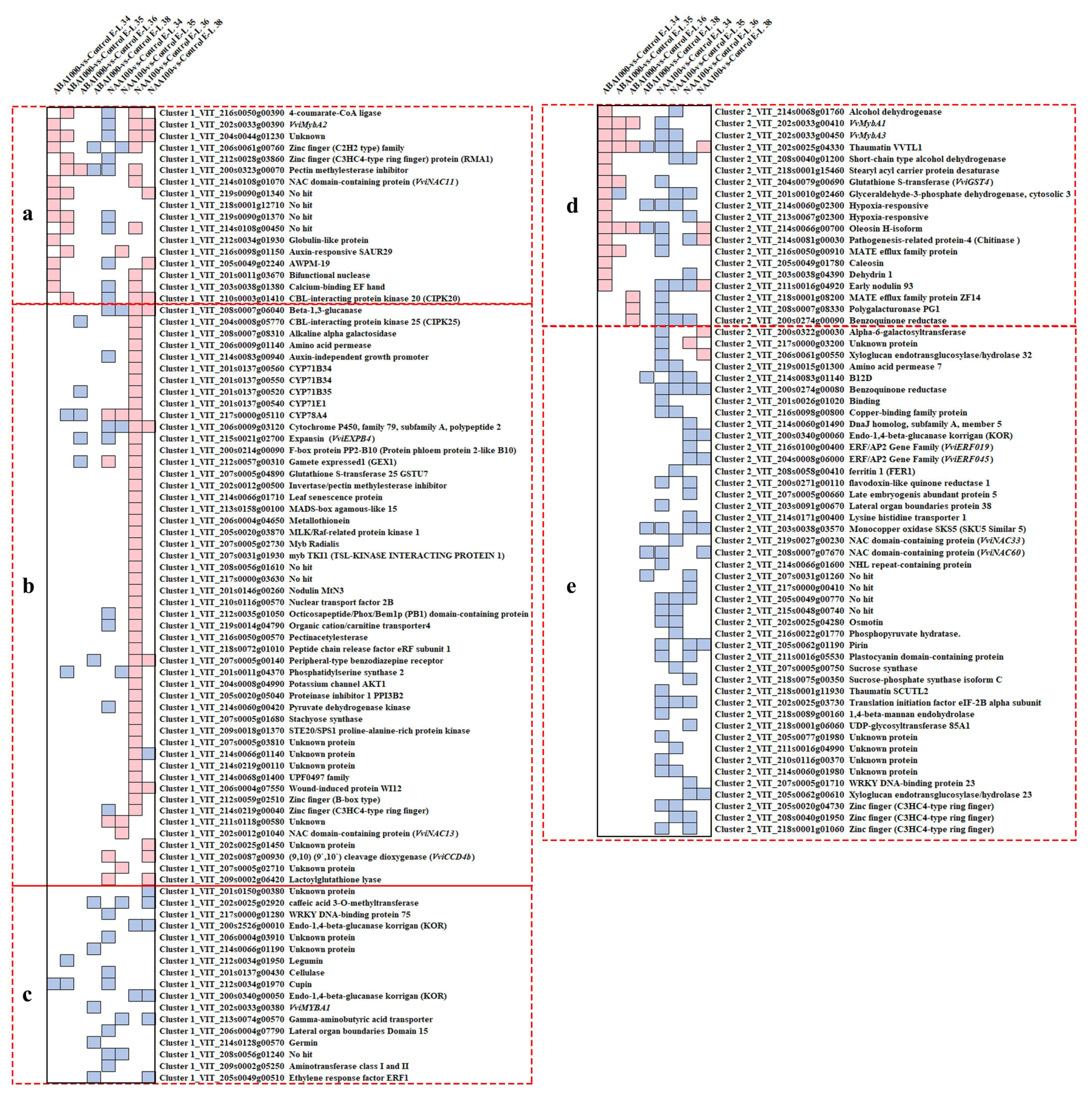
2.1.3. Strong Transcriptional Changes of Ripening Switch Genes
2.2. Regulation of ABA and NAA on the Biosynthesis and Accumulation of Norisoprenoids
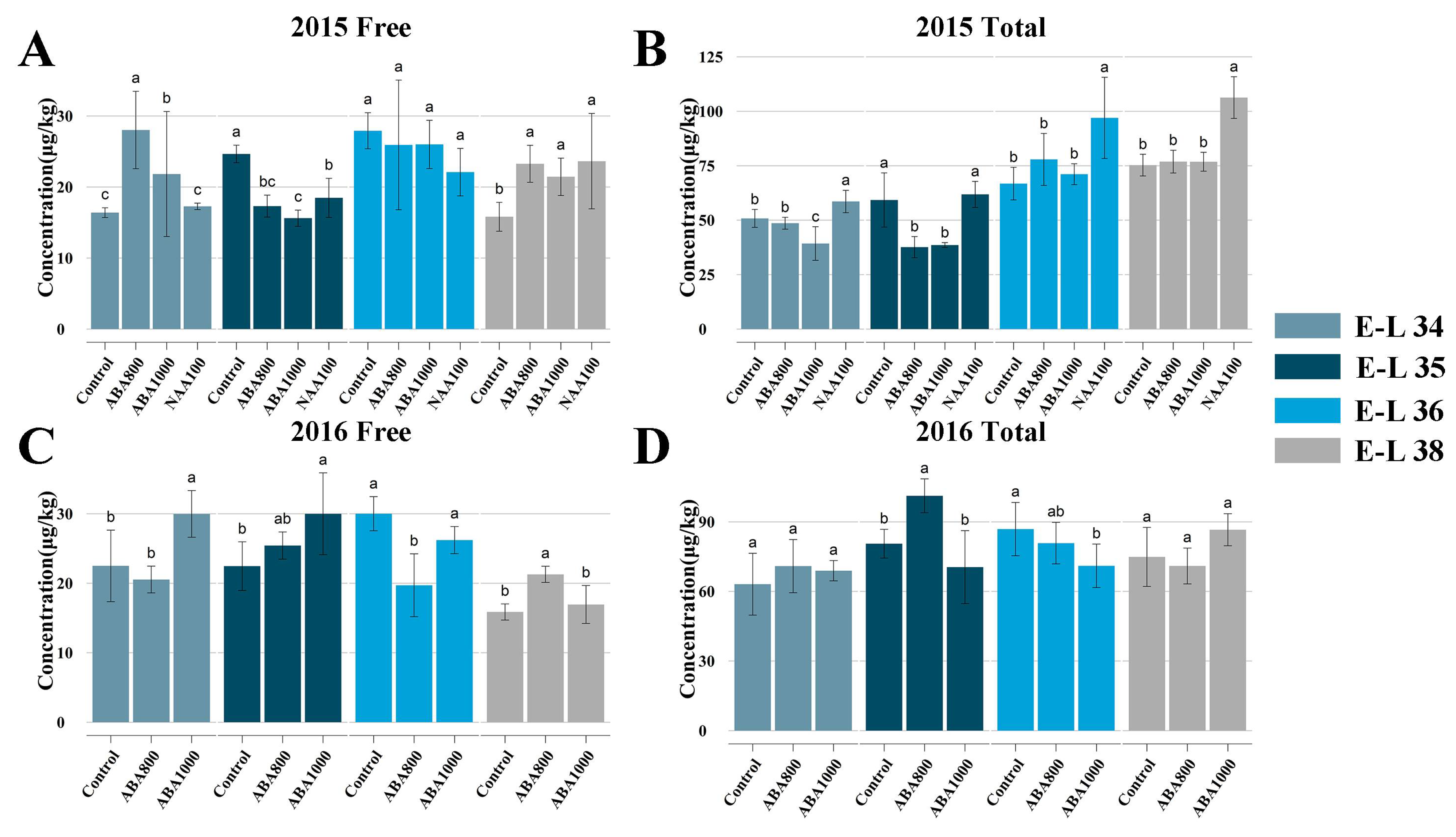
2.2.1. Effects on Norisoprenoid Production and Related Gene Expression
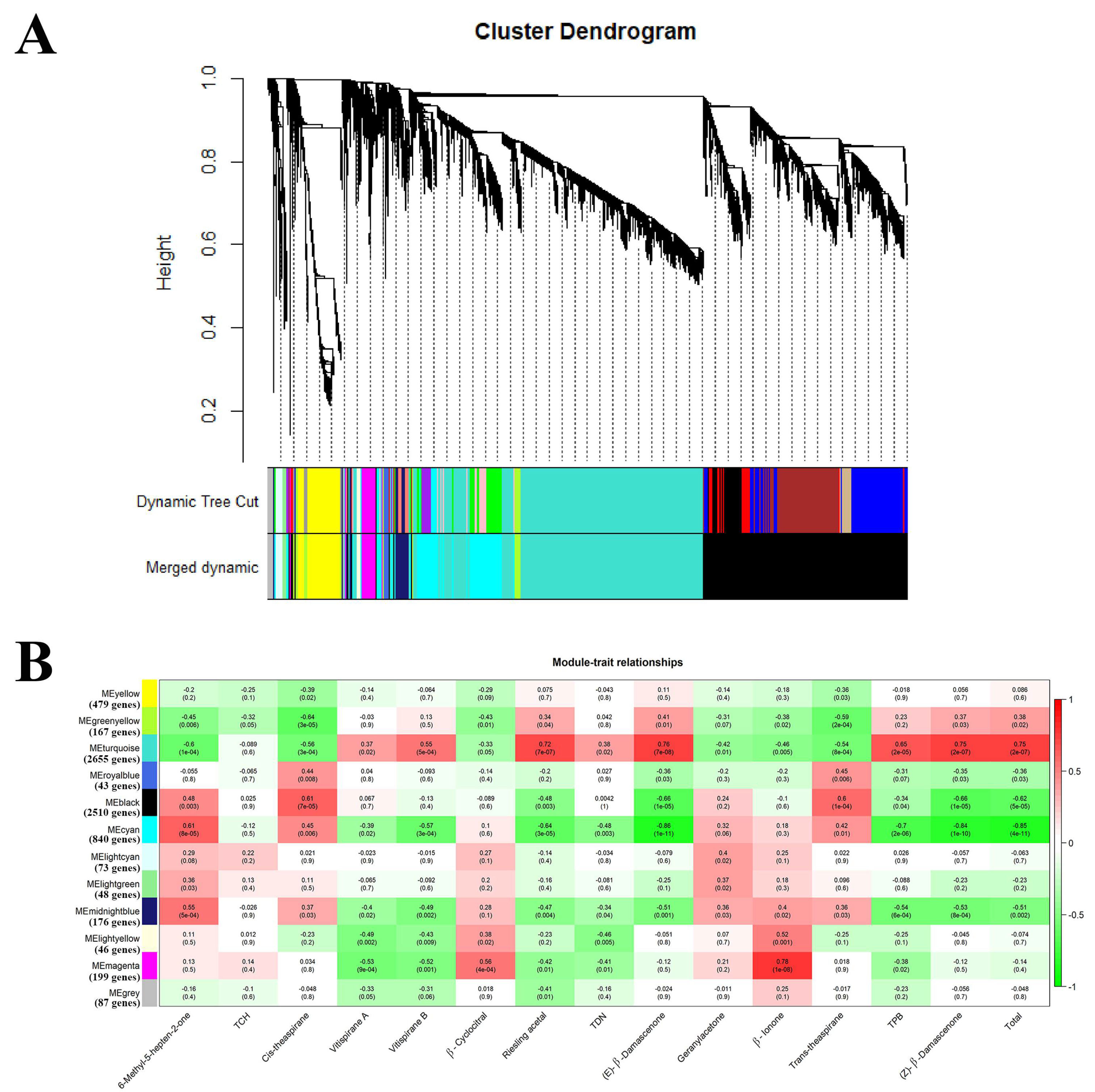
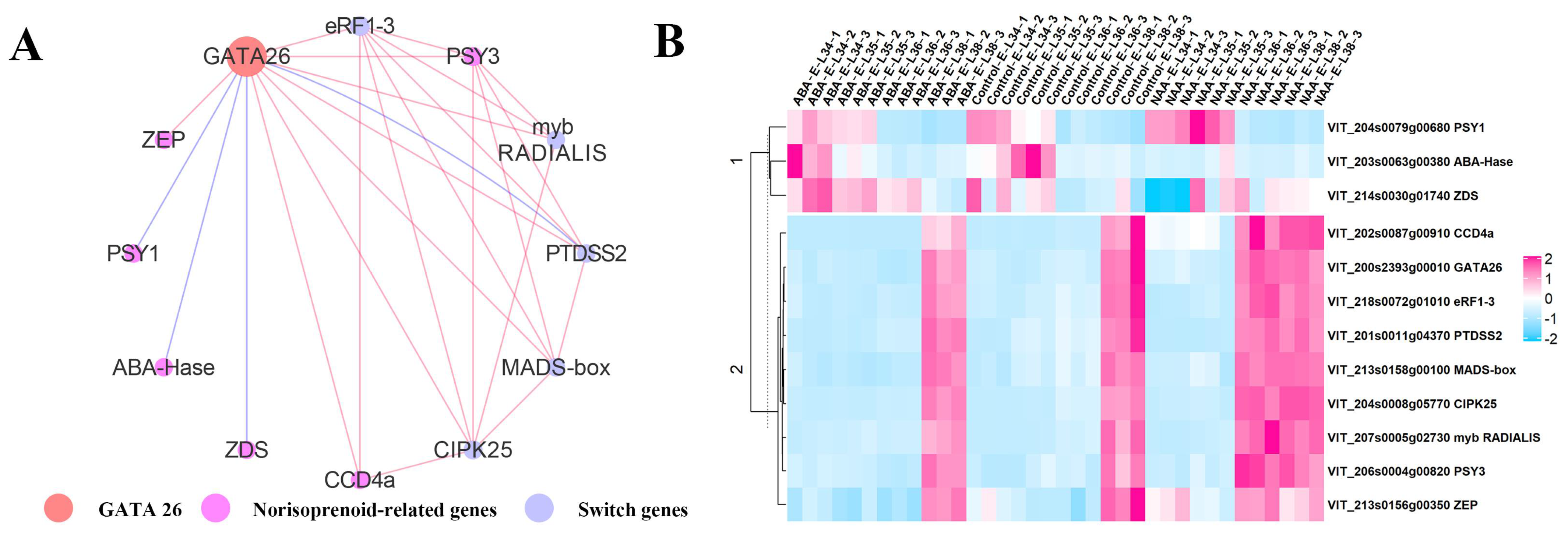
2.2.2. Acquisition of the Candidate Genes Regulating Norisoprenoid Accumulation
2.2.3. An Integrated Gene Co-Expressed and Regulatory Network Regulating Norisoprenoid Biosynthesis
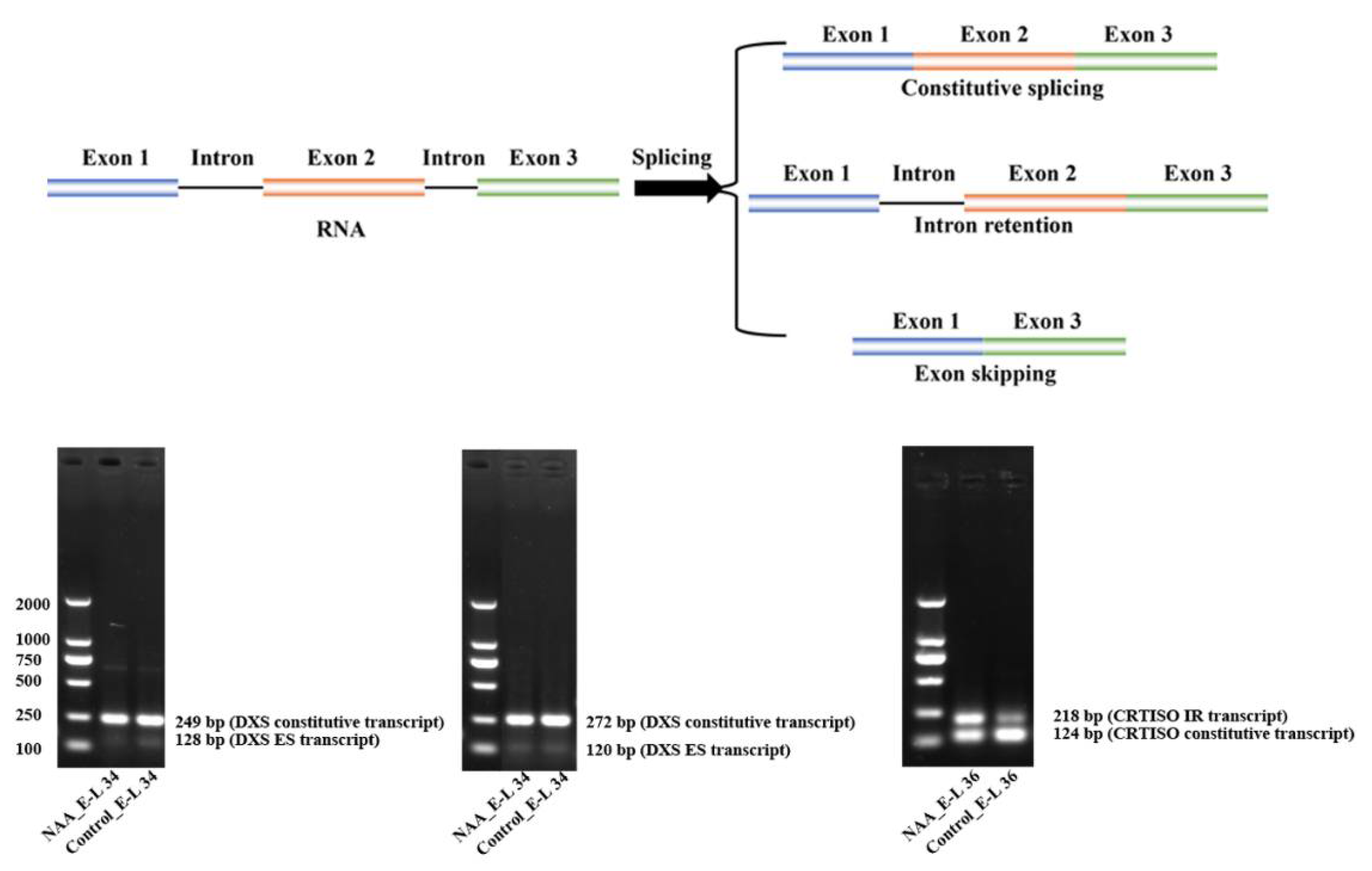
2.2.4. Potential Contribution of Differentially Alternative Splicing of VviDXS and VviCRTISO
3. Discussion
3.1. Response of ABA and IAA Biosynthesis and Signaling
3.2. Response of Norisoprenoid Biosynthesis
3.3. Potential Regulation Relating to NAA-Induced Norisoprenoid Accumulation
4. Materials and Methods
4.1. ABA and NAA Treatments and Sampling
4.2. Measurements of Total Soluble Solids and Titratable Acid
4.3. RNA Extraction and Sequencing
4.4. Extraction and Determination of ABA and IAA
4.5. Analysis of Norisoprenoids in Berries Using SPME-GC-MS
4.6. RNA Isolation, Cloning, and Expression of VviGATA26
4.7. DAP Affinity Purification Sequencing
4.8. RT-PCR Analysis of Alternative Splicing
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| AS | Alternative splicing |
| AAO | Abscisic-aldehyde oxidase |
| ABF | ABA-responsive element binding factor |
| AUX1 | Auxin influx carrier |
| AUX/IAA | Auxin/indole-3-acetic acid protein |
| ARF | Auxin response factor |
| A3SS | Alternative 3′ splice site |
| A5SS | Alternative 5′ splice site |
| CCD | Carotenoid cleavage dioxygenase |
