The Cardiometabolic Health Benefits of Sauna Exposure in Individuals with High-Stress Occupations. A Mechanistic Review
Abstract
1. Introduction
2. An Overview of Stressors and High-Stress Occupations
3. Physiological Responses to Acute Heat Exposure
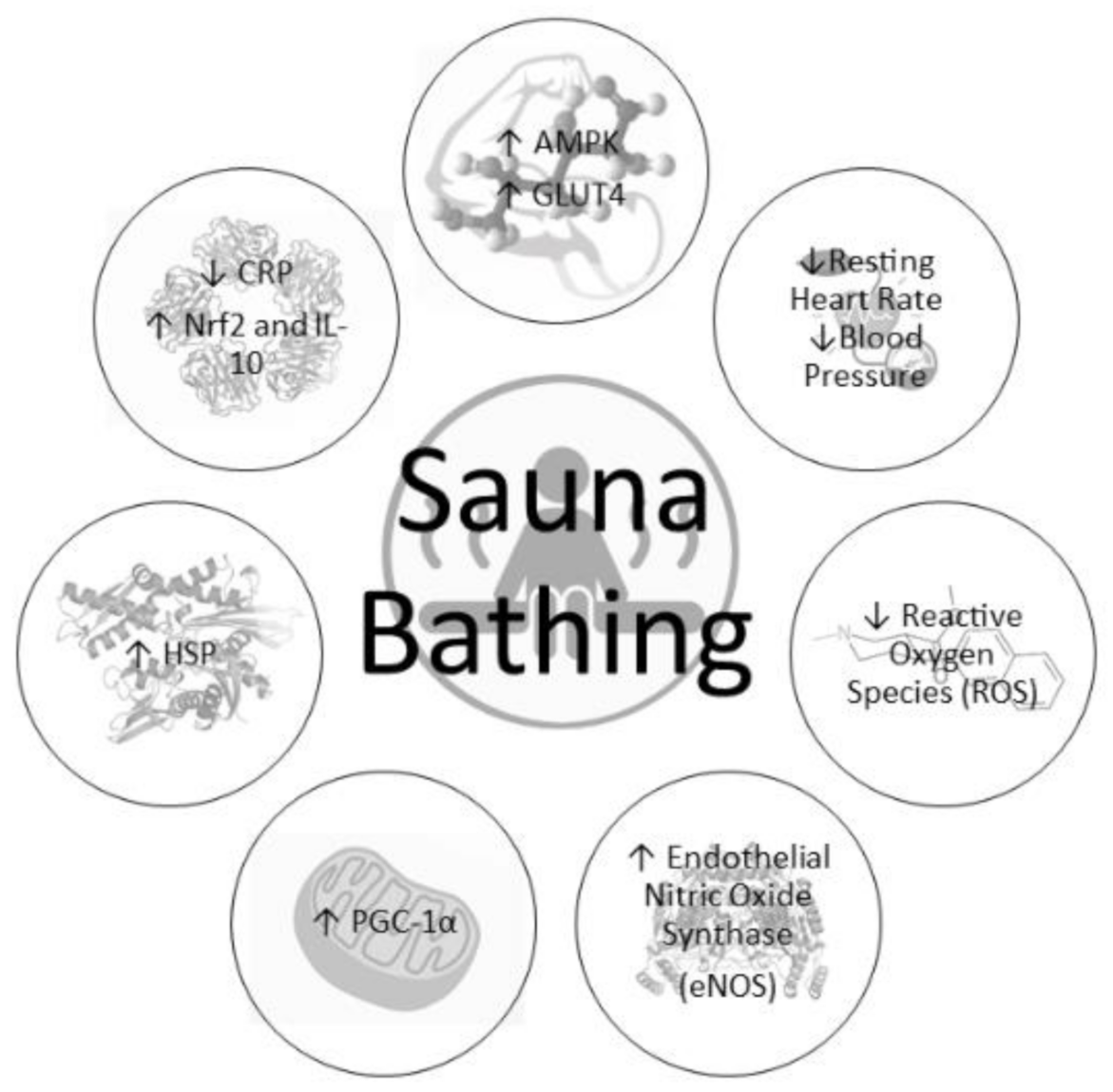
