Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
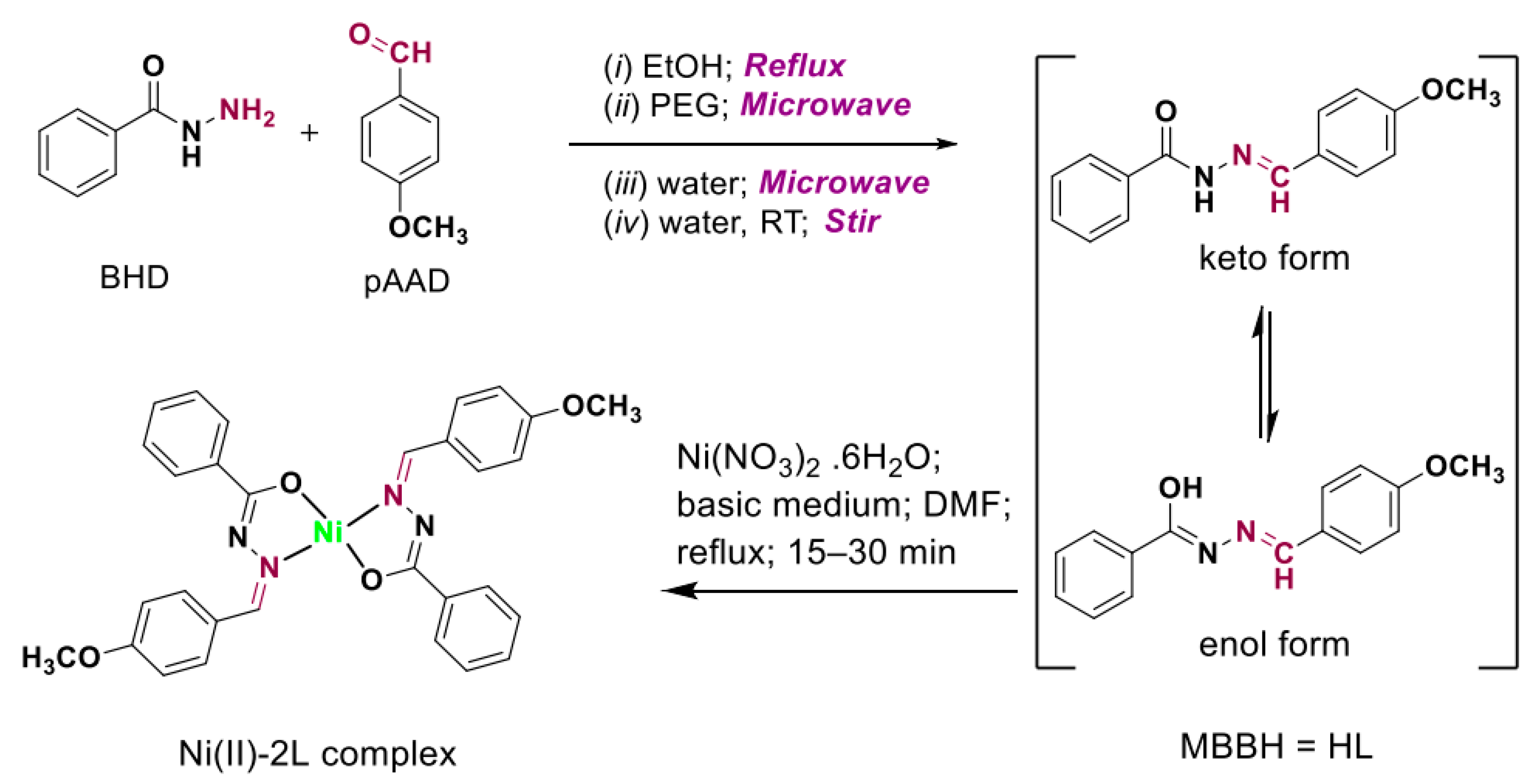
2.3. Synthesis of (Z)-N′-(4-Methoxybenzylidene) Benzohydrazide Ligand
2.4. Synthesis of (Z)-N′-(4-Methoxybenzylidene) Benzo Hydrazide Ni(II) Complex–(Ni(II)-2L)
2.5. Instrumentation
2.6. X-ray Crystallography
2.7. Biological Studies
3. Results and Discussion
3.1. Synthesis Protocols
3.2. Analytical Characterization
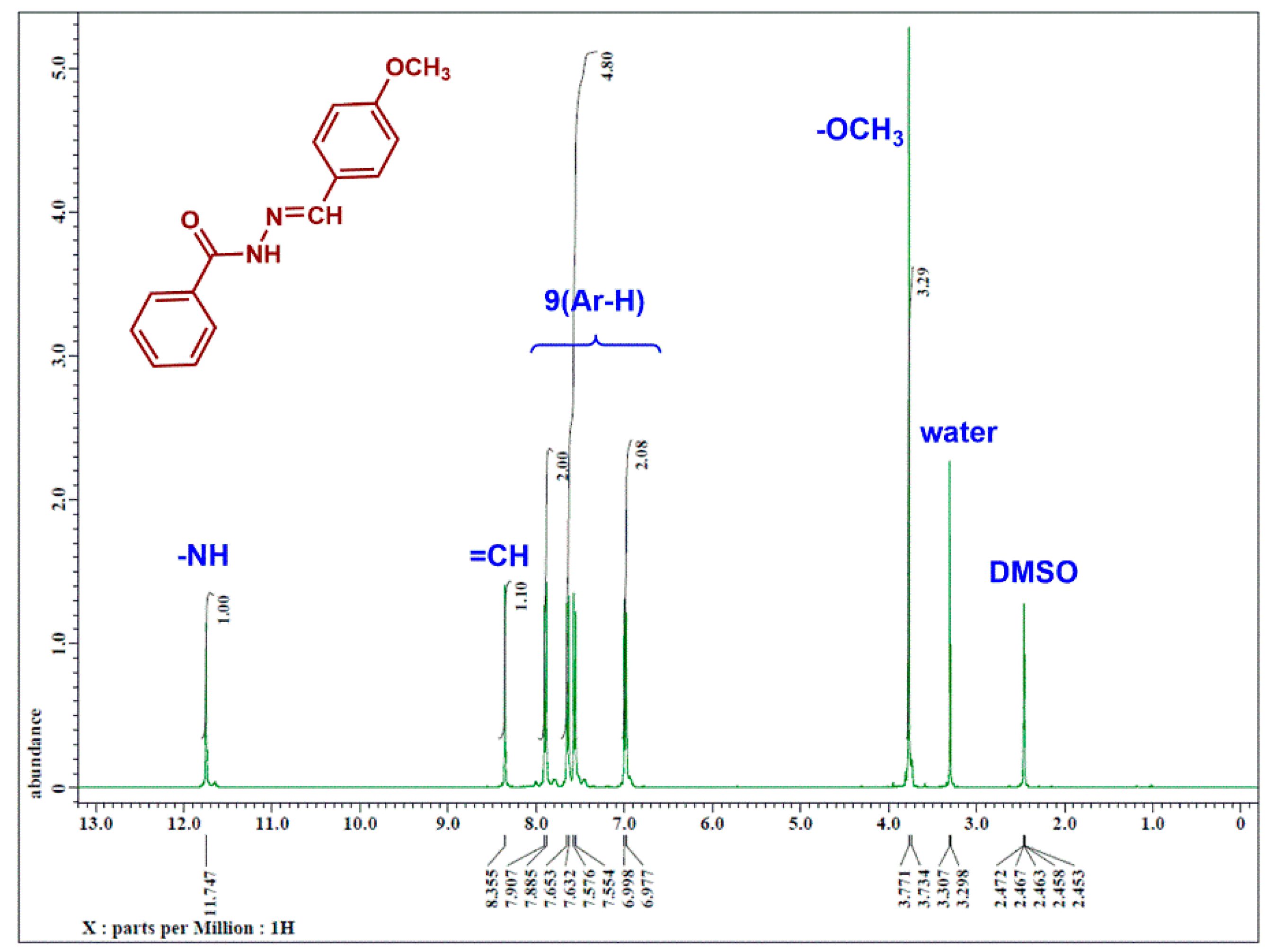
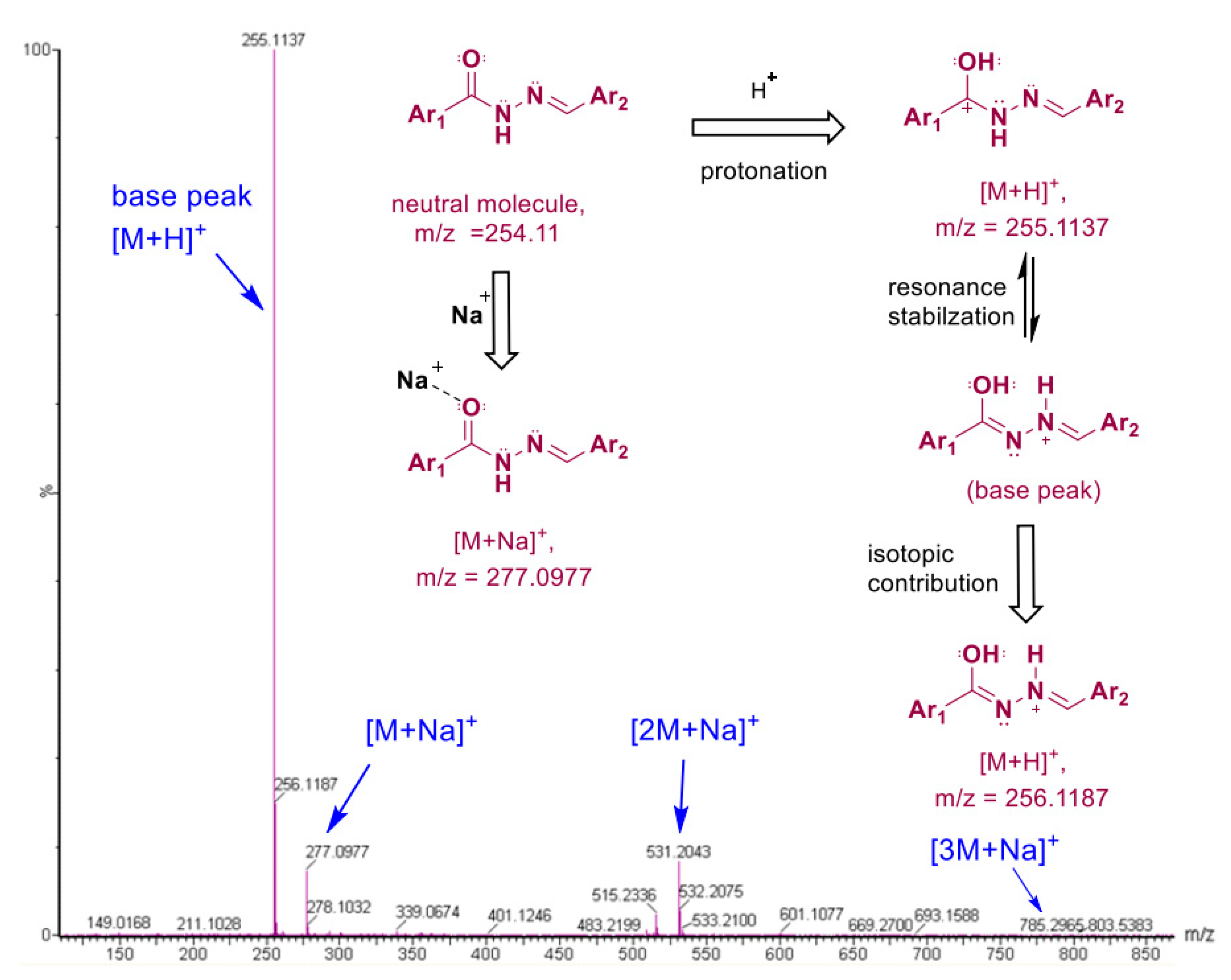
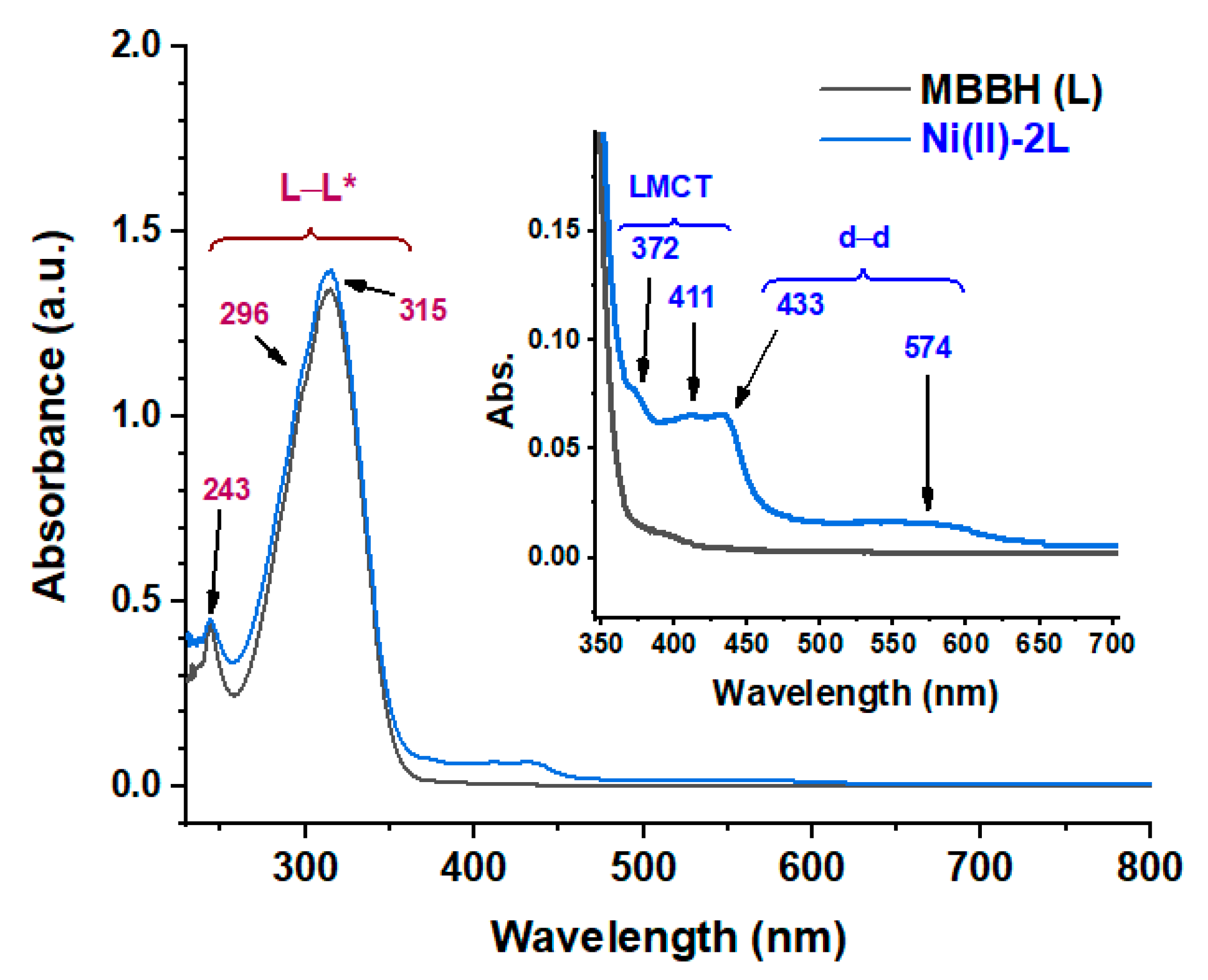
3.2.1. Spectral Analysis
3.2.2. Magnetic Moment
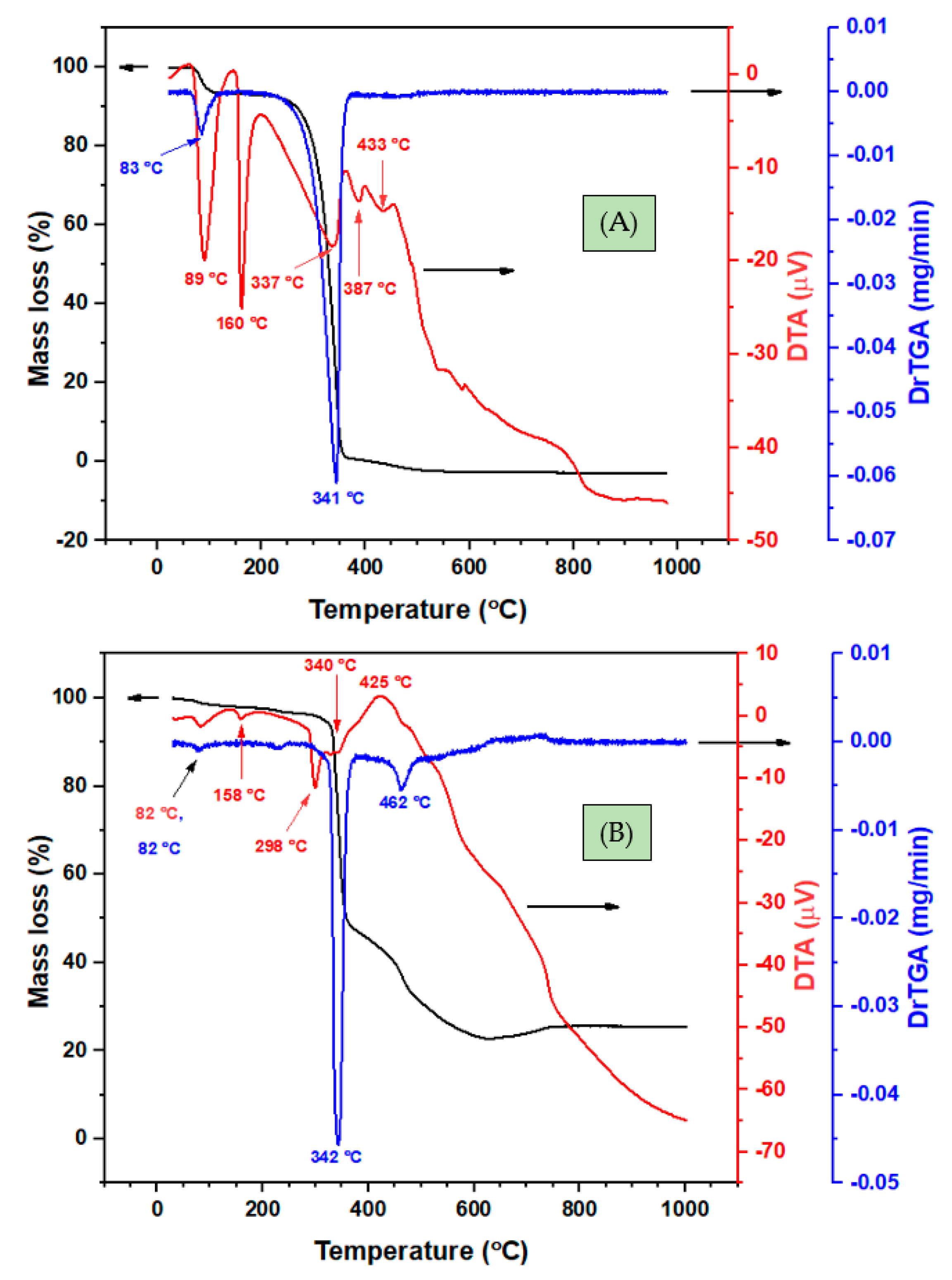
3.2.3. Thermal Analysis
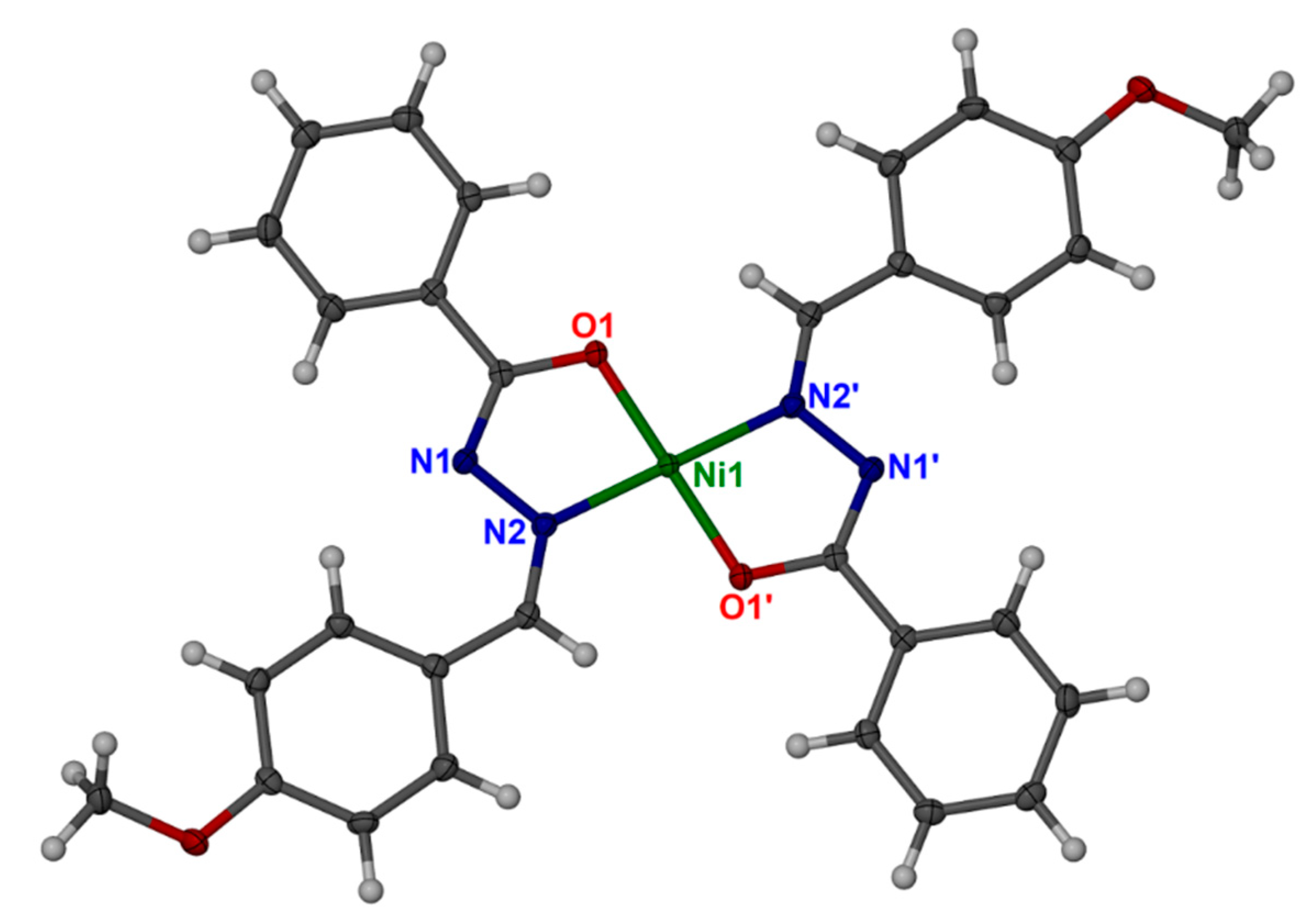
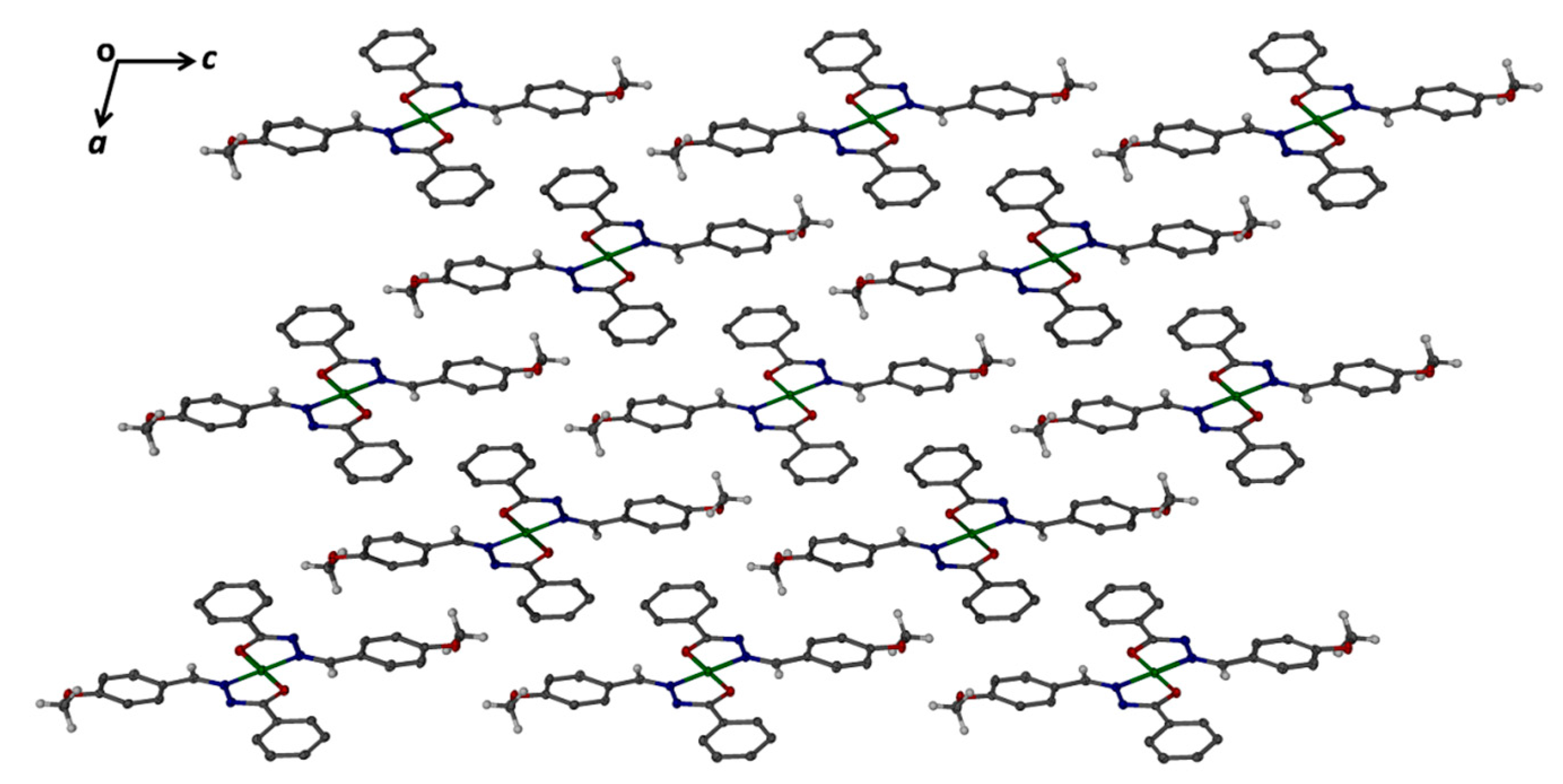
3.3. Crystal Structure
3.4. Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajarajan, M.; Senbagam, R.; Vijayakumar, R.; Balaji, S.; Manikandan, V.; Vanangamudi, G.; Thirunarayanan, G. Synthesis, spectral correlations and antimicrobial activities of substituted 4-((E)-2-benzylidenehydrazinyl) benzonitriles. Indian J. Chem. 2016, 55, 197–206. [Google Scholar]
- Chohan, Z.H.; Sherazi, S.K. Biological role of cobalt (II), copper (II) and nickel (II) metal ions on the antibacterial properties of some nicotinoyl-hydrazine derived compounds. Metal-Based Drugs 1997, 4, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Deeb, A.; El-Mariah, F.; Hosny, M. Pyridazine derivatives and related compounds. Part 13: Synthesis and antimicrobial activity of some pyridazino [3′,4′:3,4] pyrazolo [5, 1-c]-1,2,4-triazines. Bioorg. Med. Chem. Lett. 2004, 14, 5013–5017. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Sharma, N.; Kamboj, S.; Saini, V. Therapeutic potential of hydrazones as anti-inflammatory agents. Int. J. Med. Chem. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Kaushik, D.; Khan, S.A.; Chawla, G.; Kumar, S. N’-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl) methylene] 2/4-substituted hydrazides: Synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3943–3949. [Google Scholar] [CrossRef]
- Periakaruppan, P.; Abraham, R.; Mahendran, K.; Ramanathan, M. Simple synthesis of hydrazones with quorum quenching activity at room temperature in water. Environ. Chem. Lett. 2018, 16, 1063–1067. [Google Scholar] [CrossRef]
