Characterization of Two Small Heat Shock Protein Genes (Hsp17.4 and Hs20.3) from Sitodiplosis mosellana, and Their Expression Regulation during Diapause
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects
2.2. RNA Extraction, cDNA Synthesis, and gDNA Isolation
2.3. Cloning of SmHsp17.4 and SmHsp20.3
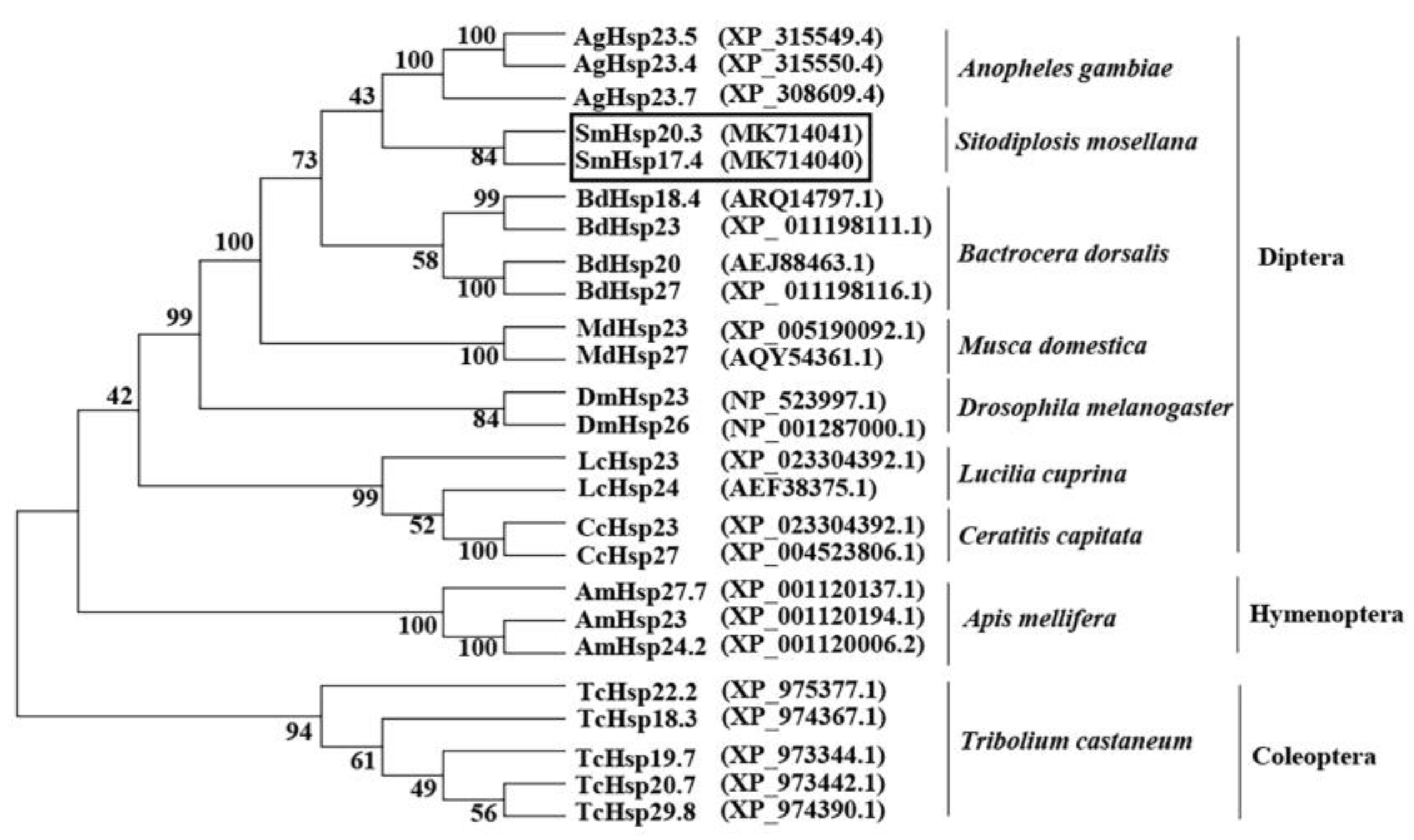
2.4. Sequence Analysis and Phylogenetic Tree Construction
2.5. Heat/Cold Shock Treatments
2.6. 20E Treatment
2.7. Expression Analysis Using RT-qPCR
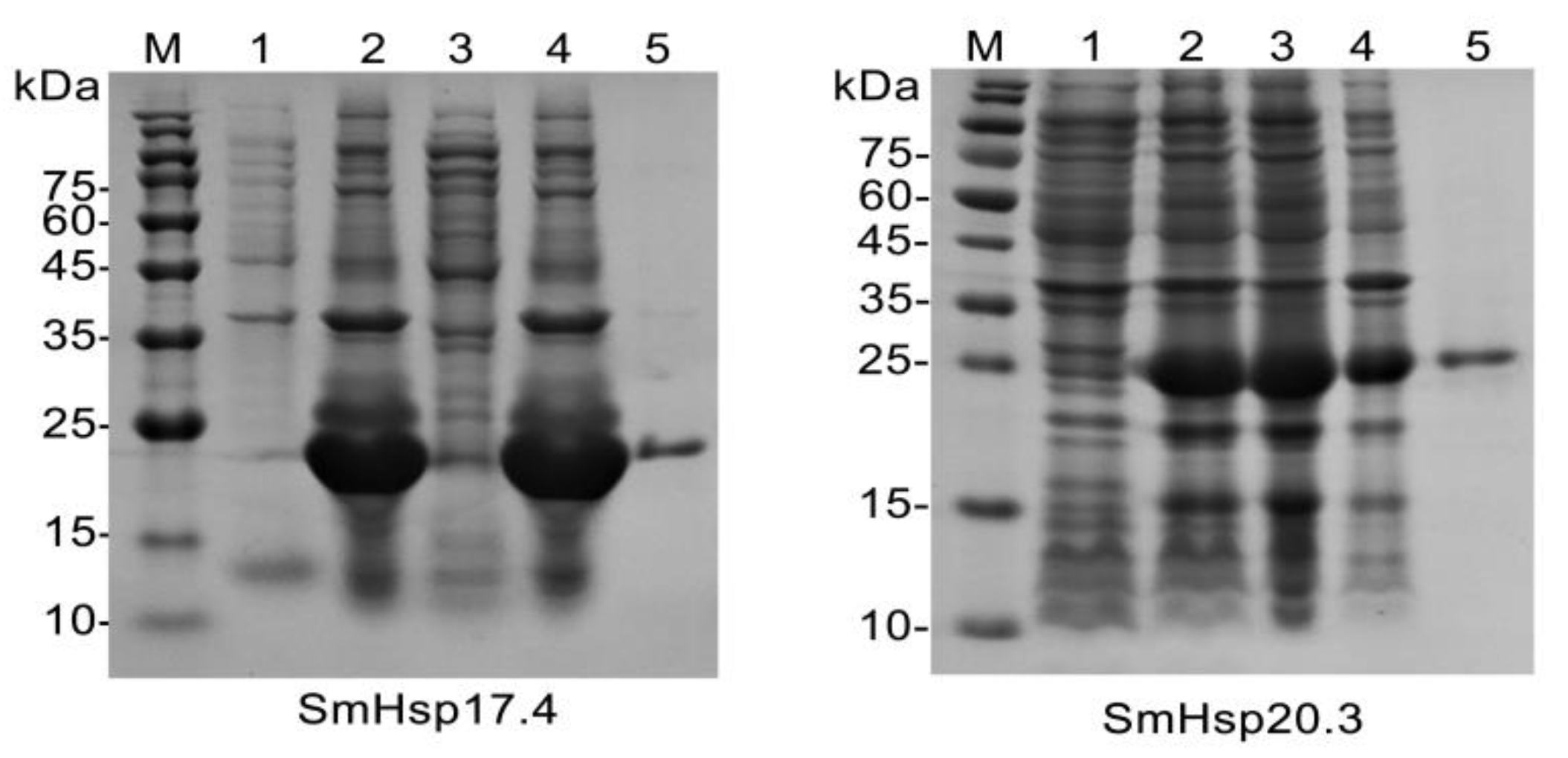
2.8. Recombinant Protein Expression and Purification
2.9. Thermal Aggregation Assays
3. Results
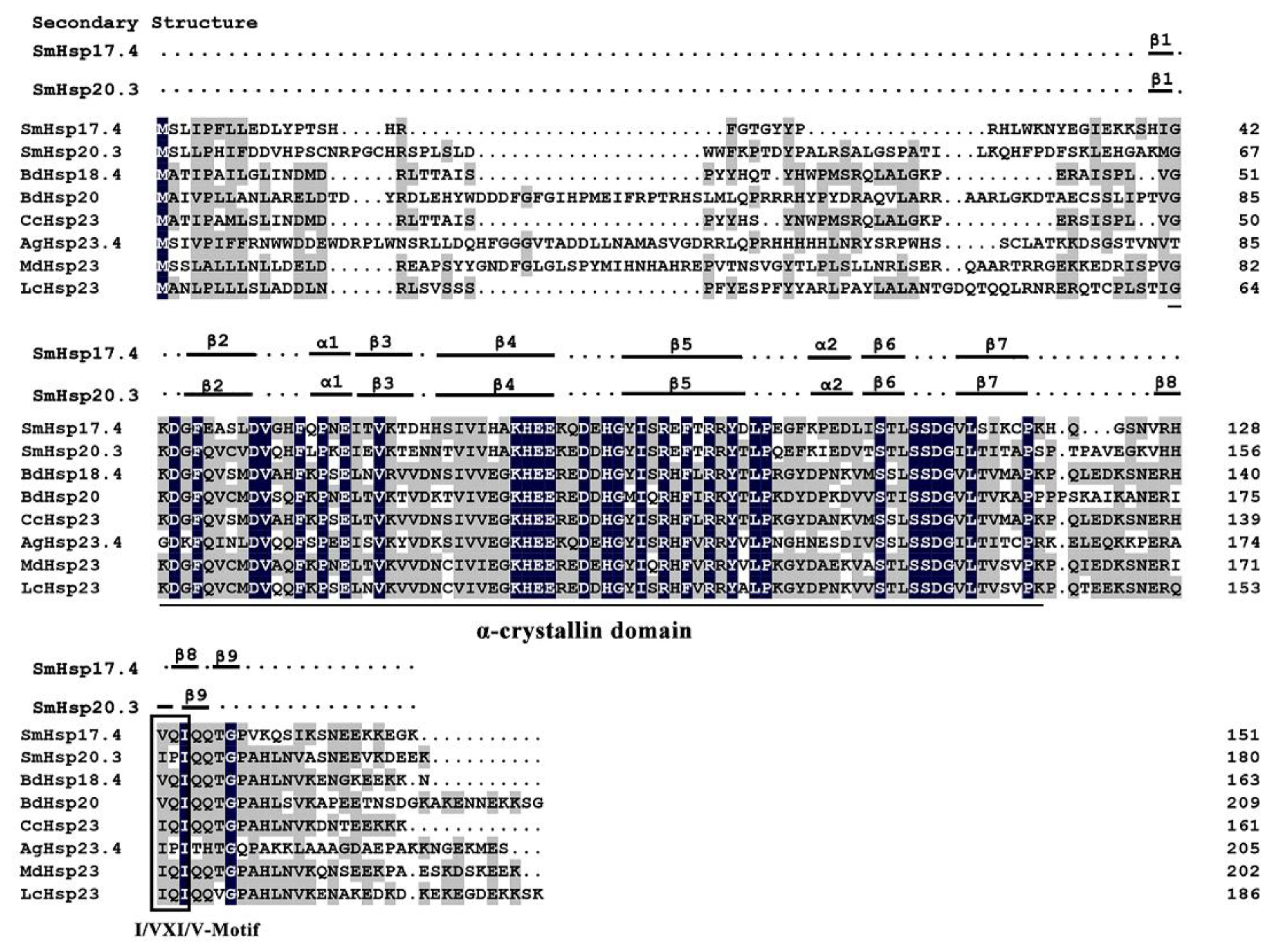
3.1. Characterization of SmHsp17.4 and SmHsp20.3
3.2. Expression of SmHsp17.4 and SmHsp20.3 during Diapause
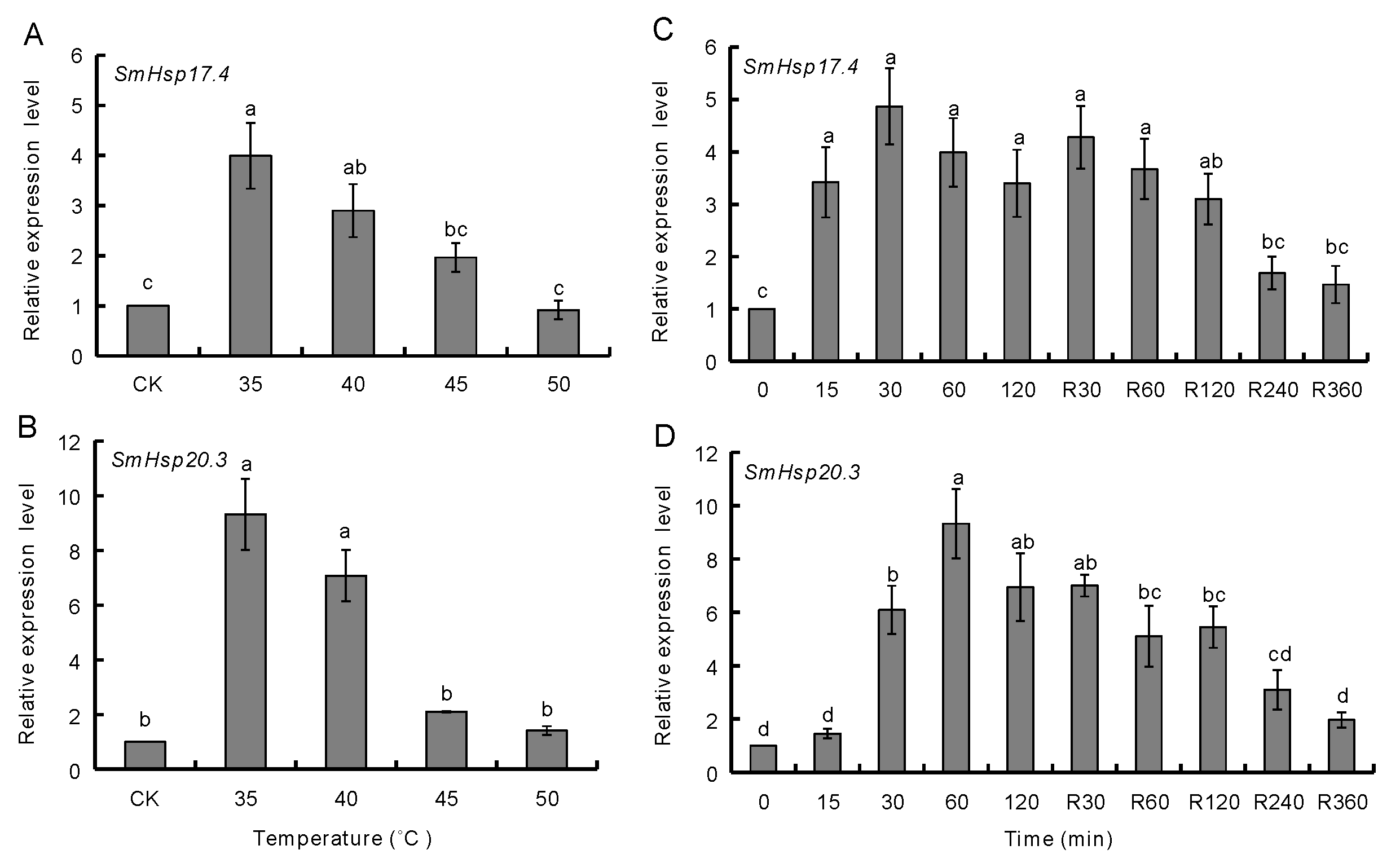
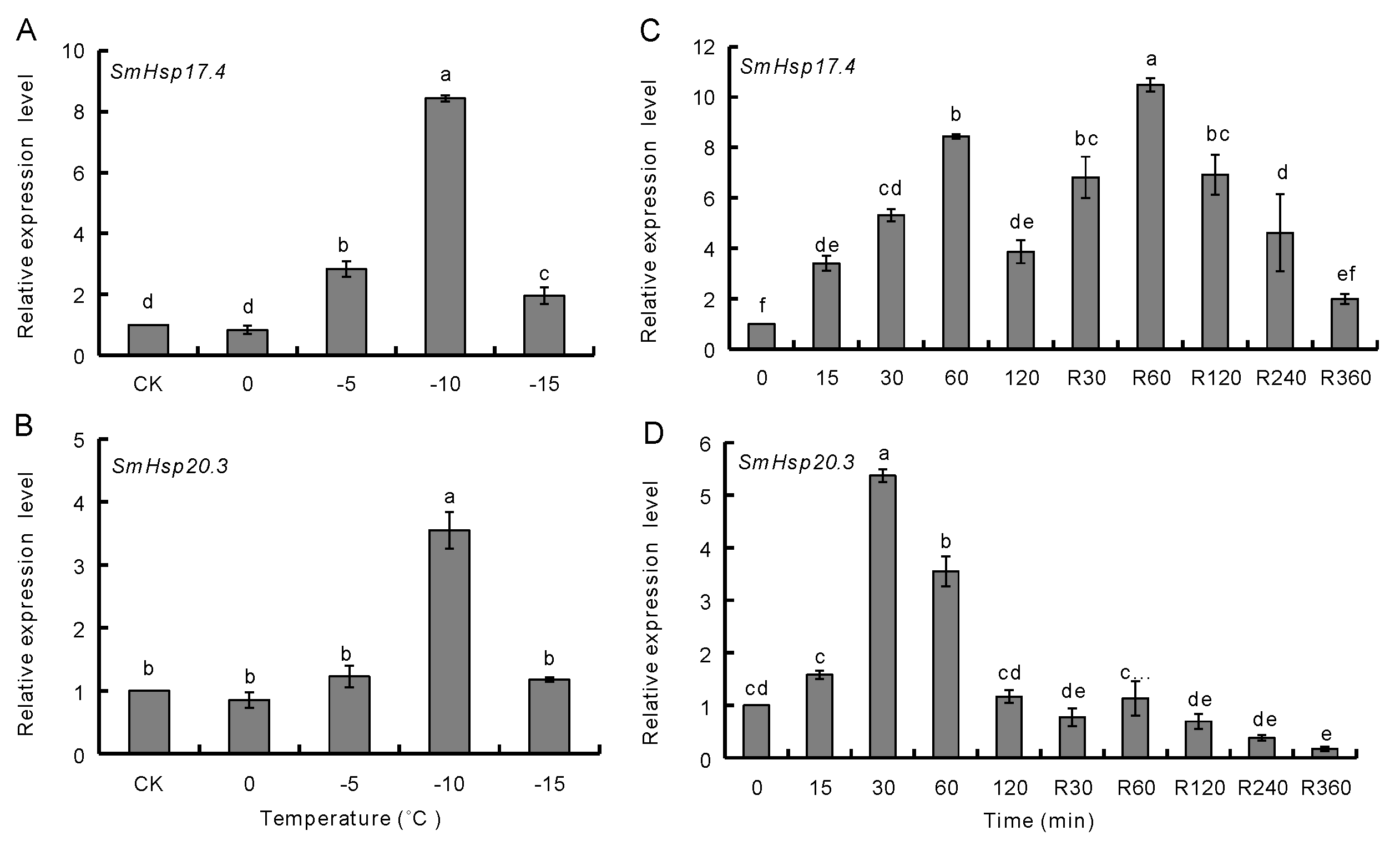
3.3. Effect of Temperature Extremes on SmHsp Expression during Diapause
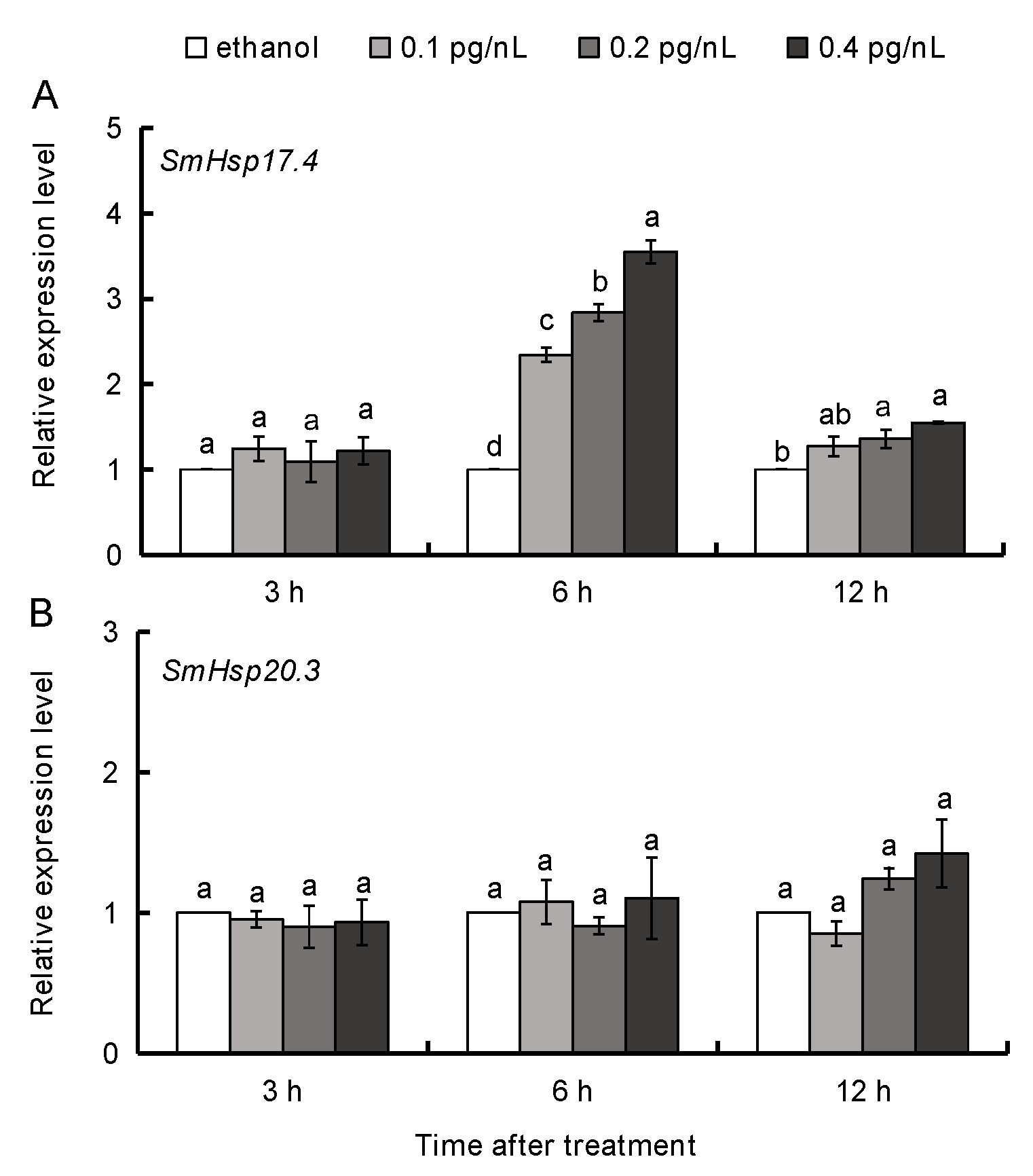
3.4. 20E Regulation of SmHsp17.4 and SmHsp20.3 during Diapause
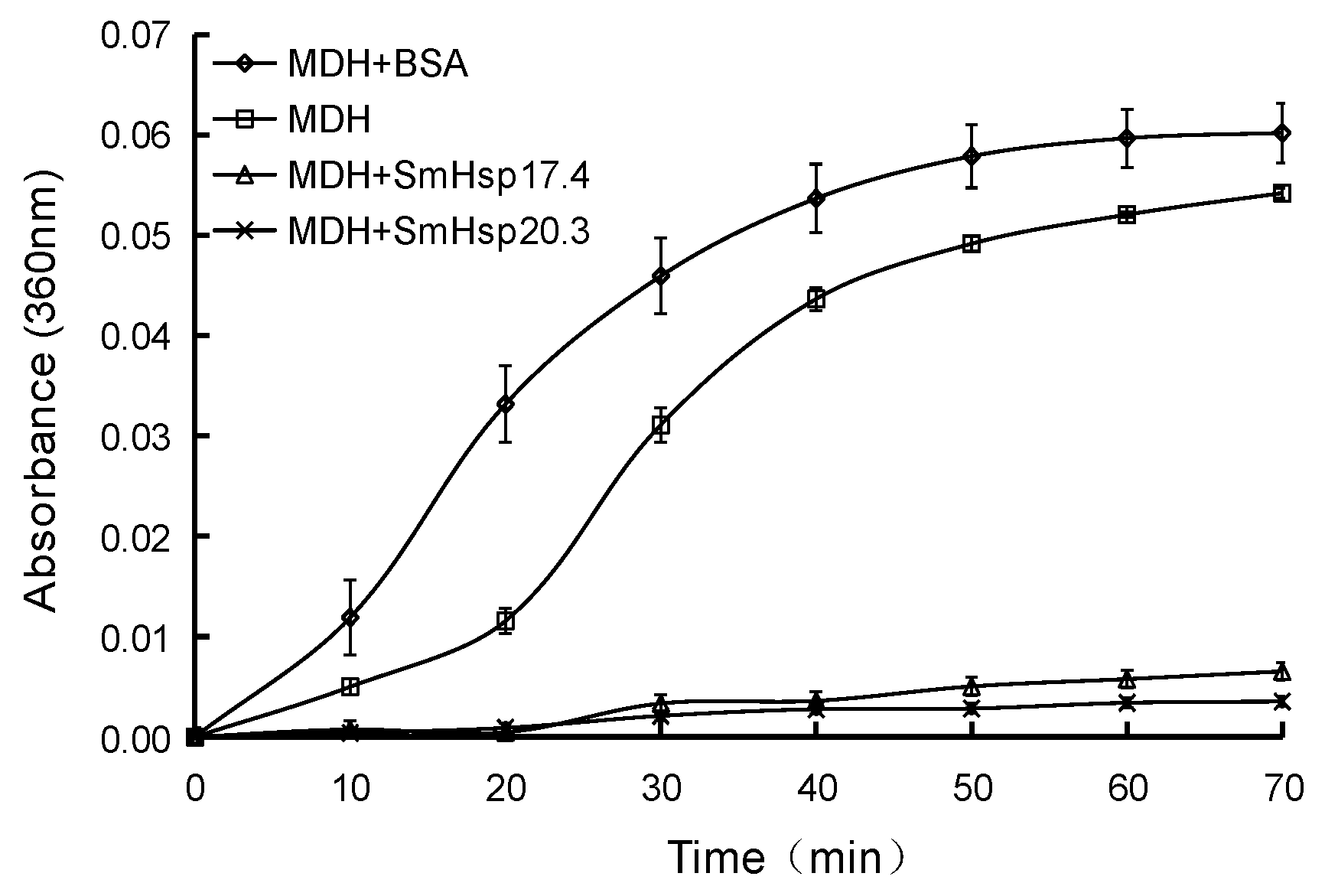
3.5. Chaperone Activity of Recombinant SmHsp17.4 and SmHsp20.3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, Y.H.; Du, Y.; Wang, J.W. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Guo, X.; Li, Y.; Gao, H.; Guo, X.; Xu, B. sHsp22.6, an intronless small heat shock protein gene, is involved in stress defence and development in Apis cerana cerana. Insect Biochem. Mol. Biol. 2014, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lei, J.; Fox, C.W.; Johnston, J.S.; Zhu-Salzman, K. Comparison of life history and genetic properties of cowpea bruchid strains and their response to hypoxia. J. Insect Physiol. 2015, 75, 5–11. [Google Scholar] [CrossRef]