| CRTISO | Prolycopene isomerase |
| DAP-seq | DNA affinity purification sequencing |
| DEG | Differentially expressed gene |
| DXS | 1-Deoxy-D-xylulose-5-phosphate synthase |
| DESG | Differentially expressed switch gene |
| ES | Exon skipping |
| GH3 | Indole-3-acetic acid-amido synthetase |
| IR | Intron retention |
| LBCY | Lycopene beta-cyclase |
| LECY | Lycopene epsilon-cyclase |
| LUT1 | Carotene epsilon-monooxygenase |
| LUT5 | beta-ring Hydroxylase |
| LBD | Lateral organ boundaries |
| MHO | 6-Methyl-5-hepten-2-one |
| MEP | 2-Methyl-D-erythritol-4-phosphate phosphate |
| MVA | Mevalonic acid |
| MXE | Mutually exclusive exon |
| NAA | 1-Naphthaleneacetic acid |
| NCED | 9-cis-Epoxycarotenoid dioxygenase |
| NSY | Neoxanthin synthase |
| RPKM | Reads per kilobases per million reads |
| SAUR | Small auxin-up RNA |
| TF | Transcription factor |
| TSS | Total soluble solids |
| TA | Titratable acidity |
| TAR | Tryptophan aminotransferase related |
| TIR1 | Transport inhibitor response 1 |
| TAA1 | Tryptophan aminotransferase arabidopsis1 |
| PSY | Phytoene synthase |
| PP2C | Protein serine/threonine phosphatase 2C |
| PDS | 15-cis-Phytoene desaturase |
| WGCNA | Weighted gene co-expression network analysis |
| XDH | Xanthoxin dehydrogenase |
| YUC | YUC flavin monooxygenase. |
| ZDS | Zeta-carotene desaturase |
| ZEP | Zeaxanthin epoxidase |
| ZISO | Zeta-carotene isomerase |
References
- Mendes-Pinto, M.M. Carotenoid breakdown products the—Norisoprenoids—In wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Williams, P.J.; Sefton, M.A.; Francis, I.L. Glycosidic precursors of varietal grape and wine flavor. In Flavour Precursors, Thermal and Enzymatic Conversions; Teranishi, R., Takeoka, G.R., Günterm, M., Eds.; ACS Symposium Series 490; American Chemical Society: Washington, DC, USA, 1992; pp. 74–86. [Google Scholar] [CrossRef]