- Cornelissen, J.P.; Van Diemen, J.H.; Groeneveld, L.R.; Haasnoot, J.G.; Spek, A.L.; Reedijk, J. Synthesis and properties of isostructural transition-metal (copper, nickel, cobalt, and iron) compounds with 7,7′,8,8′-tetracyanoquinodimethanide (1-) in an unusual monodentate coordination mode: Crystal structure of bis (3,5-bis (pyridin-2-yl)-4-amino-1,2,4-triazole) bis (7,7′,8,8′-tetracyanoquinodimethanido) copper (II). Inorg. Chem. 1992, 31, 198–202. [Google Scholar]
- Rawat, P.; Singh, R. Synthesis and study on aroylhydrazones having cyanovinylpyrrole. Arab. J. Chem. 2019, 12, 2384–2397. [Google Scholar] [CrossRef]
- Maheswari, R.; Manjula, J. Synthesis, Characterization and Biological Applications of Benzohydrazide derivatives. Int. J. Appl. Res. 2015, 1, 587–592. [Google Scholar]
- Raj, V. Review on the Pharmacological Activities of Hydrazones derivatives. EC Pharm. Sci. 2016, 2, 278–306. [Google Scholar]
- Therese, S.K.; Geethamalika, G. Synthesis, Characterization and Anti Mycobacterial Activity of Novel Hydrazones. Orient. J. Chem. 2017, 33, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2, 4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardakhan, W.W.; Sherif, S.M.; Mohareb, R.M.; Abouzied, A.S. The Reaction of Cyanoacetylhdrazine with Furan-2-Aldehyde: Novel Synthesis of Thiophene, Azole, Azine and Coumarin Derivatives and Their Antitumor Evaluation. Int. J. Org. Chem. 2012, 2, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Chandra, S.; Saraswat, B. Ni (II) and Zn (II) complexes of 2-((thiophen-2-ylmethylene) amino) benzamide: Synthesis, spectroscopic characterization, thermal, DFT and anticancer activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 200–209. [Google Scholar] [CrossRef]
- Mohareb, R.; El-Sharkawy, K.; Hussein, M.; El-Sehrawi, H. Synthesis of hydrazide-hydrazone derivatives and their evaluation of antidepressant, sedative and analgesic agents. J. Pharm. Sci. Res 2010, 2, 185–196. [Google Scholar]
- Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5 (4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; More, Y.; Vagdevi, H.; Vaidya, V.; Gadaginamath, G.; Kulkarni, V. Synthesis of new 4-(2,5-dimethylpyrrol-1-yl)/4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial, antifungal and antitubercular agents. Med. Chem. Res. 2013, 22, 1073–1089. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef]
- Pegu, R.; Mandal, R.; Guha, A.K.; Pratihar, S. A selective ratiometric fluoride ion sensor with a (2,4-dinitrophenyl) hydrazine derivative of bis (indolyl) methane and its mode of interaction. New J. Chem. 2015, 39, 5984–5990. [Google Scholar] [CrossRef]