- Tungjitwitayakul, J.; Tatun, N.; Vajarasathira, B.; Sakurai, S. Expression of Heat Shock Protein Genes in Different Developmental Stages and After Temperature Stress in the Maize Weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 1313–1323. [Google Scholar] [CrossRef]
- García-Reina, A.; Rodríguez-García, M.J.; Ramis, G.; Galian, J. Real-time cell analysis and heat shock protein gene expression in the TcA Tribolium castaneum cell line in response to environmental stress conditions. Insect Sci. 2016, 24, 358–370. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock proteins, molecular chaperones and the stress response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef] [Green Version]
- Basha, E.; O’Neill, H.; Vierling, E. Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 2012, 37, 106–117. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.W.; Caspers, G.-J.; Leunissen, J.A. Genealogy of the α-crystallin—small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef]
- Franck, E.; Madsen, O.; Van Rheede, T.; Ricard, G.; Huynen, M.A.; De Jong, W.W. Evolutionary Diversity of Vertebrate Small Heat Shock Proteins. J. Mol. Evol. 2004, 59, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Kappé, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.M.; De Jong, W.W. The human genome encodes 10 α-crystallin–related small heat shock proteins: HspB1–10. Cell Stress Chaperon 2003, 8, 53–61. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Yu, Q.-Y.; Xiang, Z.-H.; Kishino, H.; Zhang, Z. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol. Biol. 2009, 9, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, D.B.; Napoli, V.; Mazurkie, A.; Stafford, W.F.; Graceffa, P. Phosphorylation Dependence of Hsp27 Multimeric Size and Molecular Chaperone Function. J. Biol. Chem. 2009, 284, 18801–18807. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, A.P. Small stress proteins: Chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 1998, 379, 19–26. [Google Scholar]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef] [Green Version]
- Haslbeck, M. sHsps and their role in the chaperone network. Cell. Mol. Life Sci. 2002, 59, 1649–1657. [Google Scholar] [CrossRef]
- Garrido, C.; Paul, C.; Seigneuric, R.; Kampinga, H.H. The small heat shock proteins family: The long forgotten chaperones. Int. J. Biochem. Cell Biol. 2012, 44, 1588–1592. [Google Scholar] [CrossRef]
- Sun, Y.; Macrae, T.H. Small heat shock proteins: Molecular structure and chaperone function. Cell. Mol. Life Sci. 2005, 62, 2460–2476. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Stevenson, M.A.; Calderwood, S.K. Heat shock proteins and cancer vaccines: Developments in the past decade and chaperoning in the decade to come. Expert Rev. Vaccines 2011, 10, 1553–1568. [Google Scholar] [CrossRef] [Green Version]
- Macrae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.A.L.; Pavlides, S.; Tammariello, S.; Rinehart, J.; Denlinger, D. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J. Insect Physiol. 2005, 51, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.L.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137. [Google Scholar] [CrossRef] [Green Version]
- Si, F.L.; He, Z.B.; Chen, B. Cloning and expression profiling of heat shock protein DaHSP23 gene in the winter and summer diapause pupae of the onion maggot, Delia antiqua (Diptera: Anthomyiidae). Acta Entomol. Sin. 2016, 59, 402–410. [Google Scholar]
