Brain Metastases from Uterine Cervical and Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Extraction
3. Results
4. Cervical Cancer
4.1. Prevalence and Clinical Characteristics
4.2. Clinical Presentation and Diagnosis
4.3. Treatment
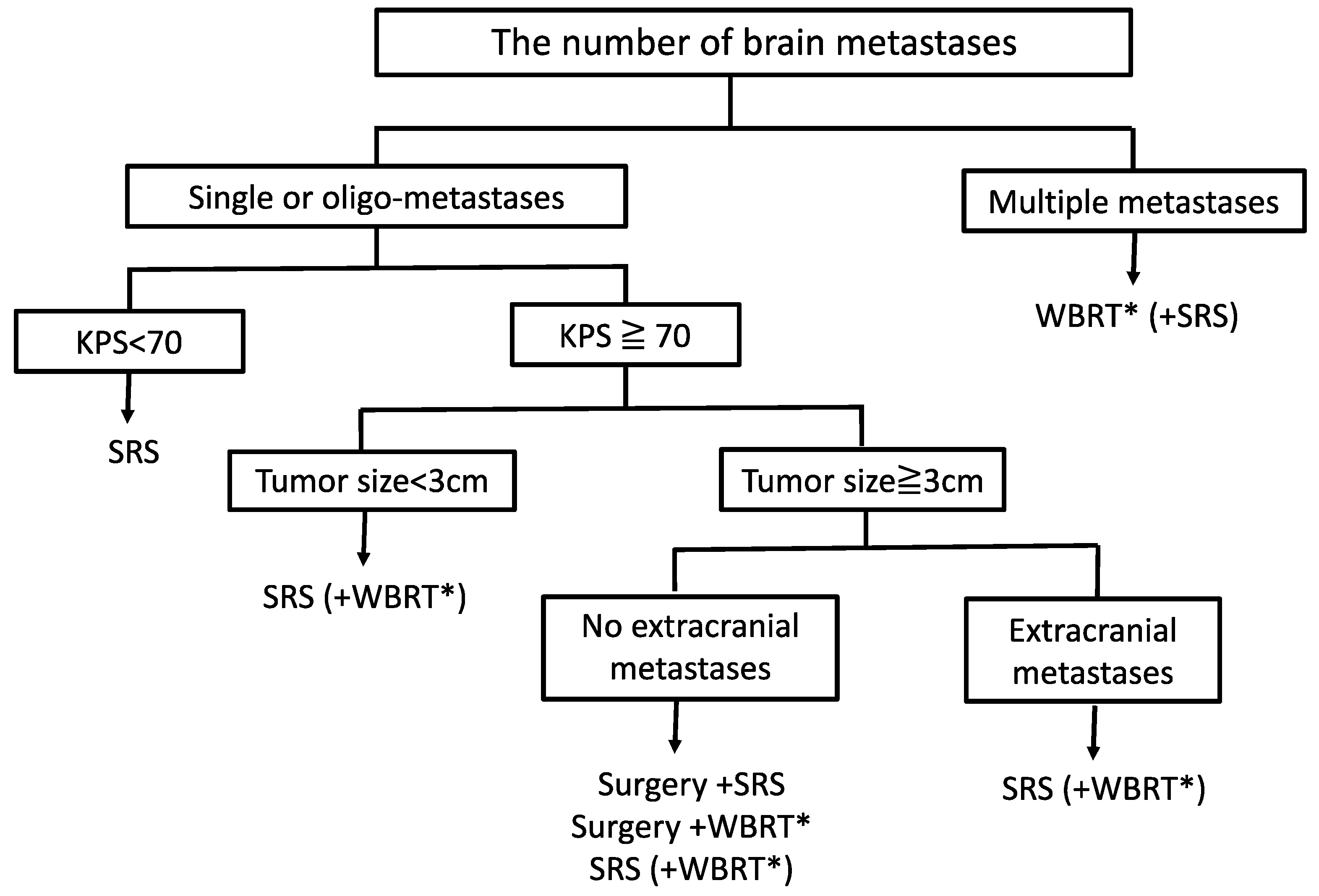
4.3.1. Whole-Brain Irradiation Therapy
4.3.2. Surgery
4.3.3. Stereotactic Radiosurgery
4.3.4. Chemotherapy
4.3.5. Multimodal Therapy
4.4. Prognostic Factors
4.5. Prognosis
5. Endometrial Cancer
5.1. Prevalence and Clinical Characteristics
5.2. Clinical Presentation and Diagnosis
5.3. Treatment
5.4. Prognostic Factors
5.5. Prognosis
6. Treatment Strategies and Future Perspectives for BMs from CC and EC
7. Limitation
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, E.; End Results Program. Cancer Stat Facts: Cervical Cancer; National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 20 December 2020).
- Surveillance, E.; End Results Program. Cancer Stat Fscts: Uterine Cancer; National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 20 December 2020).
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wei, S.; Yang, H.; Yu, Q.; Xu, M.; Guo, J.; Gao, L. Clinicopathological study of organ metastasis in endometrial cancer. Futur. Oncol. 2020, 16, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Tosoni, A.; Ermani, M.; Brandes, A.A. The pathogenesis and treatment of brain metastases: A comprehensive review. Crit. Rev. Oncol. 2004, 52, 199–215. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Ogawa, K.; Yoshii, Y.; Aoki, Y.; Nagai, Y.; Tsuchida, Y.; Toita, T.; Kakinohana, Y.; Tamaki, W.; Iraha, S.; Adachi, G.; et al. Treatment and prognosis of brain metastases from gynecological cancers. Neurol. Medico-Chir. 2008, 48, 57–63. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Buchsbaum, H.J.; Rice, A.C. Cerebral metastasis in cervical carcinoma. Am. J. Obstet. Gynecol. 1972, 114, 276–278. [Google Scholar] [CrossRef]
- Peeples, W.J.; Inalsingh, C.; Hazra, T.A.; Graft, D. The occurrence of metastasis outside the abdomen and retroperitoneal space in invasive carcinoma of the cervix. Gynecol. Oncol. 1976, 4, 307–310. [Google Scholar] [CrossRef]
- Van Nagell, J.R.; Rayburn, W.; Donaldson, E.S.; Hanson, M.; Gay, E.C.; Yoneda, J.; Marayuma, Y.; Powell, D.F. Therapeutic implications of patterns of recurrence in cancer of the uterine cervix. Cancer 1979, 44, 2354–2361. [Google Scholar] [CrossRef]
- Lefkowitz, D.; Asconapé, J.; Biller, J. Intracranial Metastases from Carcinoma of the Cervix. South. Med. J. 1983, 76, 519–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friedman, M.; Nissenbaum, M.; Lakier, R.; Browde, S. Brain metastases in early cancer of the uterine cervix. A case report. S. Afr. Med. J. 1983, 64, 498–499. [Google Scholar] [PubMed]
- Gaze, M.; Gregor, A.; Whittle, I.R.; Sellar, R.J. Calcified cerebral metastasis from cervical carcinoma. Neuroradiology 1989, 31, 291. [Google Scholar] [CrossRef] [PubMed]
- Saphner, T.; Gallion, H.H.; Van Nagell, J.R.; Kryscio, R.; Patchell, R.A. Neurologic complications of cervical cancer. A review of 2261 cases. Cancer 1989, 64, 1147–1151. [Google Scholar] [CrossRef]
- Kumar, L.; Tanwar, R.K.; Singh, S. Intracranial metastases from carcinoma cervix and review of literature. Gynecol. Oncol. 1992, 46, 391–392. [Google Scholar] [CrossRef]
- Fagundes, H.; Perez, C.A.; Grigsby, P.W.; Lockett, M.A. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int. J. Radiat. Oncol. 1992, 24, 197–204. [Google Scholar] [CrossRef]
- Cormio, G.; Pellegrino, A.; Landoni, F.; Regallo, M.; Zanetta, G.; Colombo, A.; Mangioni, C. Brain Metastases from Cervical Carcinoma. Tumori J. 1996, 82, 394–396. [Google Scholar] [CrossRef]
- Robinson, J.B.; Morris, M. Cervical Carcinoma Metastatic to the Brain. Gynecol. Oncol. 1997, 66, 324–326. [Google Scholar] [CrossRef]
- Salpietro, F.M.; Alafaci, C.; Gervasio, O.; La Rosa, G.; Baio, A.; Francolini, D.C.; Batolo, D.; Tomasello, F. Primary cervical melanoma with brain metastases. J. Neurosurg. 1998, 89, 659–666. [Google Scholar] [CrossRef]
- Senapati, S.N.; Samanta, D.R.; Giri, S.K.; Mohanty, B.K.; Nayak, C.R. Carcinoma cervix with brain metastasis. J. Indian Med. Assoc. 1998, 96, 352–353. [Google Scholar]
- Ikeda, S.-I.; Yamada, T.; Katsumata, N.; Hida, K.; Tanemura, K.; Tsunematu, R.; Ohmi, K.; Sonoda, T.; Ikeda, H.; Nomura, K. Cerebral Metastasis in Patients with Uterine Cervical Cancer. Jpn. J. Clin. Oncol. 1998, 28, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Ziainia, T.; Resnik, E. Hemiballismus and Brain Metastases from Squamous Cell Carcinoma of the Cervix. Gynecol. Oncol. 1999, 75, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Cormio, G.; Colamaria, A.; Loverro, G.; Pierangeli, E.; Di Vagno, G.; De Tommasi, A.; Selvaggi, L. Surgical Resection of a Cerebral Metastasis from Cervical Cancer: Case Report and Review of the Literature. Tumori J. 1999, 85, 65–67. [Google Scholar] [CrossRef]
- Cormio, G.; Colamaria, A.; Di Vagno, G.; De Tommasi, A.; Loverro, G.; Selvaggi, L. Surgical decompression and radiation therapy in epidural metastasis from cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 59–61. [Google Scholar] [CrossRef]
- Mahmoud-Ahmed, A.S.; Suh, J.H.; Barnett, G.H.; Webster, K.D.; Kennedy, A.W. Tumor Distribution and Survival in Six Patients with Brain Metastases from Cervical Carcinoma. Gynecol. Oncol. 2001, 81, 196–200. [Google Scholar] [CrossRef]
- El Omari-Alaoui, H.; Gaye, P.M.; Kebdani, T.; El Ghazi, E.; Benjaafar, N.; Mansouri, A.; Errihani, H.; Kettani, F.; El Ouahabi, A.; El Gueddari, B.K. Cerebellous metastases in patients with uterine cervical cancer. Two cases reports and review of the literature. Cancer/Radiothérapie 2003, 7, 317–320. [Google Scholar] [CrossRef]