- El Sayed, L.; Iskander, M. Coordination compounds of hydrazine derivatives with transition metals—III: The reaction of aroyl hydrazones with Ni (II) and Cu (II) salts. J. Inorg. Nucl. Chem. 1971, 33, 435–443. [Google Scholar] [CrossRef]
- Dimova, V.; Jankulovska, M. QSAR Modeling of Antimicrobial Activity of some p-substituted Aromatic Hydrazones. J. Scient. Ind. Res. 2017, 76, 550–555. [Google Scholar]
- Saleem, H.; Subashchandrabose, S.; Babu, N.R.; Padusha, M.S.A. Vibrational spectroscopy investigation and density functional theory calculations on (E)-N′-(4-methoxybenzylidene) benzohydrazide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 143, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Lee, D.-U. Syntheses, crystal structure, Hirshfeld surfaces, fluorescence properties, and DFT analysis of benzoic acid hydrazone Schiff bases. Acta A Mol. Biomol. Spectrosc. 2015, 145, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Jankulovska, M.; Čolančeska-Rağenović, K.; Dimova, V.; Spirevska, I.; Makreski, P. Synthesis and characterization of new p-substituted aromatic hydrazones. Org. Chem. An Ind. J. 2012, 8, 326–334. [Google Scholar]
- Gou, J.-X.; Song, M.-Z.; Fan, C.-G.; Yang, Z.-N. (E)-N′-(4-Methoxybenzylidene) benzohydrazide. Acta Cryst. E 2009, 65, 3207–3213. [Google Scholar] [CrossRef]
- Tumkevicius, S.; Mekuskiene, G.; Gefenas, V.; Vainilavicius, P. Substituent effect on proton chemical shifts of amide and azomethine groups of arylidenehydrazides of 5-substituted 2-pyrimidine-carboxylic acids and their aromatic analogs. Chemija 2005, 16, 65–68. [Google Scholar]
- Al-Qadsy, I.; Saeed, W.S.; Al-Odayni, A.-B.; Ahmed Saleh Al-Faqeeh, L.; Alghamdi, A.A.; Farooqui, M. Novel metformin-based schiff bases: Synthesis, characterization, and antibacterial evaluation. Materials 2020, 13, 514. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Butorin, S.M.; Young, A.T.; Guo, J. Nickel oxidation states and spin states of bioinorganic complexes from nickel L-edge X-ray absorption and resonant inelastic X-ray scattering. J. Phys. Chem. C 2013, 117, 24767–24772. [Google Scholar] [CrossRef]
- Maroney, M.J.; Ciurli, S. Bioinorganic Chemistry of Nickel. Multidisciplinary Digital Publishing Institute. Inorganics 2019, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Trivedi, A. A review on role of nickel in the biological system. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 719–727. [Google Scholar] [CrossRef]