- Fremdt, H.; Amendt, J.; Zehner, R. Diapause-specific gene expression in Calliphora vicina (Diptera: Calliphoridae)—A useful diagnostic tool for forensic entomology. Int. J. Leg. Med. 2013, 128, 1001–1011. [Google Scholar] [CrossRef]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.). J. Insect Physiol. 2008, 54, 1503–1510. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Xu, W.-H. Proteomic and Phosphoproteomic Analysis at Diapause Initiation in the Cotton Bollworm, Helicoverpa armigera. J. Proteome Res. 2010, 9, 5053–5064. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, J.; Peng, Y.; Liu, X.A.; Hoffmann, A.; Ma, C.-S. Stress Responses of Small Heat Shock Protein Genes in Lepidoptera Point to Limited Conservation of Function across Phylogeny. PLoS ONE 2015, 10, e0132700. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, S.-I.; Numata, H.; Goto, S.G. Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J. Insect Physiol. 2005, 51, 641–647. [Google Scholar] [CrossRef]
- Goto, S.G.; Kimura, M.T. Heat-shock-responsive genes are not involved in the adult diapause of Drosophila triauraria. Gene 2004, 326, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, R.; Ponnuvel, K.M.; Qadri, S.M.H. Expression of metabolic enzyme genes and heat-shock protein genes during embryonic development in diapause and non-diapause egg of multivoltine silkworm Bombyx mori. Biologia 2008, 63, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Mestril, R.; Schiller, P.; Amin, J.; Klapper, H.; Ananthan, J.; Voellmy, R. Heat shock and ecdysterone activation of the Drosophila melanogaster hsp23 gene; a sequence element implied in developmental regulation. EMBO J. 1986, 5, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Amin, J.; Mestril, R.; Voellmy, R. Genes for Drosophila small heat proteins are regulated differently by ecdysone. Mol. Cell. Biol. 1991, 11, 5937–5944. [Google Scholar] [PubMed] [Green Version]