4. Cardiometabolic Benefits of Chronic Heat Exposure
4.1. Cardiovascular Adaptations
4.2. Metabolic Adaptations
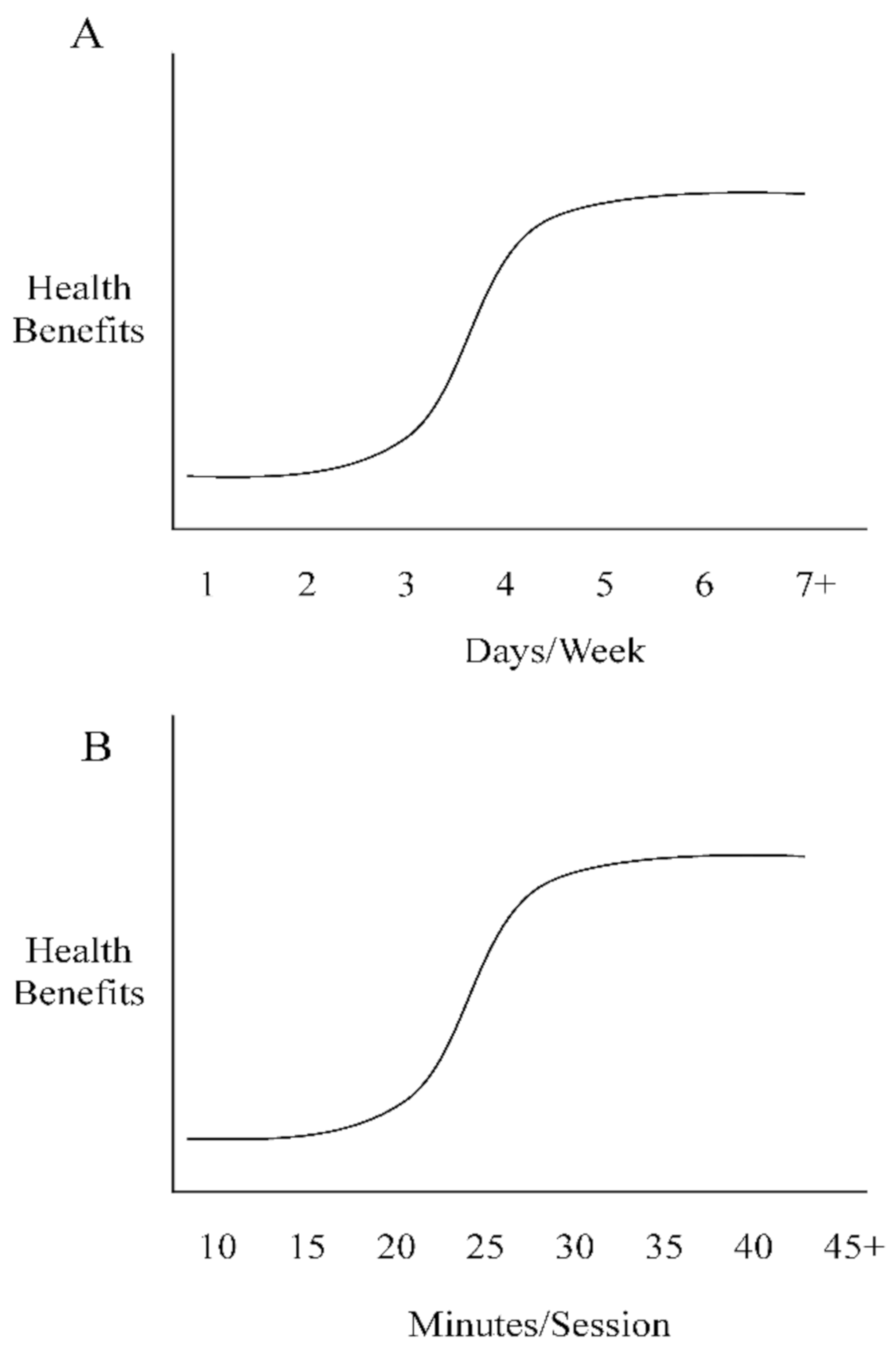
5. Practical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Heart Disease Facts. Available online: http://www.cdc.gov/heartdisease/facts.htm (accessed on 14 October 2020).