- Iskander, M.; Zayan, S.; Khalifa, M.; El-Sayed, L. Coordination compounds of hydrazine derivatives with transition metals—VI: The reaction of aroylhydrazines with nickel (II), cobalt (II) and copper (II) salts. J. Inorg. Nucl. Chem. 1974, 36, 551–556. [Google Scholar] [CrossRef]
- Wiegand, R.G. The formation of pyridoxal and pyridoxal 5-phosphate hydrazones1. J. Am. Chem. Soc. 1956, 78, 5307–5309. [Google Scholar] [CrossRef]
- Hrušková, K.; Potůčková, E.; Hergeselova, T.; Liptakova, L.; Hašková, P.; Mingas, P.; Kovaříková, P.; Šimůnek, T.; Vávrová, K. Aroylhydrazone iron chelators: Tuning antioxidant and antiproliferative properties by hydrazide modifications. Eur. J. Med. Chem. 2016, 120, 97–110. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Bernhardt, P.V.; Chin, P.; Richardson, D.R. Structural Variations and Formation Constants of First-Row Transition Metal Complexes of Biologically Active Aroylhydrazones. Eur. J. Inorg. Chem. 2003, 2003, 1145–1156. [Google Scholar] [CrossRef]
- Balachandran, K.; George, M. Oxidation with metal oxides—VI: Oxidation of benzoylhydrazones of aldehydes, ketones and 1, 2-diketones with nickel peroxide. Tetrahedron 1973, 29, 2119–2128. [Google Scholar] [CrossRef]
- Iskander, M.; Saddeck, S. Coordination compounds of hydrazine derivatives with transition metals. XIV. Nickel (II) chelates with bidentate aroylhydrazones and their reactions with heterocyclic bases. Inorg. Chim. Acta 1977, 22, 141–147. [Google Scholar] [CrossRef]
- Iskander, M.F.; El-Sayed, L.; Saddeck, S.; Abuo-Taleb, M.A. Coordination compounds of hydrazine derivatives with transition metals. Part 20. Nickel (II) chelates witho-hydroxy ando-methoxy-benzaldehyde aroylhydrazones. An example of coordination isomerism. Trans. Met. Chem. 1980, 5, 168–172. [Google Scholar] [CrossRef]
- Joseph, B.; Sithambaresan, M.; Kurup, M.P.; Ng, S.W. Bis {μ-N-[(E)-4-benzyloxy-2-oxidobenzylidene]-4-nitrobenzenecarbohydrazidato} bis [diaquanickel (II)] dimethylformamide tetrasolvate. Acta Cryst. 2014, 70, m211–m212. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-X.; Li, Q.-L.; Li, P.-P.; Zhao, J.-X.; Zhao, L. Crystal structure of bis {5-methoxy-2-((E)-((4-((E)-1-(methoxyimino) ethyl) phenyl) imino) methyl) phenolato-κ2N, O} nickel (II), C34H34N4NiO6. Z. Krist.-New Cryst. Struct. 2018, 233, 767–769. [Google Scholar] [CrossRef]