- Böttcher, C.; Boss, P.K.; Davies, C. Delaying Riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Funct. Plant Biol. 2012, 39, 745–753. [Google Scholar] [CrossRef]
- He, L.; Xu, X.-Q.; Wang, Y.; Chen, W.-K.; Sun, R.-Z.; Cheng, G.; Liu, B.; Chen, W.; Duan, C.-Q.; Wang, J. Modulation of volatile compound metabolome and transcriptome in grape berries exposed to sunlight under dry-hot climate. BMC Plant Biol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Bindon, K.A.; Dry, P.R.; Loveys, B.R. Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. J. Agric. Food Chem. 2007, 55, 4493–4500. [Google Scholar] [CrossRef]
- Mathieu, S.; Terrier, N.; Procureur, J.M.; Bigey, F.; Günata, Z. A carotenoid cleavage dioxygenase from Vitis vinifera L.: Functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef]
- Chen, W.-K.; Yu, K.-J.; Liu, B.; Lan, Y.-B.; Sun, R.-Z.; Li, Q.; He, F.; Pan, Q.-H.; Duan, C.-Q.; Wang, J. Comparison of transcriptional expression patterns of carotenoid metabolism in ‘Cabernet Sauvignon’ grapes from two regions with distinct climate. J. Plant Physiol. 2017, 213, 75–86. [Google Scholar] [CrossRef]
- Vitulo, N.; Forcato, C.; Carpinelli, E.C.; Telatin, A.; Campagna, D.; D’Angelo, M.; Zimbello, R.; Corso, M.; Vannozzi, A.; Bonghi, C. A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biol. 2014, 14, 99. [Google Scholar] [CrossRef]