- Tajran, D.; Berek, J.S. Surgical resection of solitary brain metastasis from cervical cancer. Int. J. Gynecol. Cancer 2003, 13, 368–370. [Google Scholar] [CrossRef]
- Salvati, M.; Caroli, E.; Orlando, E.R.; Nardone, A.; Frati, A.; Innocenzi, G.; Giangaspero, F. Solitary brain metastases from uterus carcinoma: Report of three cases. J. Neuro-Oncol. 2004, 66, 175–178. [Google Scholar] [CrossRef]
- Amita, M.; Sudeep, G.; Rekha, W.; Yogesh, K.; Hemant, T. Brain metastasis from cervical carcinoma—A case report. MedGenMed 2005, 7, 26. [Google Scholar]
- Nagar, Y.S.; Shah, N.; Rawat, S.; Kataria, T. Intracranial metastases from adenocarcinoma of cervix: A case report. Int. J. Gynecol. Cancer 2005, 15, 561–563. [Google Scholar] [CrossRef]
- Cordeiro, J.G.; Prevedello, D.M.-S.; Ditzel, L.F.D.S.; Pereira, C.U.; Araújo, J. Cerebral metastasis of cervical uterine cancer: Report of three cases. Arq. Neuro-Psiquiatr. 2006, 64, 300–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaussmann, A.B.; Imhoff, D.; Lambrecht, E.; Menzel, C.; Mose, S. Spontaneous Remission of Metastases of Cancer of the Uterine Cervix. Oncol. Res. Treat. 2006, 29, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Portera, C.C.; Gottesman, R.F.; Srodon, M.; Asrari, F.; Dillon, M.; Armstrong, D.K. Optic neuropathy from metastatic squamous cell carcinoma of the cervix: An unusual CNS presentation. Gynecol. Oncol. 2006, 102, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Chura, J.C.; Shukla, K.; Argenta, P.A. Brain metastasis from cervical carcinoma. Int. J. Gynecol. Cancer 2007, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Kumar, A.; Sinha, A.K.; Kumar, M.; Pandey, S.R.; Khaniya, S. Intracranial metastases from carcinoma of the cervix. Singap. Med. J. 2007, 48, 154–156. [Google Scholar]
- Iii, J.V.B.; Epstein, H.D.; Kim, R.; Micha, J.P.; Rettenmaier, M.A.; Mattison, J.A.; Goldstein, B.H. Rapid Manifestation of CNS Metastatic Disease in a Cervical Carcinoma Patient: A Case Report. Oncology 2007, 73, 273–276. [Google Scholar] [CrossRef]
- Growdon, W.B.; Lopez-Varela, E.; Littell, R.; Oliva, E.; Seiden, M.; Krasner, C.; Lee, H.; Fuller, A. Extent of extracranial disease is a powerful predictor of survival in patients with brain metastases from gynecological cancer. Int. J. Gynecol. Cancer 2007, 18, 262–268. [Google Scholar] [CrossRef]
- Rades, D.; Fischer, D.; Veninga, T.; Stalpers, L.J.; Schild, S.E. Prognostic factors for survival and intracerebral control after irradiation for brain metastases from gynecological cancer. Gynecol. Oncol. 2009, 114, 506–508. [Google Scholar] [CrossRef]
- Peters, P.; Bandi, H.; Efendy, J.; Perez-Smith, A.; Olson, S. Rapid growth of cervical cancer metastasis in the brain. J. Clin. Neurosci. 2010, 17, 1211–1212. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chu, T. Brain and Intramedullary Spinal Cord Metastasis from Squamous Cell Cervical Carcinoma. Taiwan. J. Obstet. Gynecol. 2010, 49, 525–527. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Ro, D.Y.; Park, B.J.; Kim, Y.W.; Kim, T.-E.; Jung, J.K.; Lee, J.W.; Kim, J.Y.; Han, C.W. Brain metastasis from uterine cervical cancer. J. Obstet. Gynaecol. Res. 2010, 36, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, A.; Salvati, M.; D’Elia, A.; Arcella, A.; Giangaspero, F.; Esposito, V. Single brain metastases from cervical carcinoma: Report of two cases and critical review of the literature. Neurol. Sci. 2011, 33, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.Y.; Bauer, D.F.; Shannon, C.N.; Fiveash, J.; Markert, J.M. Stereotactic radiosurgical treatment of brain metastasis of primary tumors that rarely metastasize to the central nervous system. J. Neuro-Oncol. 2012, 109, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Setoodeh, R.; Hakam, A.; Shan, Y. (Frank) Cerebral metastasis of cervical cancer, report of two cases and review of the literature. Int. J. Clin. Exp. Pathol. 2012, 5, 710–714. [Google Scholar] [PubMed]
- Lan-Fang, L.; Hai-Yan, S.; Zuo-Ming, Y.; Jian-Qing, Z.; Ya-Qing, C. Small cell neuroendocrine carcinoma of the cervix: Analysis of the prognosis and role of radiation therapy for 43 cases. Eur. J. Gynaecol. Oncol. 2012, 33, 68–73. [Google Scholar] [PubMed]
- Yuan, G.-W.; Wu, L.; Huang, M.-N.; Yao, H.-W. Analysis of 25 cases of brain metastasis from gynecological cancers. Zhonghua fu chan ke za zhi 2012, 47, 191–195. [Google Scholar] [PubMed]
- Azimirad, A.; Sarraf, Z. Parkinsonism in a Recurrent Cervical Cancer Patient: Case Report and Review of the Literature. J. Fam. Reprod. Health 2013, 7, 189–191. [Google Scholar]
- Chung, S.-B.; Jo, K.-I.; Seol, H.-J.; Nam, -H.; Lee, J.-I. Radiosurgery to palliate symptoms in brain metastases from uterine cervix cancer. Acta Neurochir. 2012, 155, 399–405. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yoo, H.J.; Lim, M.C.; Seo, S.-S.; Kang, S.; Kim, J.-Y.; Park, S.-Y. Brain metastasis in patients with uterine cervical cancer. J. Obstet. Gynaecol. Res. 2012, 39, 287–291. [Google Scholar] [CrossRef]
- Nasu, K.; Satoh, T.; Nishio, S.; Nagai, Y.; Ito, K.; Otsuki, T.; Hongo, A.; Hirashima, Y.; Ogura, T.; Shimada, M. Clinicopathologic features of brain metastases from gynecologic malignancies: A retrospective study of 139 cases (KCOG-G1001s trial). Gynecol. Oncol. 2013, 128, 198–203. [Google Scholar] [CrossRef]