- Mondal, S.; Das, C.; Ghosh, B.; Pakhira, B.; Blake, A.J.; Drew, M.G.; Chattopadhyay, S.K. Synthesis, spectroscopic studies, X-ray crystal structures, electrochemical properties and DFT calculations of three Ni (II) complexes of aroyl hydrazone ligands bearing anthracene moiety. Polyhedron 2014, 80, 272–281. [Google Scholar] [CrossRef]
- Jia, W.P.; Yang, J.G.; Li, F.; Pan, F.Y. Synthesis and Crystal Structure of a Ni (Ⅱ) Complex of 2′-[4-N, N′-(Dimethylaminobenzyl-idene)]-3,5-dihydroxybenzoylhydrazide and Its Spectral Properties. Chin. J. Inorg. Chem. 2009, 1635–1639. [Google Scholar]
- Zhang, S.-P.; Liu, Z.-D.; Chen, S.-S.; Wei, Y.; Shao, S.-C. Synthesis, characterization and crystal structure of bis (p-fluorobenzaldehyde salicylhydrazone) nickel (II). Chin. J. Inorg. Chem. 2007, 23, 1069–1071. [Google Scholar]
- Jia, W.P.; Yang, J.G.; Li, F.; Pan, F.Y. Synthesis and Crystal Structure of Ni (Ⅱ) Complex of 2′-(4-fluorobenzylidene)]-3,5-dihydroxybenzoylhydrazide and Its Spectral Properties. Chin. J. Inorg. Chem. 2008, 24, 627–630. [Google Scholar]
- SAINT Data Reduction Software. Version 6.45. Bruker Analytical X-ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- SADABS, V. 2.05; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Cryst. 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Owuama, C.I. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afric. J. Microb. Res. 2017, 11, 977–980. [Google Scholar]
- Arfan, M.; Khan, R.; Tavman, A.; Saba, S. Spectral characterization and crystal structure of 2-amino-N′-[(1Z)-1-(4-chlorophenyl) ethylidene]-benzohydrazide. J. Saud. Chem. Soc. 2016, 20, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Gluvchinsky, P.; Mockler, G.M.; Sinn, E. Nickel (II) complexes of some quadridentate Schiff-base ligands—II. Infrared spectra. Spectro. Acta A Mol. Spect. 1977, 33, 1073–1077. [Google Scholar] [CrossRef]
- Rassem, H.; Nour, A. Synthesis, Spectral Characterization and Crystal Structure of (E)-4-Hydroxy-N-(2-Methoxybenzylidene) Benzohydrazide. Aust. J. Bas. Appl. Sci. 2016, 10, 513–516. [Google Scholar]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium adduct formation efficiency in ESI source. J. Mass Spect. 2013, 48, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Crotti, A.E.; Vessecchi, R.; Lopes, J.L.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskander, M.F.; El-Sayed, L.; Salem, N.M.; Werner, R.; Haase, W. Synthesis, characterization and magnetochemical studies of dicopper (II) complexes derived from bis (N-salicylidene) dicarboxylic acid dihydrazides. J. Coord. Chem. 2005, 58, 125–139. [Google Scholar] [CrossRef]