- Potenza, E.; Racchi, M.L.; Sterck, L.; Coller, E.; Asquini, E.; Tosatto, S.C.; Velasco, R.; Van de Peer, Y.; Cestaro, A. Exploration of alternative splicing events in ten different grapevine cultivars. BMC Genom. 2015, 16, 706. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Liu, C.; Liu, G.; Li, S.; Wang, L. Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiol. 2017, 173, 1502–1518. [Google Scholar] [CrossRef]
- Meng, N.; Wei, Y.; Gao, Y.; Yu, K.; Cheng, J.; Li, X.-Y.; Duan, C.-Q.; Pan, Q.-H. Characterization of Transcriptional Expression and Regulation of Carotenoid Cleavage Dioxygenase 4b in Grapes. Front. Plant Sci. 2020, 11, 11. [Google Scholar] [CrossRef]
- Li, X.; He, L.; An, X.; Yu, K.; Meng, N.; Duan, C.-Q.; Pan, Q.-H. VviWRKY40, a WRKY Transcription Factor, Regulates Glycosylated Monoterpenoid Production by VviGT14 in Grape Berry. Genes 2020, 11, 485. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.-N.; He, L.; He, F.; Chen, W.; Duan, C.-Q.; Wang, J. Changes in global aroma profiles of Cabernet Sauvignon in response to cluster thinning. Food Res. Int. 2019, 122, 56–65. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of major ripening events during softening in grape: Turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. J. Exp. Bot. 2015, 67, 709–722. [Google Scholar] [CrossRef]
- Palumbo, M.C.; Zenoni, S.; Fasoli, M.; Massonnet, M.; Farina, L.; Castiglione, F.; Pezzotti, M.; Paci, P. Integrated Network Analysis Identifies Fight-Club Nodes as a Class of Hubs Encompassing Key Putative Switch Genes That Induce Major Transcriptome Reprogramming during Grapevine Development. Plant Cell 2014, 26, 4617–4635. [Google Scholar] [CrossRef]
- Böttcher, C.; Keyzers, R.A.; Boss, P.K.; Davies, C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J. Exp. Bot. 2010, 61, 3615–3625. [Google Scholar] [CrossRef]