- Araujo, J.L.V.; Veiga, J.C.E.; Barboza, V.R.; De Souza, N.; Mayrink, D.; Nadais, R.F.; Figueiredo, E.G. Scalp, skull and brain metastasis of squamous cell carcinoma of the cervix—A rare entity. Br. J. Neurosurg. 2013, 27, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Shepard, M.J.; Fezeu, F.; Lee, C.-C.; Sheehan, J.P. Gamma knife radiosurgery for the treatment of gynecologic malignancies metastasizing to the brain: Clinical article. J. Neuro-Oncol. 2014, 120, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Tenjarla, S.; Chawla, S.; Toy, E.P. Synchronous Presentation of Cavernous Sinus Metastasis and Cervical Cancer: A Case Report and Review of Literature. World J. Oncol. 2014, 5, 228–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Branch, B.; Henry, J.; Vecil, G.G. Brain metastases from cervical cancer—A short review. Tumori J. 2014, 100, 171–179. [Google Scholar] [CrossRef]
- Erdis, E. A rare metastatic region of cervix cancer; the brain. J. Pak. Med. Assoc. 2014, 64, 89–90. [Google Scholar]
- Kim, Y.Z.; Kwon, J.H.; Lim, S. A Clinical Analysis of Brain Metastasis in Gynecologic Cancer: A Retrospective Multi-institute Analysis. J. Korean Med. Sci. 2015, 30, 66–73. [Google Scholar] [CrossRef]
- Pyeon, S.Y.; Park, J.Y.; Ulak, R.; Seol, H.J.; Lee, J.M. Isolated brain metastasis from uterine cervical cancer: A case report and review of literature. Eur. J. Gynaecol. Oncol. 2015, 36, 602–604. [Google Scholar]
- Sato, Y.; Tanaka, K.; Kobayashi, Y.; Shibuya, H.; Nishigaya, Y.; Momomura, M.; Matsumoto, H.; Iwashita, M. Uterine cervical cancer with brain metastasis as the initial site of presentation. J. Obstet. Gynaecol. Res. 2015, 41, 1145–1148. [Google Scholar] [CrossRef]
- Gressel, G.M.; Lundsberg, L.S.; Altwerger, G.; Katchi, T.; Azodi, M.; Schwartz, P.E.; Ratner, E.S. Factors Predictive of Improved Survival in Patients With Brain Metastases from Gynecologic Cancer: A Single Institution Retrospective Study of 47 Cases and Review of the Literature. Int. J. Gynecol. Cancer 2015, 25, 1711–1716. [Google Scholar] [CrossRef]
- Walter, A.; Gunderson, C.C.; Vesely, S.K.; Algan, O.; Sughrue, M.; Slaughter, K.N.; Moore, K.N. Central nervous system metastasis in gynecologic cancer: Symptom management, prognosis and palliative management strategies. Gynecol. Oncol. 2015, 136, 472–477. [Google Scholar] [CrossRef]
- Cacho-Díaz, B.; Lorenzana-Mendoza, N.A.; Michel-Ortega, R.M.; Reyes-Soto, G.; Monroy-Sosa, A.; De León, D.C.; Martínez-Tláhuel, J.L.; Herrera-Gómez, A.; Granados-García, M. Central Nervous System Metastases in Patients with Cervical Carcinoma. Int. J. Gynecol. Cancer 2016, 26, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bandzar, S.; Atallah, H. Atypical Presentation of Cervical Carcinoma with Cerebral Metastasis. Ochsner J. 2016, 16, 548–550. [Google Scholar]
- Shin, H.K.; Kim, J.H.; Lee, D.H.; Cho, Y.H.; Kwon, D.-H.; Roh, S.W. Clinical Outcomes of Gamma Knife Radiosurgery for Metastatic Brain Tumors from Gynecologic Cancer: Prognostic Factors in Local Treatment Failure and Survival. J. Korean Neurosurg. Soc. 2016, 59, 392–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilani, M.; Williams, N.L.; Giordano, C.; Rosenblum, N.; Shi, W.; Anne, P.; Schilder, R.J.; Madiha, A.G.; Noelle, L.W.; Carolyn, G.; et al. Brain Metastases in Patients with Gynecologic Cancers: A Single Institution Experience and Review of the Literature. Open J. Obstet. Gynecol. 2016, 6, 544–552. [Google Scholar] [CrossRef]
- Divine, L.M.; Kizer, N.T.; Hagemann, A.R.; Pittman, M.E.; Chen, L.; Powell, M.A.; Mutch, D.G.; Rader, J.S.; Thaker, P.H. Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol. Oncol. 2016, 142, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dziggel, L.; Janssen, S.; Bajrovic, A.; Veninga, T.; Trang, N.T.; Khoa, M.T.; Schild, S.E.; Rades, D. Local Therapies Can Improve Intracerebral Control in Patients with Cerebral Metastasis from Gynecological Cancers. Anticancer. Res. 2016, 36, 4777–4780. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Ismail, R.; Potrebko, P.S.; Pepe, J.; Wu, M.; Saigal, K.; Biagioli, M.; Shridhar, R.; Holloway, R.; Field, M.; et al. Role of Gamma Knife® Radiosurgery for the Treatment of Brain Metastases from Gynecological Cancers. Cureus 2016, 8, e947. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shuto, T.; Sato, M. Gamma Knife Surgery for Metastatic Brain Tumors from Gynecologic Cancer. World Neurosurg. 2016, 89, 455–463. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Kim, B.-J.; Park, S.-I.; Ryu, S.-Y.; Cho, C.-K. Prognostic Importance of the Site of Recurrence in Patients with Metastatic Recurrent Cervical Cancer. Int. J. Radiat. Oncol. 2017, 98, 1124–1131. [Google Scholar] [CrossRef]
- Johnston, H.; Mctyre, E.R.; Cramer, C.K.; Lesser, G.J.; Ruiz, J.; Bourland, J.D.; Watabe, K.; Lo, H.-W.; Qasem, S.; Laxton, A.W.; et al. Stereotactic radiosurgery in the treatment of brain metastases from gynecologic primary cancer. J. Radiosurgery SBRT 2017, 5, 55–61. [Google Scholar]
- Takeshita, S.; Todo, Y.; Furuta, Y.; Okamoto, K.; Minobe, S.; Yamashiro, K.; Kato, H. Prognostic factors for patients with brain metastasis from gynecological cancer: A significance of treatment-free interval of more than 6 months. Jpn. J. Clin. Oncol. 