- Arafath, M.A.; Al-Suede, F.S.; Adam, F.; Al-Juaid, S.; Khadeer Ahamed, M.B.; Majid, A.M. Schiff base-nickel, palladium, and platinum complexes derived from N-cyclohexyl hydrazine carbothioamide and 3-hydroxy-4-methoxybenzaldehyde: Selective antiproliferative and proapoptotic effects against colorectal carcinoma. Drug. Devel. Res. 2019, 80, 778–790. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization and biological studies of metal (II) complexes of (3E)-3-[(2-{(E)-[1-(2, 4-dihydroxyphenyl) ethylidene] amino} ethyl) imino]-1-phenylbutan-1-one Schiff base. Molecules 2015, 20, 9788–9802. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, S.; Neese, F.; Bothe, E.; Bill, E.; Weyhermüller, T.; Wieghardt, K. Square planar vs tetrahedral coordination in diamagnetic complexes of nickel (II) containing two bidentate π-radical monoanions. Inorg. Chem. 2005, 44, 3636–3656. [Google Scholar] [CrossRef]
- Bouzerafa, B.; Ourari, A.; Aggoun, D.; Ruiz-Rosas, R.; Ouennoughi, Y.; Morallon, E. Novel nickel (II) and manganese (III) complexes with bidentate Schiff-base ligand: Synthesis, spectral, thermogravimetry, electrochemical and electrocatalytical properties. Res. Chem. Int. 2016, 42, 4839–4858. [Google Scholar] [CrossRef]
- Angelusiu, M.V.; Barbuceanu, S.-F.; Draghici, C.; Almajan, G.L. New Cu (II), Co (II), Ni (II) complexes with aroyl-hydrazone based ligand. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Eur. J. Med. Chem. 2010, 45, 2055–2062. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Nickel (II)-oxaloyldihydrazone complexes: Characterization, indirect band gap energy and antimicrobial evaluation. Cog. Chem. 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Kumar, D.; Chadda, S.; Sharma, J.; Surain, P. Syntheses, spectral characterization, and antimicrobial studies on the coordination compounds of metal ions with Schiff base containing both aliphatic and aromatic hydrazide moieties. Bioinorg. Chem. Appl. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Singh, V.K. Synthesis, structural and antifungal studies of Co (II), Ni (II), Cu (II) and Zn (II) complexes with new Schiff bases bearing benzimidazoles. Indian J. Chem. 1993, 32, 446–449. [Google Scholar]


| Empirical Formula | C30H26NiN4O4 |
|---|---|
| Formula weight | 565.26 |