- Kokolakis, G.; Tatari, M.; Zacharopoulou, A.; Mintzas, A.C. Thehsp27gene of the Mediterranean fruit fly, Ceratitis capitata: Structural characterization, regulation and developmental expression. Insect Mol. Biol. 2008, 17, 699–710. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, P.; Guo, X.; Xu, B. Two small heat shock protein genes in Apis cerana cerana: Characterization, regulation, and developmental expression. Gene 2014, 545, 205–214. [Google Scholar] [CrossRef]
- Gaafar, N.; Volkmar, C. Evaluation of wheat ear insects in large scale field in central Germany. Agric. Sci. 2010, 1, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Wu, Y.-Q.; Gong, Z.-J.; He, Y.-Z.; Duan, Y.; Jiang, Y.-L. Long-Distance Wind-Borne Dispersal of Sitodiplosis mosellana Géhin (Diptera: Cecidomyiidae) in Northern China. J. Insect Behav. 2012, 26, 120–129. [Google Scholar] [CrossRef]
- Shrestha, G.; Reddy, G.V.P. Field efficacy of insect pathogen, botanical, and jasmonic acid for the management of wheat midge Sitodiplosis mosellana and the impact on adult parasitoid Macroglenes penetrans populations in spring wheat. Insect Sci. 2019, 26, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Long, Z.R.; Feng, A.R.; Cheng, W.N. Effects of initial population number, wheat varieties and precipitation on infestation of Sitodiplosis mosellana (Diptera: Cecidomyiidae). Acta Agric. Boreali Occident. Sin. 2015, 24, 165–171. [Google Scholar]