- Joseph, P.N.; Violanti, J.M.; Donahue, R.; Andrew, M.E.; Trevisan, M.; Burchfiel, C.M.; Dorn, J. Police Work and Subclinical Atherosclerosis. J. Occup. Environ. Med. 2009, 51, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Teehan, D.; Farioli, A.; Baur, D.M.; Smith, D.; Kales, S.N. Sudden Cardiac Death Among Firefighters ≤45 Years of Age in the United States. Am. J. Cardiol. 2013, 112, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Kales, S.N.; Soteriades, E.S.; Christoudias, S.G.; Christiani, D.C. Firefighters and on-duty deaths from coronary heart disease: A case control study. Environ. Health 2003, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, T.L.; Clough, V.M. Occupational Health Concerns of Firefighting. Annu. Rev. Public Health 1992, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, Y.-J.; Lee, S.-W.; Bang, C.-H.; Lee, G.; Lee, J.-K.; Kwan, J.-S.; Huh, Y.-S. Changes of oxidative/antioxidative parameters and DNA damage in firefighters wearing personal protective equipment during treadmill walking training. J. Phys. Ther. Sci. 2016, 28, 3173–3177. [Google Scholar] [CrossRef]
- Walker, A.J.; Keene, T.; Argus, C.K.; Driller, M.; Guy, J.H.; Rattray, B. Immune and inflammatory responses of Australian firefighters after repeated exposures to the heat. Ergonomics 2015, 58, 2032–2039. [Google Scholar] [CrossRef]
- Irving, S.; Orr, R.; Pope, R. Profiling the occupational tasks and physical conditioning of specialist police. Int. J. Ex. Sci. 2019, 12, 173–186. [Google Scholar] [CrossRef]
- Marzabadi, E.A.; Fesharaki, M.G. Effective factors on occupational stress in military personnel. J. Mil. Med. 2011, 13, 1–6. [Google Scholar]
- Poston, W.S.C.; Haddock, C.K.; Jahnke, S.; Jitnarin, N.; Day, R.S. An examination of the benefits of health promotion programs for the national fire service. BMC Public Health 2013, 13, 805. [Google Scholar] [CrossRef]
- Hartley, T.A.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Violanti, J.M. Health disparities in police officers: Comparisons to the U.S. general population. Int. J. Emerg. Ment. Health Hum. Resil. 2011, 13, 211–220. [Google Scholar]
- Hunter, A.L.; Shah, A.S.; Langrish, J.P.; Raftis, J.B.; Lucking, A.J.; Brittan, M.; Venkatasubramanian, S.; Stables, C.L.; Stelzle, D.; Marshall, J.; et al. Fire Simulation and Cardiovascular Health in Firefighters. Circulation 2017, 135, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Farioli, A.; Christophi, C.A.; Quarta, C.; Kales, S.N. Incidence of Sudden Cardiac Death in a Young Active Population. J. Am. Hear. Assoc. 2015, 4, e001818. [Google Scholar] [CrossRef] [PubMed]
- National Law Enforcement Officers Memorial Fund. April 2011. Available online: http://www.nleomf.org (accessed on 1 November 2020).
- Calvert, G.M.; Merling, J.W.; Burnett, C.A. Ischemic Heart Disease Mortality and Occupation among 16- to 60-Year-Old Males. J. Occup. Environ. Med. 1999, 41, 960–966. [Google Scholar] [CrossRef]
- Dubrow, R.; Burnett, C.A.; Gute, D.M.; Brockert, J.E. Ischemic Heart Disease and Acute Myocardial Infarction Mortality Among Police Officers. J. Occup. Environ. Med. 1988, 30, 650–654. [Google Scholar] [CrossRef]
- McAllister, M.J.; Martaindale, M.H.; Rentería, L.I. Active Shooter Training Drill Increases Blood and Salivary Markers of Stress. Int. J. Environ. Res. Public Health 2020, 17, 5042. [Google Scholar] [CrossRef]
- United States Army. Health of the Force: Create a Healthier Force for Tomorrow. 2015. Available online: https://www.army.mil/e2/c/downloads/419337.pdf (accessed on 1 November 2020).
- Barlas, F.H.W.; Higgins, W.B.; Pflieger, J.C.; Diecker, K. Department of Defense Health Related Behaviors Survey of Active Duty Military Personnel: Military Health System; Department of Defense: Fairfax, VA, USA, 2013.