| Temperature/K | 100(2) |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 11.6165(9) |
| b/Å | 6.4540(5) |
| c/Å | 17.9785(14) |
| α/° | 90 |
| β/° | 106.395(3) |
| γ/° | 90 |
| Volume/Å3 | 1293.09(17) |
| Z | 2 |
| Density (calculated)/(g/cm3) | 1.452 |
| μ/mm−1 | 0.795 |
| F(000) | 588.0 |
| Crystal size/mm3 | 0.210 × 0.200 × 0.180 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 6.74 to 49.998 |
| Index ranges | −13 ≤ h ≤ 13, −7 ≤ k ≤ 7, −21 ≤ l ≤ 21 |
| Reflections collected | 15,298 |
| Independent reflections | 2283 [Rint = 0.0353, Rsigma = 0.0221] |
| Data/restraints/parameters | 2283/0/179 |
| Goodness-of-fit on F2 | 1.054 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0245, wR2 = 0.0568 |
| Final R indexes [all data] | R1 = 0.0290, wR2 = 0.0605 |
| Largest diff. peak/hole/e Å−3 | 0.27/−0.25 |
| Comp. | Synthesis Strategy | Physical Properties | |||||
|---|---|---|---|---|---|---|---|
| Methods | Type | Medium | Reaction Time | Color | Melting Point | Yield (%) | |
| HL | Method (i) | Reflux | EtOH | 2 hrs | White | 153 ± 3 | 98 |
| Method (ii) | Microwave | PEG | 2 min | Light-brown | 152 ± 2 | 79 | |
| Method (iii) | Microwave | Water | 20 sec | White | 156 ± 2 | 99 | |
| Method (iv) | Stir | Water | 30 sec | White | 156 ± 2; (DTA, 160) | 99 | |
| Ni(II)-2L complex | Reflux | DMF | 15 min | Orange crystal | >300; (DTA, 298) | 87 | |
| Microwave-assisted synthesis | DMF | 90 sec | Orange microcrystals | >300 | 98 | ||
| Compound | Chemical Formula | MW | Elemental Analysis (wt %); Found (Calculated) | |||
|---|---|---|---|---|---|---|
| C | H | N | O | |||
| HL | C15H14N2O2 | 254.29 | 70.93 (70.85) | 5.62 (5.55) | 11.10 (11.02) | 12.60 (12.58) |
| Ni(II)-2L | C30H26N4NiO4 | 565.26 | 64.05 (63.75) | 4.75 (4.64) | 9.94 (9.91) | 11.35 (11.32) |
| Compound | Abs (a.u.) | λ (nm) | ɛ (M−1cm−1) | Assignment | μeff (BM) | Geometry |
|---|---|---|---|---|---|---|
| HL | 0.445 | 243 | 148 | π–π* | - | - |
| 1.022 | 296 | 341 | π–π* | |||
| 1.344 | 315 | 448 | n–π* | |||
| Ni(II)-2L | 0.445 | 243 | 148 | π–π* | 0 | Square planar |
| 1.085 | 296 | 362 | π–π* | |||
| 1.394 | 315 | 465 | n–π* | |||
| 0.081 | 372 | 27 | LMCT | |||
| 0.066 | 411 | 22 | LMCT | |||
| 0.066 | 433 | 22 | 1A1g → 1A2g | |||
| 0.017 | 574 | 6 | 1A1g → 1B1g |
| Comp. | Step | Temp. (°C) | DrTGA max (°C) | DTA Max (°C) | Mass Loss (%) | Evolved Moieties | Residue (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Endo | Exo | Obs. | Calc. | ||||||