- Cheng, W.; Long, Z.; Zhang, Y.; Liang, T.; Zhu-Salzman, K. Effects of temperature, soil moisture and photoperiod on diapause termination and post-diapause development of the wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae). J. Insect Physiol. 2017, 103, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doane, J.; Olfert, O. Seasonal development of wheat midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae), in Saskatchewan, Canada. Crop. Prot. 2008, 27, 951–958. [Google Scholar] [CrossRef]
- Cheng, W.N.; Li, J.J.; Li, Y.P.; Li, X.L.; Wu, J.X.; Wang, H.L. Quantitative analysis of ecdysteroid in adults and the pre-diapause, diapause and post-diapause larvae of wheat blossom midge, Sitodiplosis Mosellana Gehin. Acta Phytophy. Sin. 2009, 36, 163–167. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Mei, X.-D.; Feng, J.-N.; Berg, B.G.; Zhang, Y.; Guo, Y.-Y. Characterization of three pheromone-binding proteins (PBPs) of Helicoverpa armigera (Hübner) and their binding properties. J. Insect Physiol. 2012, 58, 941–948. [Google Scholar] [CrossRef]
- Pérez-Morales, D.; Ostoa-Saloma, P.; Espinoza, B. Trypanosoma cruzi SHSP16: Characterization of an α-crystallin small heat shock protein. Exp. Parasitol. 2009, 123, 182–189. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Shaw, D.C. Heat-Stable Proteins and Abscisic Acid Action in Barley Aleurone Cells. Plant Physiol. 1989, 91, 1520–1526. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Li, W.; Mao, Q.; Chang, Z. Disulfide bonds convert small heat shock protein Hsp16.3 from a chaperone to a non-chaperone: Implications for the evolution of cysteine in molecular chaperones. Biochem. Biophys. Res. Commun. 2003, 308, 627–635. [Google Scholar] [CrossRef]