- McAllister, M.J.; Pigg, B.L.; Renteria, L.I.; Waldman, H.S. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: A 4-week randomized pre-post pilot study. Nutr. Res. 2020, 75, 32–43. [Google Scholar] [CrossRef]
- Waldman, H.S.; Smith, J.W.; Lamberth, J.; Fountain, B.J.; Bloomer, R.J.; Butawan, M.B.; McAllister, M.J. A 28-Day Carbohydrate-Restricted Diet Improves Markers of Cardiovascular Disease in Professional Firefighters. J. Strength Cond. Res. 2020, 34, 2785–2792. [Google Scholar] [CrossRef]
- Waldman, H.S.; Smith, J.W.; Lamberth, J.; Fountain, B.J.; McAllister, M.J. A 28-Day Carbohydrate-Restricted Diet Improves Markers of Cardiometabolic Health and Performance in Professional Firefighters. J. Strength Cond. Res. 2019, 33, 3284–3294. [Google Scholar] [CrossRef]
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and Real-World Settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef]
- Thomas, D.M.; Martin, C.K.; Redman, L.M.; Heymsfield, S.B.; Lettieri, S.; Levine, J.A.; Bouchard, C.; Schoeller, D.A. Effect of dietary adherence on the body weight plateau: A mathematical model incorporating intermittent compliance with energy intake prescription. Am. J. Clin. Nutr. 2014, 100, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.S.; Medd, E.R.; Adamo, K.B.; Goldfield, G.S.; Prud’Homme, D.; Kenny, G.P.; Sigal, R.J. Top 10 practical lessons learned from physical activity interventions in overweight and obese children and adolescents. Appl. Physiol. Nutr. Metab. 2013, 38, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Szygula, Z.; Pałka, T.; Pilch, P.; Cison, T.; Wiecha, S.; Tota, Ł. COMPARISON OF PHYSIOLOGICAL REACTIONS AND PHYSIOLOGICAL STRAIN IN HEALTHY MEN UNDER HEAT STRESS IN DRY AND STEAM HEAT SAUNAS. Biol. Sport 2014, 31, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Leppäluoto, J. Human thermoregulation in sauna. Ann. Clin. Res. 1988, 20, 240–243. [Google Scholar]
- Kunutsor, S.K.; Laukkanen, T.; Laukkanen, J.A. Sauna bathing reduces the risk of respiratory diseases: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 1107–1111. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Mäkikallio, T.H.; Khan, H.; Laukkanen, T.; Kauhanen, J.; Laukkanen, J.A. Sauna bathing reduces the risk of venous thromboembolism: A prospective cohort study. Eur. J. Epidemiol. 2019, 34, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.K.; Khan, H.; Willeit, P.; Zaccardi, F.; Laukkanen, J.A. Sauna bathing is associated with reduced cardiovascular mortality and improves risk prediction in men and women: A prospective cohort study. BMC Med. 2018, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Gryka, D.; Pilch, W.; Szarek, M.; Szygula, Z.; Tota, Ł. The effect of sauna bathing on lipid profile in young, physically active, male subjects. Int. J. Occup. Med. Environ. Health 2014, 27, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.; Renquist, B.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Selye, M.H. The general adaptation syndrome and the diseases of adaptation. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Webb, H.E.; Evans, R.K.; McCleod, K.A.; Tangsilsat, S.E.; Kamimori, G.H.; Acevedo, E.O. Psychological stress during exercise: Immunoendocrine and oxidative responses. Exp. Biol. Med. 2010, 235, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A.; Blumenthal, J.A.; Kaplan, J. Impact of Psychological Factors on the Pathogenesis of Cardiovascular Disease and Implications for Therapy. Circulation 1999, 99, 2192–2217. [Google Scholar] [CrossRef]
