CD200 Change Is Involved in Neuronal Death in Gerbil Hippocampal CA1 Field Following Transient Forebrain Ischemia and Postischemic Treatment with Risperidone Displays Neuroprotection without CD200 Change
Abstract
:1. Introduction
2. Results
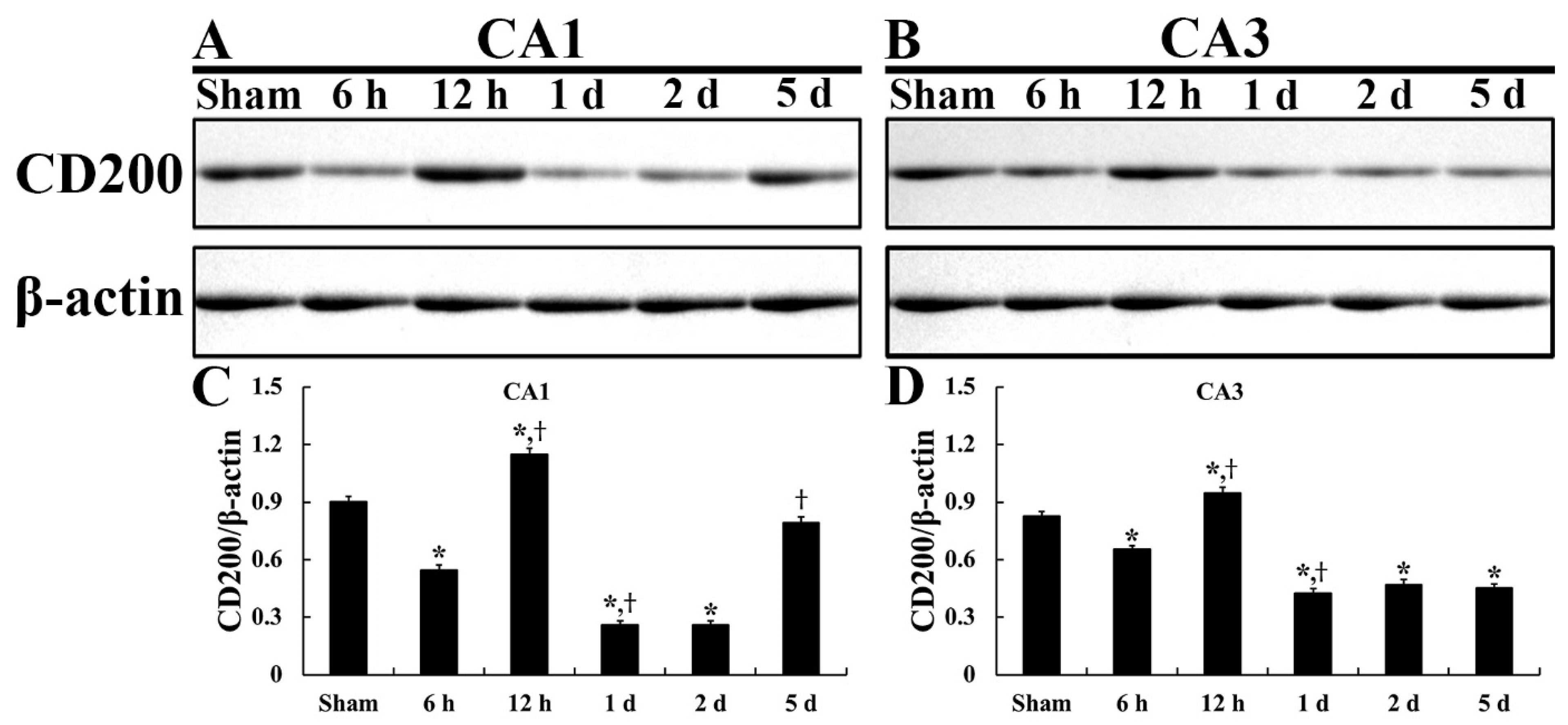
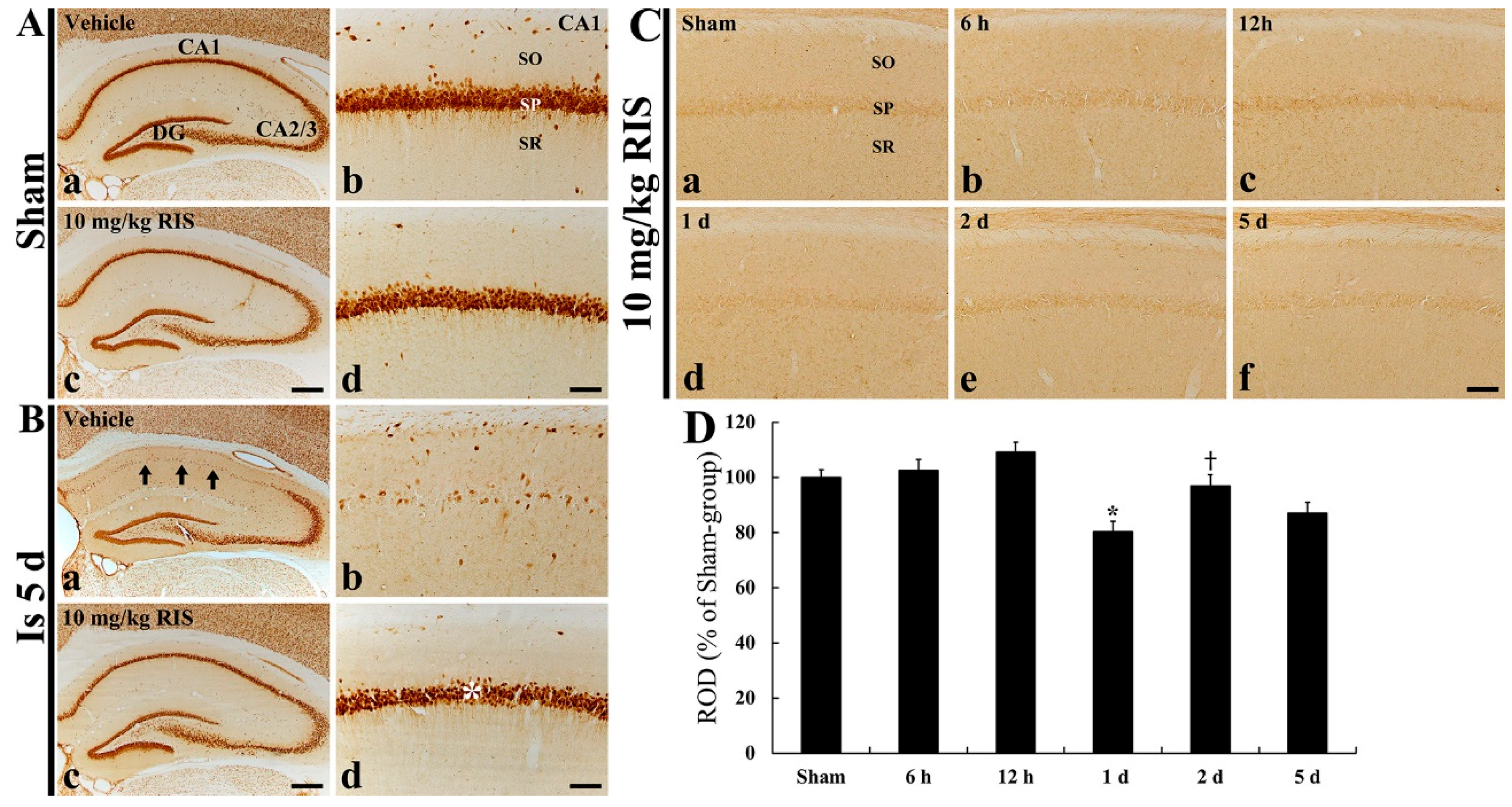