- Zhao, J.J.; Li, X.J.; Liu, Z.Z.; Cheng, W.N.; Zhu, K.Y. Cloning of heat shock protein gene SmHsp40 and its expression during diapause and under extreme temperatures stress in Sitodiplosis mosellana (Diptera: Cecidomyiidae). Acta Entomol. Sin. 2018, 61, 1253–1263. [Google Scholar]
- Bagneris, C.; Bateman, O.A.; Naylor, C.E.; Cronin, N.; Boelens, W.C.; Keep, N.H.; Slingsby, C. Crystal Structures of α-Crystallin Domain Dimers of αB-Crystallin and Hsp20. J. Mol. Biol. 2009, 392, 1242–1252. [Google Scholar] [CrossRef]
- De Jong, W.W.A.; Leunissen, J.E.; Voorter, C. Evolution of the alpha-crystallin/small heat-shock protein family. Mol. Biol. Evol. 1993, 10, 103–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhao, J.; Zhu-Salzman, K. Cloning and characterization of Methoprene-tolerant (Met) and Krüppel homolog 1 (Kr-h1) genes in the wheat blossom midge, Sitodiplosis mosellana. Insect Sci. 2020, 27, 292–303. [Google Scholar] [CrossRef]
- Barnes, H.F. Studies of fluctuations in insect populations. Ⅷ. The wheat blossom midge on broadbalk, 1932–1940, with a discussion of the results obtained 1927–1940. J. Anim. Ecol. 1941, 10, 94–120. [Google Scholar]
- Yuan, F. The Wheat Blossom Midges Sitodiplosis Mosellana (Gehin) and Contarinia Tritici (Kirby): Their Plague Principle and Control; Science Press: Beijing, China, 2004. [Google Scholar]


| Primer Name | Sequence (5′ to 3′) | Purpose |
|---|---|---|
| Hsp17.4-5′-outer | CTTGTTTCTCTTCGTGTTTGGCATG | 5′ RACE |
| Hsp17.4-5′-inner | GTGACGCTTCGAAACCATCCTTG | |
| Hsp20.3-5′-outer | GATCGCAAAGCTGGATAATCGGTTG | |
| Hsp20.3-5′-inner | CGTCAAAAATGTGTGGCAATAGAGAC | |
| Hsp17.4-3′-outer | CTACTCTCTCATCTGATGGTGTTCTCTC | 3′ RACE |
| Hsp17.4-3′-inner | CAGAGCATCAAGAGCAACGAGGAG | |