- Webb, H.E.; Weldy, M.L.; Fabianke-Kadue, E.C.; Orndorff, G.R.; Kamimori, G.H.; Acevedo, E.O. Psychological stress during exercise: Cardiorespiratory and hormonal responses. Eur. J. Appl. Physiol. 2008, 104, 973–981. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Degan, D.; Ornello, R.; Tiseo, C.; Carolei, A.; Sacco, S.; Pistoia, F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018, 24, 1485–1501. [Google Scholar] [CrossRef]
- Biro, S.; Masuda, A.; Kihara, T.; Tei, C. Clinical Implications of Thermal Therapy in Lifestyle-Related Diseases. Exp. Biol. Med. 2003, 228, 1245–1249. [Google Scholar] [CrossRef]
- Hafen, P.S.; Preece, C.N.; Sorensen, J.R.; Hancock, C.R.; Hyldahl, R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J. Appl. Physiol. 2018, 125, 1447–1455. [Google Scholar] [CrossRef]
- Hannuksela, M.L.; Ellahham, S. Benefits and risks of sauna bathing. Am. J. Med. 2001, 110, 118–126. [Google Scholar] [CrossRef]
- Iguchi, M.; Littmann, A.E.; Chang, S.-H.; Wester, L.A.; Knipper, J.S.; Shields, R. Heat Stress and Cardiovascular, Hormonal, and Heat Shock Proteins in Humans. J. Athl. Train. 2012, 47, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I.T.; Laukkanen, J.A. Endocrine effects of sauna bath. Curr. Opin. Endocr. Metab. Res. 2020, 11, 15–20. [Google Scholar] [CrossRef]
- Ketelhut, S.; Ketelhut, R. The blood pressure and heart rate during sauna bath correspond to cardiac responses during submaximal dynamic exercise. Complement. Ther. Med. 2019, 44, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.K.; Zaccardi, F.; Lee, E.; Willeit, P.; Khan, H.; Laukkanen, J.A. Acute effects of sauna bathing on cardiovascular function. J. Hum. Hypertens. 2017, 32, 129–138. [Google Scholar] [CrossRef]
- Kukkonen-Harjula, K.; Kauppinen, K. Health effects and risks of sauna bathing. Int. J. Circumpolar Health 2006, 65, 195–205. [Google Scholar] [CrossRef]
- Kukkonen-Harjula, K.; Oja, P.; Laustiola, K.; Vuori, I.; Jolkkonen, J.; Siitonen, S.; Vapaatalo, H. Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur. J. Appl. Physiol. 1989, 58, 543–550. [Google Scholar] [CrossRef]
- Hofmann, C.; Katus, H.A.; Doroudgar, S. Protein Misfolding in Cardiac Disease. Circulation 2019, 139, 2085–2088. [Google Scholar] [CrossRef]
- Yamada, P.M.; Amorim, F.T.; Moseley, P.; Robergs, R.; Schneider, S.M. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J. Appl. Physiol. 2007, 103, 1196–1204. [Google Scholar] [CrossRef]
- Singh, R.; Kølvraa, S.; Bross, P.; Christensen, K.; Bathum, L.; Gregersen, N.; Tan, Q.; Rattan, S.I.S. Anti-inflammatory heat shock protein 70 genes are positively associated with human survival. Curr. Pharm. Des. 2010, 16, 796–801. [Google Scholar] [CrossRef]
- Costello, J.T.; Rendell, R.A.; Furber, M.; Massey, H.; Tipton, M.; Young, J.S.; Corbett, J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine 2018, 110, 277–283. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Wu, H.-P.; Chen, C.-K.; Chung, K.; Tseng, J.-C.; Hua, C.-C.; Liu, Y.-C.; Chuang, D.-Y.; Yang, C.-H. Serial cytokine levels in patients with severe sepsis. Inflamm. Res. 2009, 58, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Laukkanen, T. Sauna bathing and systemic inflammation. Eur. J. Epidemiol. 2017, 33, 351–353. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.; Rifai, N. C-reactive protein and cardiovascular disease: A review of risk prediction and interventions. Clin. Chim. Acta 2002, 317, 1–15. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Dasu, M.R.; Ciobanu, D.; Reusch, J.E.; Jialal, I. C-Reactive Protein Decreases Interleukin-10 Secretion in Activated Human Monocyte-Derived Macrophages via Inhibition of Cyclic AMP Production. Arter. Thromb. Vasc. Biol. 2006, 26, 2469–2475. [Google Scholar] [CrossRef]