2017, 47, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Takahashi, H.; Hasegawa, Y.; Higuchi, F.; Takahashi, M.; Makino, K.; Takagaki, M.; Akimoto, J.; Okuda, T.; On Behalf of the Committee of Brain Tumor Registry of Japan Supported by the Japan Neurosurgical Society; et al. A nationwide multi-institutional retrospective study to identify prognostic factors and develop a graded prognostic assessment system for patients with brain metastases from uterine corpus and cervical cancer. BMC Cancer 2017, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Fetcko, K.; Gondim, D.D.; Bonnin, J.M. Cervical cancer metastasis to the brain: A case report and review of literature. Surg. Neurol. Int. 2017, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Janssen, S.; Bajrovic, A.; Veninga, T.; Fischer, D.; Schild, S.E. A New Scoring Tool to Assess Overall Survival in Patients With Intracerebral Metastases From Gynecological Cancers. Int. J. Gynecol. Cancer 2017, 27, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Hansen, H.C.; Schild, S.E.; Rades, D. An Instrument for Estimating the 6-Month Survival Probability After Whole-brain Irradiation Alone for Cerebral Metastases from Gynecological Cancer. Anticancer. Res. 2018, 38, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, M.J.; Hasan, S.; Fuhrer, R.; Krivak, T.; Aziz, K.; Wegner, R.E. Linear accelerator-based radiosurgery and hypofractionated stereotactic radiotherapy for brain metastasis secondary to gynecologic malignancies: A single institution series examining outcomes of a rare entity. Gynecol. Oncol. Rep. 2018, 25, 19–23. [Google Scholar] [CrossRef]
- Bi, Y.; Li, L. Pathologically confirmed brain metastases from primary uterine cervical tumors: Two cases and a literature review. World J. Surg. Oncol. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Grant, M.S.; Stepp, W.H.; Clark, L.H. Clinical characteristics of CNS metastases from primary gynecologic cancers. Gynecol. Oncol. Rep. 2019, 30, 100518. [Google Scholar] [CrossRef]
- Sadik, Z.H.A.; Beerepoot, L.V.; Hanssens, P.E.J. Efficacy of gamma knife radiosurgery in brain metastases of primary gynecological tumors. J. Neuro-Oncol. 2019, 142, 283–290. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.K.; Heo, M.H.; Kim, J.Y. The prognostic factors influencing overall survival in uterine cervical cancer with brain metastasis. Korean J. Intern. Med. 2019, 34, 1324–1332. [Google Scholar] [CrossRef]
- Nasioudis, D.; Persaud, A.; Taunk, N.K.; Latif, N.A. Brain Metastases from Gynecologic Malignancies. Am. J. Clin. Oncol. 2020, 43, 418–421. [Google Scholar] [CrossRef]
- Gardner, A.B.; Charo, L.M.; Mann, A.K.; Kapp, D.S.; Eskander, R.N.; Chan, J.K. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin. Exp. Metastasis 2019, 37, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Nguyen, T.; Janssen, S.; Schild, S.E. An Instrument to Guide Physicians when Estimating the Survival of Elderly Patients with Brain Metastasis from Gynecological Cancer. Anticancer. Res. 2020, 40, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Salibi, B.S.; Beltaos, E. Endometrial adenocarcinoma with cerebral metastasis and subdural ossification. Wis. Med. J. 1972, 71, 255–258. [Google Scholar] [PubMed]
- Nakano, K.K.; Schoene, W.C. Endometrial carcinoma with a predominant clear-cell pattern with metastases to the adrenal, posterior mediastinum, and brain. Am. J. Obstet. Gynecol. 1975, 122, 529–530. [Google Scholar] [CrossRef]
- Hacker, R.J.; Fox, J.L. Surgical Treatment of Brain Stem Carcinoma. Neurosurgery 1980, 6, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.M.; Graf, C.J. Nontraumatic Subdural Hematoma Secondary to Dural Metastasis: Case Report and Review of the Literature. Neurosurgery 1982, 11, 678–680. [Google Scholar] [CrossRef]
- Aalders, J.; Abeler, V.; Kolstad, P. Recurrent adenocarcinoma of the endometrium: A clinical and histopathological study of 379 patients. Gynecol. Oncol. 1984, 17, 85–103. [Google Scholar] [CrossRef]
- Ritchie, W.W.; Messmer, J.M.; Whitley, D.P.; Gopelrud, D.R. Uterine Carcinoma Metastatic to the Larynx. Laryngoscope 1985, 95, 97–98. [Google Scholar] [CrossRef]
- Savage, J.; Subby, W.; Okagaki, T. Adenocarcinoma of the endometrium with trophoblastic differentiation and metastases as choriocarcinoma: A case report. Gynecol. Oncol. 1987, 26, 257–262. [Google Scholar] [CrossRef]
- Sawada, M.; Ozaki, M.; Inagaki, M.; Yamasaki, M.; Nakagawa, H.; Inoue, T.; Terada, N.; Wada, A. Long-term Survival after Brain Metastasis from Endometrial Cancer. Jpn. J. Clin. Oncol. 1990, 20, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Brezinka, C.; Fend, F.; Huter, O.; Plattner, A. Cerebral metastasis of endometrial carcinoma. Gynecol. Oncol. 1990, 38, 278–281. [Google Scholar] [CrossRef]
- Kottke-Marchant, K.; Estes, M.; Nunez, C. Early brain metastases in endometrial carcinoma. Gynecol. Oncol. 1991, 41, 67–73. [Google Scholar] [CrossRef]
- Thomas, H.; Lambert, H. Solitary cerebral metastases from gynaecological malignancy: The case for radical therapy. Clin. Oncol. 1992, 4, 133–134. [Google Scholar] [CrossRef]
- De Porre, P.M.; Tjokrowardojo, A.J.S. Brain metastases of endometrial carcinoma. Case report and review of literature. Strahlenther. Onkol. 1992, 168, 100–101. [Google Scholar]
- Wroński, M.; Zakowski, M.; Arbit, E.; Hoskins, W.J.; Galicich, J.H. Endometrial cancer metastasis to brain: Report of two cases and a review of the literature. Surg. Neurol. 1993, 39, 355–359. [Google Scholar] [CrossRef]
- Ruelle, A.; Zuccarello, M.; Andrioli, G. Brain metastasis from endometrial carcinoma. Report of two cases. Neurosurg. Rev. 1994, 17, 83–87. [Google Scholar] [CrossRef]
- De Witte, O.; Lefranc, F.; Salmon, I.; Violon, P.; Brotchi, J. Cerebral metastases of gynecological origin. Neurochirurgie 1996, 42, 216–220. [Google Scholar]
- Salvati, M.; Cervoni, L.; Raguso, M. Therapeutic considerations in solitary cerebral metastases from uterine carcinoma. Minerva Ginecol. 1998, 50, 445–447. [Google Scholar]
- Martínez-Mañas, R.M.; Brell, M.; Rumià, J.; Ferrer, E. Brain Metastases in Endometrial Carcinoma. Gynecol. Oncol. 1998, 70, 282–284. [Google Scholar] [CrossRef]