| Hsp20.3-3′-outer | CAAACGGGACCAGCTCACTTGAATG | |
| Hsp20.3-3′-inner | AGCAACGAAGAAGTGAAAGACGAAG | |
| Hsp17.4-F1 | AGTCGAATCTAAAGCATTCC | Full-length cDNA validation and gDNA cloning |
| Hsp17.4-R1 | GGTCCTTTATATTGATTGAAATTTAC | |
| Hsp20.3-F1 | TTATACGAATCGTTAACGAAAC | |
| Hsp20.3-R1 | AATGAATTTCAAAATTCGCTCTTAG | |
| Hsp17.4-F2 | CGGGATCCATGTCGTTGATTCCATTCC (BamHI) | Prokaryotic expression |
| Hsp17.4-R2 | CCCAAGCTTGGGTTACTTTCCCTCTTTTTTC (HindIII) | |
| Hsp20.3-F2 | CGGGATCCATGTCTCTATTGCCACACA (BamHI) | |
| Hsp20.3-R2 | CCCAAGCTTGGGTTATTTTTCTTCGTCTTTC (HindIII) | |
| Hsp17.4-q-F | GAGCACGGTTACATTTCGC | qPCR |
| Hsp17.4-q-R | CTCCTCGTTGCTCTTGATGCTCTGTTTGACT | |
| Hsp20.3-q-F | AAGCCGTCCTTGCCCATTT | |
| Hsp20.3-q-R | ATTATCGCTTGACTGGTGG | |
| GAPDH-q-F | CCATCAAAGCAAGCAAGA | |
| GAPDH-q-R | CAGCACGGAGCACAAGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, Q.; Zhang, G.; Zhu-Salzman, K.; Cheng, W. Characterization of Two Small Heat Shock Protein Genes (Hsp17.4 and Hs20.3) from Sitodiplosis mosellana, and Their Expression Regulation during Diapause. Insects 2021, 12, 119. https://doi.org/10.3390/insects12020119
Zhao J, Huang Q, Zhang G, Zhu-Salzman K, Cheng W. Characterization of Two Small Heat Shock Protein Genes (Hsp17.4 and Hs20.3) from Sitodiplosis mosellana, and Their Expression Regulation during Diapause. Insects. 2021; 12(2):119. https://doi.org/10.3390/insects12020119
Chicago/Turabian StyleZhao, Jiajia, Qitong Huang, Guojun Zhang, Keyan Zhu-Salzman, and Weining Cheng. 2021. "Characterization of Two Small Heat Shock Protein Genes (Hsp17.4 and Hs20.3) from Sitodiplosis mosellana, and Their Expression Regulation during Diapause" Insects 12, no. 2: 119. https://doi.org/10.3390/insects12020119