| HL | 1 | 66–108 | 83 | 89 | - | 9.5 | 9.6 | 1.5 H2O, moisture | - |
| 2 | 250–380 | 341 | 337, 387, 433 | - | 90.5 | 90.4 | 100% ligand | ||
| Ni(II)-2L | 1 | 55–296 | 82, 158 | 82, 225 | - | 4.6 | 5.3 | 2(-CH3) | NiC6H6; Obs. 22.8, Calc. 24.1 |
| 2 | 297–379 | 342 | 340, 372 | - | 42.3 | 42.1 | 2[C7H5NO] | ||
| 3 | 380–627 | 462 | 425 | multi | 30.4 | 28.3 | C8H4N2O2 | ||
| Bond Lengths | Bond Angles | |||||
|---|---|---|---|---|---|---|
| Atom | Atom | Length (Å) | Atom | Atom | Atom | Angle (˚) |
| Ni1 | O1 | 1.8403(11) | O1 | Ni1 | O11 | 180.0 |
| Ni1 | O11 | 1.8403(11) | O1 | Ni1 | N2 | 83.70(5) |
| Ni1 | N2 | 1.8703(13) | O11 | Ni1 | N2 | 96.30(5) |
| Ni1 | N21 | 1.8703(13) | O1 | Ni1 | N21 | 96.30(5) |
| O1 | C7 | 1.3016(19) | O11 | Ni1 | N21 | 83.70(5) |
| N1 | N2 | 1.3976(19) | N2 | Ni1 | N21 | 180.0 |
| Compound | Minimal Inhibition Concentration (MIC) (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Bacteria Species | Fungal Species | ||||||
| Gram-Positive | Gram-Negative | ||||||
| S. aureus | S. pyogenes | E. coli | P. aeruginosa | C. albicans | A. niger | A. clavatus | |
| HL | 500 | 250 | 62.5 | 125 | 500 | 1000 | 1000 |
| Ni(II)-2L | 125 | 62.5 | 125 | 250 | >1000 | 500 | 500 |
| Gentamycin | 0.25 | 0.5 | 0.05 | 1 | - | - | - |
| Nystatin | - | - | - | - | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qadsy, I.; Al-Odayni, A.-B.; Saeed, W.S.; Alrabie, A.; Al-Adhreai, A.; Al-Faqeeh, L.A.S.; Lama, P.; Alghamdi, A.A.; Farooqui, M. Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex. Crystals 2021, 11, 110. https://doi.org/10.3390/cryst11020110
Al-Qadsy I, Al-Odayni A-B, Saeed WS, Alrabie A, Al-Adhreai A, Al-Faqeeh LAS, Lama P, Alghamdi AA, Farooqui M. Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex. Crystals. 2021; 11(2):110. https://doi.org/10.3390/cryst11020110
Chicago/Turabian StyleAl-Qadsy, Inas, Abdel-Basit Al-Odayni, Waseem Sharaf Saeed, Ali Alrabie, Arwa Al-Adhreai, Lena Ahmed Saleh Al-Faqeeh, Prem Lama, Abdulaziz Ali Alghamdi, and Mazahar Farooqui. 2021. "Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex" Crystals 11, no. 2: 110. https://doi.org/10.3390/cryst11020110
APA StyleAl-Qadsy, I., Al-Odayni, A.-B., Saeed, W. S., Alrabie, A., Al-Adhreai, A., Al-Faqeeh, L. A. S., Lama, P., Alghamdi, A. A., & Farooqui, M. (2021). Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene)benzohydrazide–Nickel(II)] Complex. Crystals, 11(2), 110. https://doi.org/10.3390/cryst11020110