- Ogawa, K.; Toita, T.; Kakinohana, Y.; Kamata, M.; Moromizato, H.; Nagai, Y.; Higashi, M.; Kanazawa, K.; Yoshii, Y. Palliative radiation therapy for brain metastases from endometrial carcinoma: Report of two cases. Jpn. J. Clin. Oncol. 1999, 29, 498–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crispino, M.; Tira, A.; Volpi, D.; Olivetti, L. Solitary cerebral metastasis of endometrial carcinoma. A case report. La Radiol. Medica 2000, 100, 515–517. [Google Scholar]
- Petru, E.; Lax, S.; Kurschel, S.; Gücer, F.; Sutter, B. Long-term survival in a patient with brain metastases preceding the diagnosis of endometrial cancer. J. Neurosurg. 2001, 94, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Sewak, S.; Muggia, F.; Zagzag, D. Endometrial carcinoma with cerebellar metastasis: A case report and review of the literature. J. Neuro-Oncol. 2002, 58, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, S.; Ohara, M.; Itoh, K.; Shiozawa, T.; Konishi, I. Successful treatment with stereotactic radiosurgery for brain metastases of endometrial carcinoma: A case report and review of the literature. Int. J. Gynecol. Cancer 2003, 13, 71–76. [Google Scholar] [CrossRef]
- Elliott, K.S.; Borowsky, M.E.; Lee, Y.-C.; Rao, C.; Abulafia, O. Prolonged survival in recurrent endometrial carcinoma to the brain. Gynecol. Oncol. 2004, 95, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Gien, L.T.; Kwon, J.S.; D’Souza, D.P.; Radwan, J.S.; Hammond, J.; Sugimoto, A.K.; Carey, M.S. Brain metastases from endometrial carcinoma: A retrospective study. Gynecol. Oncol. 2004, 93, 524–528. [Google Scholar] [CrossRef]
- N’Kanza, A.L.; Jobanputra, S.; Farmer, P.; Lovecchio, J.; Yelon, J.A.; Rudloff, U. Central nervous system involvement from malignant mixed Müllerian tumor (MMMT) of the uterus. Arch. Gynecol. Obstet. 2005, 273, 63–68. [Google Scholar] [CrossRef]
- Llaneza-Coto, A.P.; Seco-Navedo, M.; Fernandez-Garcia, T.; Redondo-Onia, M. Brain metastases of endometrial carcinoma in young woman. Prog. Obstet. Ginecol. 2006, 49, 82–84. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chen, C.H.; Chow, S.-N. Brain metastases from early stage endometrial carcinoma 8 years after primary treatment: Case report and review of the literature. Acta Obstet. Gynecol. Scand. 2006, 85, 890–891. [Google Scholar] [CrossRef]
- Orru, S.; Lay, G.; Dessi, M.; Murtas, R.; Deidda, M.A.; Amichetti, M. Brain Metastases from Endometrial Carcinoma: Report of Three Cases and Review of the Literature. Tumori J. 2007, 93, 112–117. [Google Scholar] [CrossRef]
- Sohaib, S.; Houghton, S.; Meroni, R.; Rockall, A.G.; Blake, P.; Reznek, R. Recurrent endometrial cancer: Patterns of recurrent disease and assessment of prognosis. Clin. Radiol. 2007, 62, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.; Kondziolka, D.; Mongia, S.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Management of brain metastases from ovarian and endometrial carcinoma with stereotactic radiosurgery. Cancer 2008, 113, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Reyns, N.; Pasquier, D.; Blond, S. Bilateral Thalamic Metastases in Endometrial Adenocarcinoma. Eur. Neurol. 2008, 59, 330. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Chang, H.T. Intracranial and scalp metastasis of endometrial carcinoma. Med. Sci. Monit. 2008, 14, 87–88. [Google Scholar]
- Al-Mujaini, A.; Gans, M.; Deschênes, J. Cortical visual loss consequent to brain metastases from an endometrial carcinoma. Can. J. Ophthalmol. 2008, 43, 486. [Google Scholar] [CrossRef]
- Forster, M.D.; Dedes, K.J.; Sandhu, S.; Frentzas, S.; Kristeleit, R.; Ashworth, A.; Poole, C.J.; Weigelt, B.; Kaye, S.B.; Molife, L.R. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat. Rev. Clin. Oncol. 2011, 8, 302–306. [Google Scholar] [CrossRef]
- Gulsen, S.; Terzi, A. Multiple brain metastases in a patient with uterine papillary serous adenocarcinoma: Treatment options for this rarely seen metastatic brain tumor. Surg. Neurol. Int. 2013, 4, 111. [Google Scholar] [CrossRef]
- Romero, A.C.; Cantos, A.C.; Rodriguez, I.R.; Rueda, A.M. Brain metastases from endometrial carcinoma: Case report with literature review. Rep. Pract. Oncol. Radiother. 2013, 18, S228. [Google Scholar] [CrossRef]
- Yoshida, A.; Okamoto, N.; Tozawa-Ono, A.; Koizumi, H.; Kiguchi, K.; Ishizuka, B.; Kumai, T.; Suzuki, N. Proteomic analysis of differential protein expression by brain metastases of gynecological malignancies. Hum. Cell 2013, 26, 56–66. [Google Scholar] [CrossRef]
- Nassir, M.; Roth, A.; Gasimli, K.; Braicu, E.I.; Fotopoulou, C.; Mawrin, C.; Badakhshi, H.; Warnke, J.-P.; Sehouli, J. Is endometrial cancer really a neurophobic tumor? A case report and review of the literature. Anticancer Res. 2014, 34, 249–257. [Google Scholar] [PubMed]
- Sierra, T.; Nguyen, L.; Mascitelli, J.; Kalir, T.; Fishman, D.A. Brain metastasis from uterine serous carcinoma: A case report and review of literature. Gynecol. Oncol. Rep. 2015, 11, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Kouhen, F.; Afif, M.; El Kabous, M.; Raiss, F.; Benhmidou, N.; Majjaoui, S.; Elkacemi, H.; Kebdani, T.; Benjaafar, N. Métastase cérébrale d’un cancer de l’endomètre: À propos d’un cas et une revue de la literature. Pan Afr. Med. J. 2015, 20, 68. [Google Scholar] [CrossRef]
- Uccella, S.; Morris, J.M.; Multinu, F.; Cliby, W.A.; Podratz, K.C.; Gostout, B.S.; Dowdy, S.C.; Ghezzi, F.; Makdisi, P.B.; Keeney, G.L.; et al. Primary brain metastases of endometrial cancer: A report of 18 cases and review of the literature. Gynecol. Oncol. 2016, 142, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kimyon, G.; Turan, T.; Basaran, D.; Turkmen, O.; Karalok, A.; Tasci, T.; Tulunay, G.; Kose, M.F. Is Neurosurgery with Adjuvant Radiotherapy an Effective Treatment Modality in Isolated Brain Involvement from Endometrial Cancer: From Case Report to Analysis. Int. J. Gynecol. Cancer 2017, 27, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kasper, E.; Ippen, F.; Wong, E.; Uhlmann, E.; Floyd, S.; Mahadevan, A. Stereotactic radiosurgery for brain metastasis from gynecological malignancies. Oncol. Lett. 2017, 13, 1525–1528. [Google Scholar] [CrossRef]