- Fortes, A.M.; Teixeira, R.T.; Agudelo-Romero, P. Complex interplay of hormonal signals during grape berry ripening. Molecules 2015, 20, 9326–9343. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aus. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- He, L.; Ren, Z.-Y.; Wang, Y.; Fu, Y.-Q.; Li, Y.; Meng, N.; Pan, Q.-H. Variation of Growth-to-Ripening Time Interval Induced by Abscisic Acid and Synthetic Auxin Affecting Transcriptome and Flavor Compounds in Cabernet Sauvignon Grape Berry. Plants 2020, 9, 630. [Google Scholar] [CrossRef]
- Boneh, U.; Biton, I.; Zheng, C.; Schwartz, A.; Ben-Ari, G. Characterization of potential ABA receptors in Vitis vinifera. Plant Cell Rep. 2012, 31, 311–321. [Google Scholar] [CrossRef]
- Klingler, J.P.; Batelli, G.; Zhu, J.-K. ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp. Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef]
- Lau, S.; Jürgens, G.; De Smet, I. The Evolving Complexity of the Auxin Pathway. Plant Cell 2008, 20, 1738–1746. [Google Scholar] [CrossRef]
- Ishimaru, M.; Kobayashi, S. Expression of a xyloglucan endo-transglycosylase gene is closely related to grape berry softening. Plant Sci. 2002, 162, 621–628. [Google Scholar] [CrossRef]
- Nunan, K.J.; Davies, C.; Robinson, S.P.; Fincher, G.B. Expression patterns of cell wall-modifying enzymes during grape berry development. Planta 2001, 214, 257–264. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.-x.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Casado, C.G.; Heredia, A. Structure and dynamics of reconstituted cuticular waxes of grape berry cuticle (Vitis vinifera L.). J. Exp. Bot. 1999, 50, 175–182. [Google Scholar] [CrossRef]