- Garrett, A.T.; Creasy, R.; Rehrer, N.J.; Patterson, M.J.; Cotter, J.D. Effectiveness of short-term heat acclimation for highly trained athletes. Eur. J. Appl. Physiol. 2011, 112, 1827–1837. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Chemical hormesis: Its historical foundations as a biological hypothesis. Hum. Exp. Toxicol. 2000, 19, 2–31. [Google Scholar] [CrossRef]
- Laukkanen, T.; Khan, H.; Zaccardi, F.; Laukkanen, J.A. Association between Sauna Bathing and Fatal Cardiovascular and All-Cause Mortality Events. JAMA Intern. Med. 2015, 175, 542. [Google Scholar] [CrossRef]
- Zaccardi, F.; Laukkanen, T.; Willeit, P.; Kunutsor, S.K.; Kauhanen, J.; Laukkanen, J.A. Sauna Bathing and Incident Hypertension: A Prospective Cohort Study. Am. J. Hypertens. 2017, 30, 1120–1125. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, M.; Nozawa, T.; Ihori, H.; Shida, T.; Ohori, T.; Suzuki, T.; Matsuki, A.; Yasumura, S.; Inoue, H. Repeated sauna therapy improves myocardial perfusion in patients with chronically occluded coronary artery-related ischemia. Int. J. Cardiol. 2013, 167, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Khan, H.; Zaccardi, F.; Laukkanen, T.; Willeit, P.; Laukkanen, J.A. Sauna bathing reduces the risk of stroke in Finnish men and women: A prospective cohort study. Neurology 2018, 90, e1937–e1944. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Black, M.A.; Dawson, E.A.; Minson, C.T.; Newcomer, S.C.; Laughlin, M.H.; Cable, N.T.; Green, D.J. Impact of Shear Rate Modulation on Vascular Function in Humans. Hypertension 2009, 54, 278–285. [Google Scholar] [CrossRef]
- Tei, C.; Tanaka, N. Thermal vasodilation as a treatment of congestive heart failure: A novel approach. J. Cardiol. 1996, 27, 29–30. [Google Scholar]
- McMurray, J.J.; Ezekowitz, J.A.; Lewis, B.S.; Gersh, B.J.; Van Diepen, S.; Amerena, J.; Bartunek, J.; Commerford, P.; Oh, B.-H.; Harjola, V.-P.; et al. Left Ventricular Systolic Dysfunction, Heart Failure, and the Risk of Stroke and Systemic Embolism in Patients With Atrial Fibrillation: Insights from the ARISTOTLE trial. Circ. Hear. Fail. 2013, 6, 451–460. [Google Scholar] [CrossRef]
- Tei, C.; Tanaka, N. Comprehensive Therapy for Congestive Heart Failure: A Novel Approach Incorporating Thermal Vasodilation. Intern. Med. 1996, 35, 67–69. [Google Scholar] [CrossRef][Green Version]
- Kihara, T.; Biro, S.; Imamura, M.; Yoshifuku, S.; Takasaki, K.; Ikeda, Y.; Otuji, Y.; Minagoe, S.; Toyama, Y.; Tei, C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2002, 39, 754–759. [Google Scholar] [CrossRef]
- Cheng, J.L.; Macdonald, M.J. Effect of heat stress on vascular outcomes in humans. J. Appl. Physiol. 2019, 126, 771–781. [Google Scholar] [CrossRef]
- Hasenour, C.M.; Berglund, E.D.; Wasserman, D.H. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol. Cell. Endocrinol. 2013, 366, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK Enhances Insulin-Stimulated GLUT4 Regulation via Lowering Membrane Cholesterol. Endocrinol. 2012, 153, 2130–2141. [Google Scholar] [CrossRef]
- Liu, C.-T.; Brooks, G.A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 2012, 112, 354–361. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Pilch, W.; Szyguła, Z.; Klimek, A.T.; Pałka, T.; Cisoń, T.; Pilch, P.; Torii, M. Changes in the lipid profile of blood serumin women taking sauna baths of various duration. Int. J. Occup. Med. Environ. Health 2010, 23, 167–174. [Google Scholar] [CrossRef]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Banko, M.R.; Brunet, A. AMP-activated Protein Kinase and FoxO Transcription Factors in Dietary Restriction-induced Longevity. Ann. N. Y. Acad. Sci. 2009, 1170, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The Crosstalk Between Nrf2 and AMPK Signal Pathways Is Important for the Anti-Inflammatory Effect of Berberine in LPS-Stimulated Macrophages and Endotoxin-Shocked Mice. Antioxid. Redox Signal 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Steinbrenner, H. Cellular adaptation to xenobiotics: Interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 2017, 13, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, K.; Liu, L.; Liu, H.; Zhao, F.