- Rades, D.; Dziggel, L.; Schild, S.E. A Specific Survival Score for Patients Receiving Local Therapy for Single Brain Metastasis from a Gynecological Malignancy. In Vivo 2018, 32, 825–828. [Google Scholar] [CrossRef]
- Moroney, M.; Wheeler, L.J.; Corr, B.R. Clinical presentation of brain metastases from endometrial carcinoma: A case series. Gynecol. Oncol. Rep. 2019, 28, 79–83. [Google Scholar] [CrossRef]
- Bodurka-Bevers, D.; Morris, M.; Eifel, P.J.; Levenback, C.; Bevers, M.W.; Lucas, K.R.; Wharton, J. Posttherapy Surveillance of Women with Cervical Cancer: An Outcomes Analysis. Gynecol. Oncol. 2000, 78, 187–193. [Google Scholar] [CrossRef]
- Elit, L.; Fyles, A.W.; Devries, M.C.; Oliver, T.K.; Fung-Kee-Fung, M. Follow-up for women after treatment for cervical cancer: A systematic review. Gynecol. Oncol. 2009, 114, 528–535. [Google Scholar] [CrossRef]
- Barajas, R.F.; Cha, S. Metastasis in Adult Brain Tumors. Neuroimaging Clin. N. Am. 2016, 26, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Nagao, S.; Yamaguchi, S. Leptomeningeal metastases arising from gynecological cancers. Int. J. Clin. Oncol. 2019, 25, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Weithman, A.M.; Morrison, G.; Ingram, E.A. Meningeal metastasis of squamous-cell carcinoma of the uterine cervix: Case report and review of the literature. Diagn. Cytopathol. 1987, 3, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Aboulafia, D.M.; Taylor, L.P.; Crane, R.D.; Yon, J.L.; Rudolph, R.H. Carcinomatous Meningitis Complicating Cervical Cancer: A Clinicopathologic Study and Literature Review. Gynecol. Oncol. 1996, 60, 313–318. [Google Scholar] [CrossRef]
- Rentinck, M.; Schrijver, H.M.; Kneppers, E.; Zijlmans, J.; Van Groeningen, C. Carcinomatous meningitis in cancer of the uterine cervix. J. Neuro-Oncol. 2004, 70, 87–90. [Google Scholar] [CrossRef]
- Wuntakal, R.; Maheshwari, A.; Kerkar, R.A.; Kane, S.V.; Tongaonkar, H.B. Carcinoma of uterine cervix primarily presenting as carcinomatous meningitis: A case report. Aust. N. Z. J. Obstet. Gynaecol. 2004, 44, 268–269. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, S.; Alexander, M. Carcinomatous meningitis occurring prior to a diagnosis of large cell neuroendocrine carcinoma of the uterine cervix. J. Postgrad. Med. 2004, 50, 311–312. [Google Scholar]
- Kastritis, E.; Moulopoulos, L.A.; Politi, E.; Kostis, E.; Pissakas, G.; Dimopoulos, M.; Bamias, A. Intramedullary spinal cord and leptomeningeal metastases in a patient with carcinoma of the uterine cervix. Gynecol. Oncol. 2006, 102, 124–127. [Google Scholar] [CrossRef]
- Han, L.; Bhan, R.; Johnson, S.; Zak, I.; Husain, M.; Al-Abbadi, M.A. Leptomeningeal metastasis in a patient with squamous cell carcinoma of the uterine cervix: Report of a case and review of the literature. Diagn. Cytopathol. 2007, 35, 660–662. [Google Scholar] [CrossRef]
- Ignatius, R.T.; Wills, S.M.; Nadeau, L.; DePeralta-Venturina, M.; Weiner, S. Leptomeningeal Carcinomatosis Due to Squamous Cell Carcinoma of the Uterine Cervix Associated With HPV-45. J. Clin. Oncol. 2008, 26, 154–156. [Google Scholar] [CrossRef]
- Asensio, N.; Luis, A.; Costa, I.; Oliveira, J.; Vaz, F. Meningeal Carcinomatosis and Uterine Carcinoma: Three Different Clinical Settings and Review of the Literature. Int. J. Gynecol. Cancer 2009, 19, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, N.; Sameshima, H.; Osato, K.; Fukushima, K.; Sato, Y.; Ikenoue, T. Carcinomatous meningitis from adenocarcinoma of the uterine cervix: A case report and literature review. J. Obstet. Gynaecol. Res. 2010, 36, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, S.; Nishio, E.; Torii, Y.; Kawamura, K.; Oe, S.; Kato, R.; Hasagawa, K.; Abe, M.; Kuroda, M.; Udagawa, Y. A case of primary uterine cervical neuroendocrine tumor with meningeal carcinomatosis confirmed by diagnostic imaging and autopsy. Int. J. Clin. Oncol. 2010, 16, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Teke, F.; Senem, Y.T.; Memik, T.; Yahya, T.; Zuhat, U.; Bekir, E.; Suleyman, A.; Mustafa, U. The impact of the stage and tumor size on rare brain metastasis of cervical cancer. Turk. Neurosurg. 2015, 26, 818–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piura, E.; Piura, B. Brain metastases from cervical carcinoma: Overview of pertinent literature. Eur. J. Gynaecol. Oncol. 2012, 33, 567–573. [Google Scholar] [PubMed]
- Nieder, C.; Spanne, O.; Mehta, M.P.; Grosu, A.-L.; Geinitz, H. Presentation, patterns of care, and survival in patients with brain metastases. Cancer 2010, 117, 2505–2512. [Google Scholar] [CrossRef]
- Au, Y.M.K.; Suppiah, S.; Nater, A.; Jalali, R.; Zadeh, G. New Approches to the Management of Primary and Secondary CNS Tumors; Morgan, L.R., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Schroeder, T.; Bittrich, P.; Kuhne, J.F.; Noebel, C.; Leischner, H.; Fiehler, J.; Schroeder, J.; Schoen, G.; Gellißen, S. Mapping distribution of brain metastases: Does the primary tumor matter? J. Neuro-Oncol. 2020, 147, 229–235. [Google Scholar] [CrossRef]
- Elit, L.; Fyles, A.W.; Oliver, T.K.; Devries-Aboud, M.C.; Fung-Kee-Fung, M. Follow-up for women after treatment for cervical cancer. Curr. Oncol. 2010, 17, 65–69. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Posner, J.B. Headaches in patients with brain tumors: A study of 111 patients. Neurology 1993, 43, 1678. [Google Scholar] [CrossRef]