- Ziliotto, F.; Corso, M.; Rizzini, F.M.; Rasori, A.; Botton, A.; Bonghi, C. Grape berry ripening delay induced by a pre-véraison NAA treatment is paralleled by a shift in the expression pattern of auxin-and ethylene-related genes. BMC Plant Biol. 2012, 12, 185. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Wang, H.; Yin, X.; Li, X.; Wang, L.; Zheng, Y.; Xu, X.; Zhang, Y.; Wang, X. Genome-wide identification, evolution and expression analysis of the grape (Vitis vinifera L.) zinc finger-homeodomain gene family. Int. J. Mol. Sci. 2014, 15, 5730–5748. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef]
- Neto, F.J.D.; Junior, A.P.; Borges, C.V.; Cunha, S.R.; Callili, D.; Lima, G.P.P.; Roberto, S.R.; Leonel, S.; Tecchio, M.A. The Exogenous Application of Abscisic Acid Induce Accumulation of Anthocyanins and Phenolic Compounds of the ‘Rubi’Grape. Am. J. Plant Sci. 2017, 8, 2422–2432. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid increases carotenoid and chlorophyll concentrations in leaves and fruit of two tomato genotypes. J. Am. Soc. Hortic. Sci. 2014, 139, 261–266. [Google Scholar] [CrossRef]
- Haisel, D.; Pospíšilová, J.; Synková, H.; Schnablová, R.; Baťková, P. Effects of abscisic acid or benzyladenine on pigment contents, chlorophyll fluorescence, and chloroplast ultrastructure during water stress and after rehydration. Photosynthetica 2006, 44, 606–614. [Google Scholar] [CrossRef]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato Lycopene Biosynthesis through Virus-Induced Gene Silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA Family of Transcription Factors in Arabidopsis and Rice. Plant Physiol. 2004, 134, 1718–1732. [Google Scholar] [CrossRef]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; von Lintig, J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Fraser, P.D.; Enfissi, E.M.A.; Bramley, P.M. Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch. Biochem. Biophys. 2009, 483, 196–204. [Google Scholar] [CrossRef]