-Q.; Liu, J. Nuclear factor-like factor 2-antioxidant response element signaling activation by tert-butylhydroquinone attenuates acute heat stress in bovine mammary epithelial cells. J. Dairy Sci. 2016, 99, 9094–9103. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Tharrett, S.J.; Peterson, J.A. American College of Sports Medicine Health/Fitness Facility Standards and Guidelines, 4th ed.; Human Kinetics: Champaign, IL, USA, 2012; Chapter 5; p. 170. [Google Scholar]
- Milunsky, A.; Ulcickas, M.; Rothman, K.J.; Willett, W.; Jick, S.S.; Jick, H. Maternal Heat Exposure and Neural Tube Defects. JAMA 1992, 268, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.-H.; Messerlian, C.; Williams, P.L.; Ford, J.B.; Souter, I.; Hauser, R.; Chavarro, J.E. Type of underwear worn and markers of testicular function among men attending a fertility center. Hum. Reprod. 2018, 33, 1749–1756. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Sartini, B.; Cosci, I.; Patassini, C.; Carraro, U.; Foresta, C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum. Reprod. 2013, 28, 877–885. [Google Scholar] [CrossRef]
- Haddock, C.K.; Jahnke, S.A.; Poston, W.S.C.; Jitnarin, N.; Kaipust, C.M.; Tuley, B.; Hyder, M.L. Alcohol use among firefighters in the Central United States. Occup. Med. 2012, 62, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Obst, J.D.D.P.L. Does Joining the Police Service Drive You to Drink? A longitudinal study of the drinking habits of police recruits. Drugs Educ. Prev. Policy 2001, 8, 347–357. [Google Scholar] [CrossRef][Green Version]
- Green, K.T.; Beckham, J.C.; Youssef, N.; Elbogen, E.B. Alcohol misuse and psychological resilience among U.S. Iraq and Afghanistan era veterans. Addict. Behav. 2014, 39, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Nakazato, M.; Kihara, T.; Minagoe, S.; Tei, C. Repeated Thermal Therapy Diminishes Appetite Loss and Subjective Complaints in Mildly Depressed Patients. Psychosom. Med. 2005, 67, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.W.; Lowry, C.A.; Mehl, M.R.; Allen, J.J.B.; Kelly, K.L.; Gartner, D.E.; Medrano, A.; Begay, T.K.; Rentscher, K.; White, J.J.; et al. Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 789–795. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, K.N.; Killen, L.G.; O’Neal, E.K.; Waldman, H.S. The Cardiometabolic Health Benefits of Sauna Exposure in Individuals with High-Stress Occupations. A Mechanistic Review. Int. J. Environ. Res. Public Health 2021, 18, 1105. https://doi.org/10.3390/ijerph18031105
Henderson KN, Killen LG, O’Neal EK, Waldman HS. The Cardiometabolic Health Benefits of Sauna Exposure in Individuals with High-Stress Occupations. A Mechanistic Review. International Journal of Environmental Research and Public Health. 2021; 18(3):1105. https://doi.org/10.3390/ijerph18031105
Chicago/Turabian StyleHenderson, Kaemmer N., Lauren G. Killen, Eric K. O’Neal, and Hunter S. Waldman. 2021. "The Cardiometabolic Health Benefits of Sauna Exposure in Individuals with High-Stress Occupations. A Mechanistic Review" International Journal of Environmental Research and Public Health 18, no. 3: 1105. https://doi.org/10.3390/ijerph18031105
APA StyleHenderson, K. N., Killen, L. G., O’Neal, E. K., & Waldman, H. S. (2021). The Cardiometabolic Health Benefits of Sauna Exposure in Individuals with High-Stress Occupations. A Mechanistic Review. International Journal of Environmental Research and Public Health, 18(3), 1105. https://doi.org/10.3390/ijerph18031105