- Schultheiss, T.; Kun, L.; Ang, K.; Stephens, L. Radiation response of the central nervous system. Int. J. Radiat. Oncol. 1995, 31, 1093–1112. [Google Scholar] [CrossRef]
- Brown, P.D.; Ahluwalia, M.S.; Khan, O.H.; Asher, A.L.; Wefel, J.S.; Gondi, V. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J. Clin. Oncol. 2018, 36, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.; Wong, F.; Swenerton, K.; Pike, J.; Manji, M.; McMurtrie, E.; Acker, B.; Le Riche, J. Small Cell Carcinoma of the Cervix Treated with Concurrent Radiotherapy, Cisplatin, and Etoposide. Gynecol. Oncol. 1995, 56, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Deavers, M.T.; Jhingran, A.; Ramirez, P.T.; Levenback, C.; Eifel, P.J. Small cell neuroendocrine carcinoma of the cervix: Outcome and patterns of recurrence. Gynecol. Oncol. 2004, 93, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Bae, K.; Gore, E.M.; Movsas, B.; Wong, S.J.; Meyers, C.A.; Bonner, J.A.; Schild, S.E.; Gaspar, L.E.; Bogart, J.A.; et al. Phase III Trial of Prophylactic Cranial Irradiation Compared with Observation in Patients with Locally Advanced Non–Small-Cell Lung Cancer: Neurocognitive and Quality-of-Life Analysis. J. Clin. Oncol. 2011, 29, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.H.; Bae, K.; Komaki, R.; Meyers, C.; Movsas, B.; Le Pechoux, C.; Werner-Wasik, M.; Videtic, G.M.; Garces, Y.I.; Choy, H. Primary Analysis of a Phase II Randomized Trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of Different Total Doses and Schedules of Prophylactic Cranial Irradiation on Chronic Neurotoxicity and Quality of Life for Patients with Limited-Disease Small-Cell Lung Cancer. Int. J. Radiat. Oncol. 2011, 81, 77–84. [Google Scholar] [CrossRef]
- Roy, S.; Ko, J.J.; Bahl, G. Small cell carcinoma of cervix: A population-based study evaluating standardized provincial treatment protocols. Gynecol. Oncol. Rep. 2019, 27, 54–59. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Sneed, P.K.; Lamborn, K.R.; Forstner, J.M.; McDermott, M.W.; Chang, S.; Park, E.; Gutin, P.H.; Phillips, T.L.; Wara, W.M.; Larson, D.A. Radiosurgery for brain metastases: Is whole brain radiotherapy necessary? Int. J. Radiat. Oncol. 1999, 43, 549–558. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Vermorken, J.B. The role of chemotherapy in squamous cell carcinoma of the uterine cervix: A review. Int. J. Gynecol. Cancer 1993, 3, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Oberhoff, C.; Kieback, D.; Würstlein, R.; Deertz, H.; Sehouli, J.; Van Soest, C.; Hilfrich, J.; Mesrogli, M.; Von Minckwitz, G.; Staab, H.; et al. Topotecan chemotherapy in patients with breast cancer and brain metastases: Results of a pilot study. Onkologie 2001, 24, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Zighelboim, I.; Taylor, N.P.; Powell, M.A.; Gibb, R.K.; Rader, J.S.; Mutch, D.G.; Grigsby, P.W. Outcomes in 24 selected patients with stage IVB cervical cancer and excellent performance status treated with radiotherapy and chemotherapy. Radiat. Med. 2006, 24, 625–630. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative Radiotherapy in the Treatment of Single Metastases to the Brain. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Vecht, C.J.; Haaxma-Reiche, H.; Noordijk, E.M.; Padberg, G.W.; Voormolen, J.H.C.; Hoekstra, F.H.; Tans, J.T.J.; Lambooij, N.; Metsaars, J.A.L.; Wattendorff, A.R.; et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery. Ann. Neurol. 1993, 33, 583–590. [Google Scholar] [CrossRef]
- Kayama, T.; Sato, S.; Sakurada, K.; Mizusawa, J.; Nishikawa, R.; Narita, Y.; Sumi, M.; Miyakita, Y.; Kumabe, T.; Sonoda, Y.; et al. Effects of Surgery with Salvage Stereotactic Radiosurgery Versus Surgery With Whole-Brain Radiation Therapy in Patients With One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 3282–3289. [Google Scholar] [CrossRef]
- Zhang, T.W.; Palma, D.; D’Souza, D.; Velker, V.; Mendez, L.C. Stereotactic Ablative Radiotherapy for Recurrent or Metastatic Gynecological Cancer: Extending Lives? Curr. Treat. Options Oncol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Zindler, J.D.; Rodrigues, G.; Haasbeek, C.J.; De Haan, P.F.; Meijer, O.W.; Slotman, B.J.; Lagerwaard, F.J. The clinical utility of prognostic scoring systems in patients with brain metastases treated with radiosurgery. Radiother. Oncol. 2013, 106, 370–374. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A New Prognostic Index and Comparison to Three Other Indices for Patients with Brain Metastases: An Analysis of 1,960 Patients in the RTOG Database. Int. J. Radiat. Oncol. 2008, 70, 510–514. [Google Scholar] [CrossRef]
- Takayanagi, A.; Florence, T.J.; Hariri, O.R.; Armstrong, A.; Yazdian-Anari, P.; Sumida, A.; Quadri, S.A.; Cohen, J.G.; Tehrani, O.S. Brain metastases from cervical cancer reduce longevity independent of overall tumor burden. Surg. Neurol. Int. 2019, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Dincoglan, F.; Beyzadeoglu, M.; Sager, O.; Oysul, K.; Sirin, S.; Surenkok, S.; Gamsiz, H.; Uysal, B.; Demiral, S.; Dirican, B. Image-guided positioning in intracranial non-invasive stereotactic radiosurgery for the treatment of brain metastasis. Tumori J. 2012, 98, 630–635. [Google Scholar] [CrossRef]
- Niibe, Y.; Karasawa, K.; Nakamura, O.; Shinoura, N.; Okamoto, K.; Yamada, R.; Fukino, K.; Tanaka, Y. Survival benefit of stereotactic radiosurgery for metastatic brain tumors in patients with controlled primary lesions and no other distant metastases. Anticancer. Res. 2003, 23, 4157–4159. [Google Scholar] [PubMed]