- McQuinn, R.P.; Gapper, N.E.; Gray, A.G.; Zhong, S.; Takayuki, T.; Fei, Z.; Fernie, A.R.; Giovannoni, J.J. Manipulation of ZDS in Tomato Exposes Carotenoid- and ABA-Specific Effects on Fruit Development and Ripening. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Ito, Y.; Kitagawa, M.; Ihashi, N.; Yabe, K.; Kimbara, J.; Yasuda, J.; Ito, H.; Inakuma, T.; Hiroi, S.; Kasumi, T. DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J. 2008, 55, 212–223. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Ba, L.J.; Shan, W.; Xiao, Y.Y.; Lu, W.J.; Kuang, J.F.; Chen, J.Y. A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. Plant J. 2018, 96, 1191–1205. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The Citrus Transcription Factor CsMADS6 Modulates Carotenoid Metabolism by Directly Regulating Carotenogenic Genes. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef]
- Marquez, Y.; Brown, J.W.; Simpson, C.; Barta, A.; Kalyna, M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome Res. 2012, 22, 1184–1195. [Google Scholar] [CrossRef]
- Wang, B.-B.; Brendel, V. Genomewide comparative analysis of alternative splicing in plants. Proc. Natl. Acad. Sci. USA 2006, 103, 7175–7180. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Cao, J.; Li, M.; Chen, J.; Liu, P.; Li, Z. Effects of MeJA on Arabidopsis metabolome under endogenous JA deficiency. Sci. Rep. 2016, 6, 37674. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem. 2016, 192, 633–641. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.-K.; Gao, X.-T.; He, L.; Yang, X.-H.; He, F.; Duan, C.-Q.; Wang, J. Rootstock-Mediated Effects on Cabernet Sauvignon Performance: Vine Growth, Berry Ripening, Flavonoids, and Aromatic Profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Shen, Z.; Zhang, Y.; Wu, X.; Wang, J.; Sa, G.; Zhang, Y.; Zhang, H.; Deng, C.; Liu, J.; et al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J. Exp. Bot. 2019, 71, 1527–1539. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Meng, N.; Castellarin, S.D.; Wang, Y.; Sun, Q.; Li, X.-Y.; Dong, Z.-G.; Tang, X.-P.; Duan, C.-Q.; Pan, Q.-H. Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin. Int. J. Mol. Sci. 2021, 22, 1420. https://doi.org/10.3390/ijms22031420
He L, Meng N, Castellarin SD, Wang Y, Sun Q, Li X-Y, Dong Z-G, Tang X-P, Duan C-Q, Pan Q-H. Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin. International Journal of Molecular Sciences. 2021; 22(3):1420. https://doi.org/10.3390/ijms22031420
Chicago/Turabian StyleHe, Lei, Nan Meng, Simone D. Castellarin, Yu Wang, Qi Sun, Xiang-Yi Li, Zhi-Gang Dong, Xiao-Ping Tang, Chang-Qing Duan, and Qiu-Hong Pan. 2021. "Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin" International Journal of Molecular Sciences 22, no. 3: 1420. https://doi.org/10.3390/ijms22031420
APA StyleHe, L., Meng, N., Castellarin, S. D., Wang, Y., Sun, Q., Li, X.-Y., Dong, Z.-G., Tang, X.-P., Duan, C.-Q., & Pan, Q.-H. (2021). Combined Metabolite and Transcriptome Profiling Reveals the Norisoprenoid Responses in Grape Berries to Abscisic Acid and Synthetic Auxin. International Journal of Molecular Sciences, 22(3), 1420. https://doi.org/10.3390/ijms22031420