- Piura, E.; Piura, B. Brain Metastases from Endometrial Carcinoma. ISRN Oncol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henriksen, E. The lymphatic dissemination in endometrial carcinoma. Am. J. Obstet. Gynecol. 1975, 123, 570–576. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Levenback, C.F.; Beller, U.; Kavanagh, J.J. Role of surgical resection for lung, liver, and central nervous system metastases in patients with gynecological cancer: A literature review. Int. J. Gynecol. Cancer 2004, 14, 399–422. [Google Scholar] [CrossRef]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 Trials of Stereotactic Radiosurgery with or without Whole-Brain Radiation Therapy for 1 to 4 Brain Metastases: Individual Patient Data Meta-Analysis. Int. J. Radiat. Oncol. 2015, 91, 710–717. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Boucher, Y.; Duda, D.G.; Martin, J.D.; Seano, G.; Ancukiewicz, M.; Barry, W.T.; Goel, S.; Lahdenrata, J.; Isakoff, S.J.; et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. USA 2015, 112, 14325–14330. [Google Scholar] [CrossRef]
- Lim, Z.L.; Puttick, S.; Houston, Z.H.; Thurecht, K.J.; Croft, P.K.; Mahler, S.M.; Rose, S.; Jeffree, R.; Mazzieri, R.; Dolcetti, R.; et al. Innovative Therapeutic Strategies for Effective Treatment of Brain Metastases. Int. J. Mol. Sci. 2019, 20, 1280. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
| N | N | ||
|---|---|---|---|
| Median age at the initial Cx diagnosis (y) | 48 (29–87) | Symptoms (%) (n = 136) | |
| Headaches | 62(45.6) | ||
| Incidence (%) | 0.63 (0.1–2.2) | Syncope/seizures | 19 (14.0) |
| Ataxia | 16 (11.8) | ||
| Histology (%) (n = 224) | Nausea/vomiting | 15 (10.3) | |
| SCC | 141 (62.9) | Visual disturbance | 12 (8.8) |
| AC | 44 (19.6) | Weakness | 12 (8.8) |
| ASC | 11 (4.9) | Altered mental status | 9 (6.6) |
| SCNEC | 28 (12.5) | Dizziness | 7 (5.1) |
| Confusion | 7 (5.1) | ||
| FIGO stage at diagnosis of BMs (%) (n = 208) | Speech impairment | 7 (5.1) | |
| I | 53 (25.5) | Paresthesia | 2 |
| II | 69 (33.0) | Facial twitching | 2 |
| III | 41 (19.7) | Tinnitus | 1 |
| IV | 45 (21.6) | Tremor | 1 |
| Hemiballismus | 1 | ||
| Interval Cx to BM (m) | 24 (−0.25–386) | ||
| Treatments | |||
| Other metastases (%) (n = 528) | WBRT alone | 83 | |
| No | 188 (35.6) | SRS alone | 49 |
| Yes | 340 (64.3) | Surgery alone | 17 |
| Surgery plus WBRT | 28 | ||
| Single BM or Multiple BMs (%) (n = 246) | Surgery plus SRS | 2 | |
| Single | 103 (41.8) | SRS plus WBRT | 9 |
| Multiple | 143 (58.1) | BSC | 13 |
| Site of BM (%) (n = 165) | Median survival (mo) | 5 (0.5–120) | |
| cerebrum | 121(73.3) | ||
| parietal lobe | 27 (16.4) | ||
| frontal lobe | 21 (12.7) | ||
| occipital lobe | 16 (9.7) | ||
| temporal lobe | 15 (9.1) | ||
| unknown | 42 | ||
| cerebellum | 44(26.7) |
| N | N | ||
|---|---|---|---|
| Median age at the initial EC diagnosis (y) | 61 (32–84) | Site of BM (%) (n = 195) | |
| cerebrum | 143 (68.2) | ||
| Incidence (%) | 0.7 (0.2–1.2) | frontal lobe | 47 (24.1) |
| parietal lobe | 46 (23.6) | ||
| Histology (%) (n = 391) | occipital lobe | 30 (15.4) | |
| AC | 319 (81.6) | temporal lobe | 15 (7.7) |
| ASC | 15 (3.8) | unknown | 22 |
| SCC | 2 (0.6) | cerebellum | 50 (25.6) |
| UD | 7 (1.8) | ||
| SCNEC | 3 (0.8) | Symptoms (%) (n = 113) | |
| CS | 31 (8.0) | Headaches | 31 (27.4) |
| LS | 8 (2.0) | Weakness | 25 (22.1) |
| AS | 2 (0.5) | Syncope/seizures | 14 (12.4) |
| Sarcoma | 4 (1.0) | Visual disturbance | 11 (9.7) |
| Ataxia | 10 (8.8) | ||
| Histological grade (%) (n = 192) | Altered mental status | 8 (7.1) | |
| Grade1 | 17 (8.9) | Dizziness | 8 (7.1) |
| Grade2 | 32 (16.7) | Speech impairment | 8 (7.1) |
| Grade3 | 143 (74.5) | Confusion | 7 (6.2) |
| Hemiparesis | 7 (6.2) | ||
| FIGO stage at diagnosis of BMs (%) (n = 253) | Nausea/vomiting | 3 | |
| I | 57 (22.5) | Numbness | 3 |
| II | 23 (9.1) | Dysarthria | 2 |
| III | 94 (37.2) | Hyponatremia | 2 |
| IV | 79 (31.2) | Strokes | 2 |
| Memory loss | 1 | ||
| Interval EC to BM (m) | 18 (−3–216) | ||
| Treatments | |||
| Other metastases (%) (n = 770) | WBRT alone | 89 | |
| No | 424 (55.0) | SRS alone | 66 |
| Yes | 346 (45.0) | Surgery alone | 25 |
| Surgery plus WBRT | 56 | ||
| Single BM or Multiple BMs (%) (n = 411) | Surgery plus SRS | 2 | |
| Single | 187 (45.5) | SRS plus WBRT | 5 |
| Multiple | 224 (54.5) | BSC | 35 |
| Median survival (mo) | 7.5 (0.1–171) |
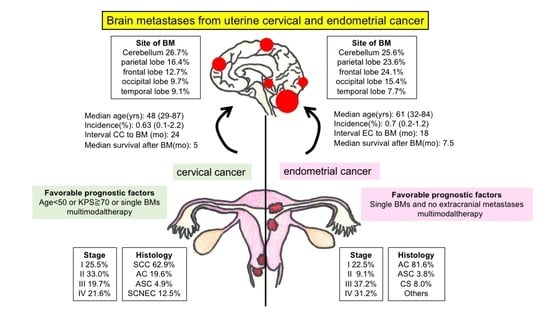
| Favorable/Poor/Unknown | Prognostic Factors |
|---|---|
| Favorable | Age < 50 or Karnofsky performance status ≧ 70 or single BMs multimodal therapy |
| Poor | Multiple BMs and extracranial metastases |
| Unknown | The presence of extracranial metastases |
| Favorable/Poor/ Unknown | Prognostic Factors |
|---|---|
| Favorable | Single BMs and no extracranial metastases, multimodal therapy |
| Poor | Multiple BMs and extracranial metastases |
| Unknown | Stage, the number of metastases, the presence of extracranial metastases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, M.K.; Tanase, Y.; Uno, M.; Ishikawa, M.; Kato, T. Brain Metastases from Uterine Cervical and Endometrial Cancer. Cancers 2021, 13, 519. https://doi.org/10.3390/cancers13030519
Kato MK, Tanase Y, Uno M, Ishikawa M, Kato T. Brain Metastases from Uterine Cervical and Endometrial Cancer. Cancers. 2021; 13(3):519. https://doi.org/10.3390/cancers13030519
Chicago/Turabian StyleKato, Mayumi Kobayashi, Yasuhito Tanase, Masaya Uno, Mitsuya Ishikawa, and Tomoyasu Kato. 2021. "Brain Metastases from Uterine Cervical and Endometrial Cancer" Cancers 13, no. 3: 519. https://doi.org/10.3390/cancers13030519
APA StyleKato, M. K., Tanase, Y., Uno, M., Ishikawa, M., & Kato, T. (2021). Brain Metastases from Uterine Cervical and Endometrial Cancer. Cancers, 13(3), 519. https://doi.org/10.3390/cancers13030519