2.1. CD200 Levels
2.2. DND
2.3. Microgliosis (Microglial Activation)
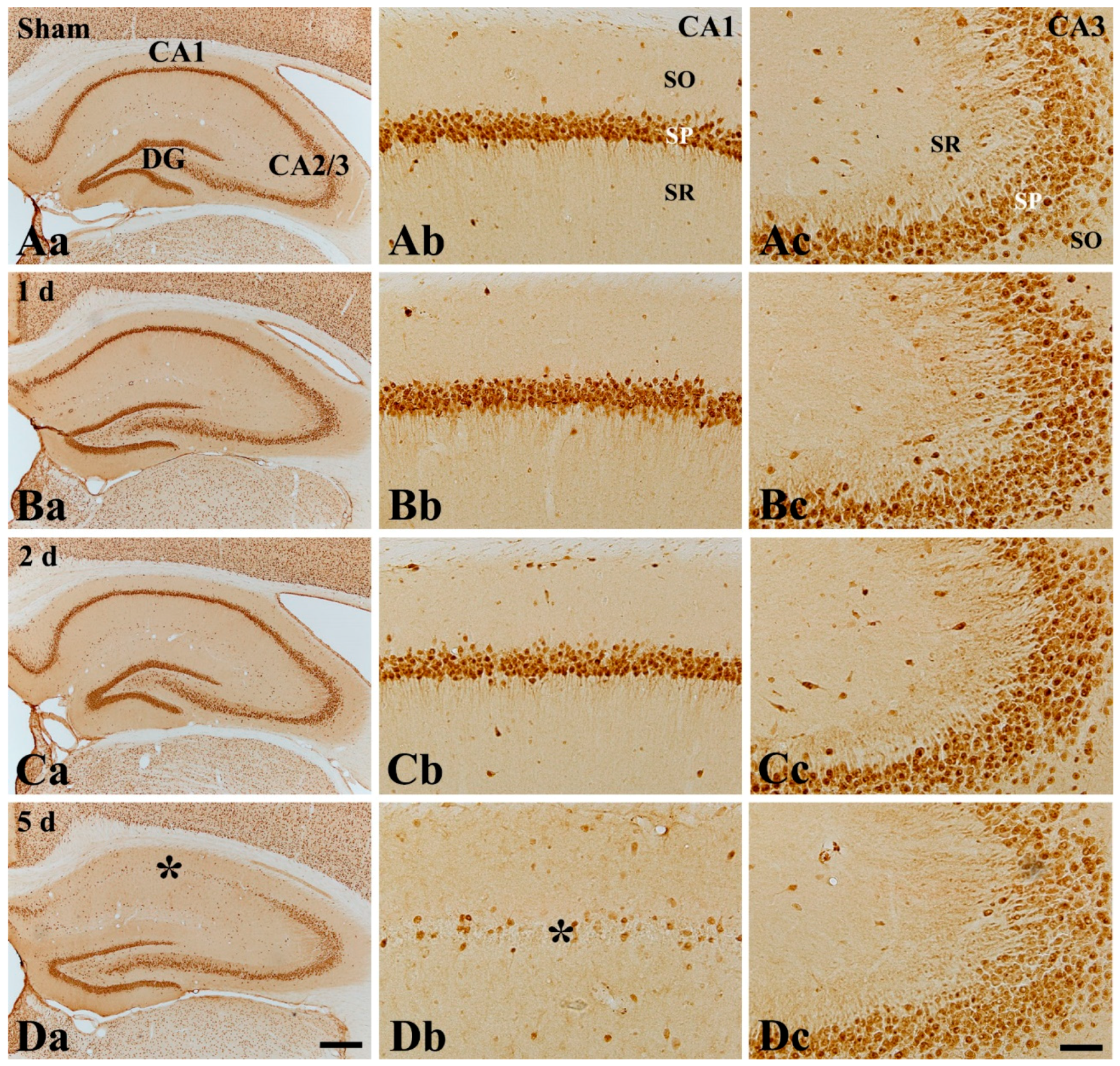
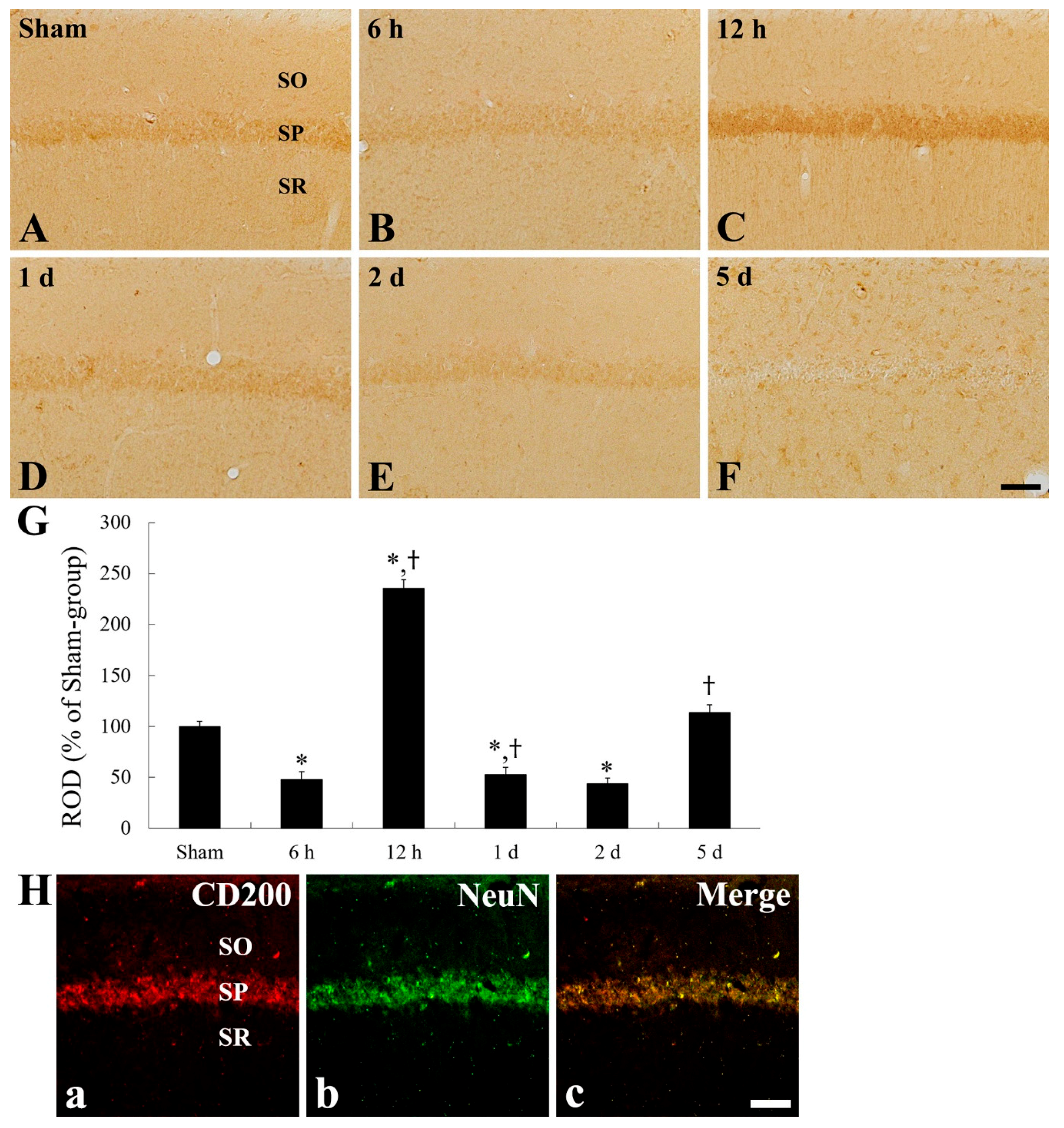
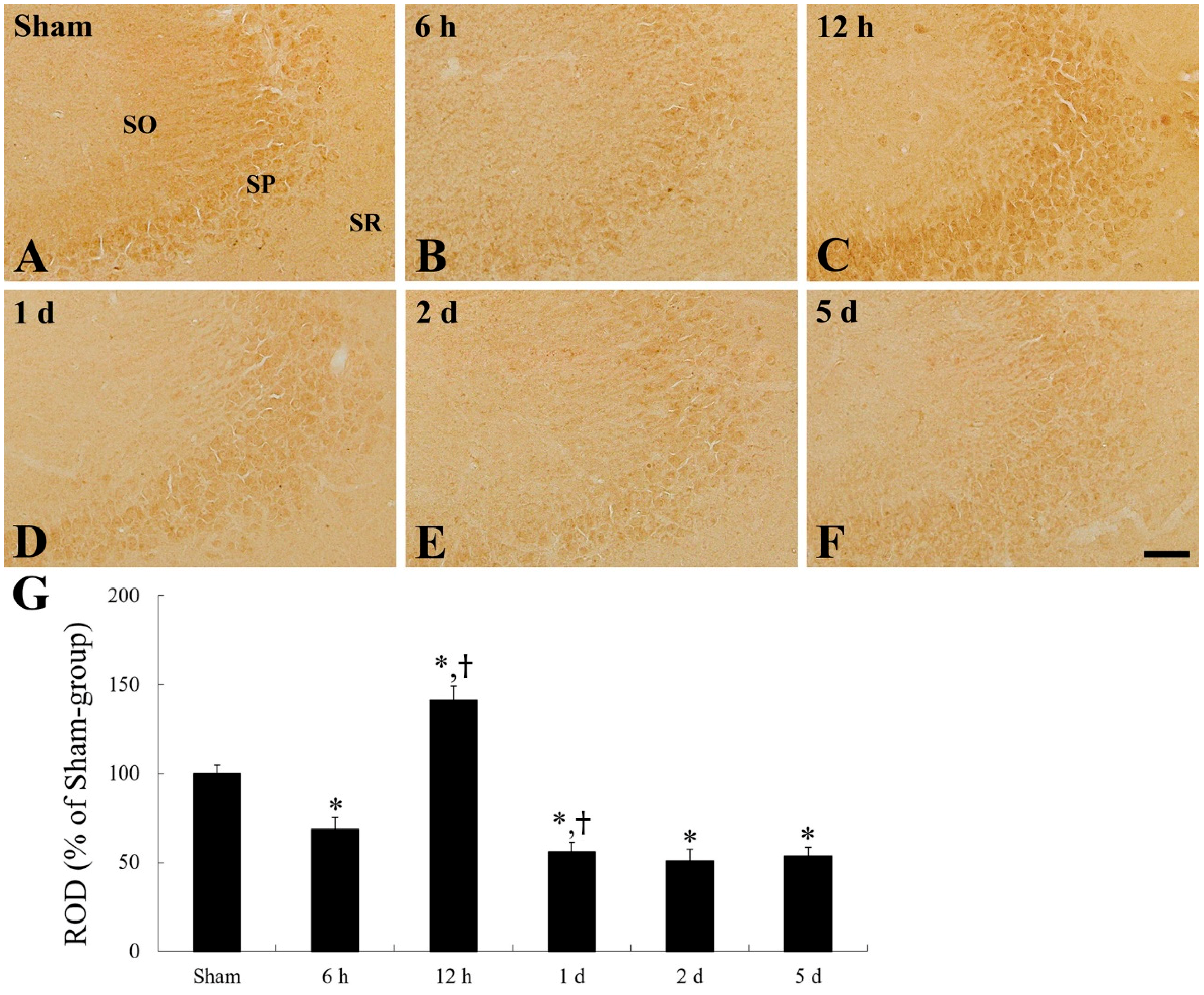
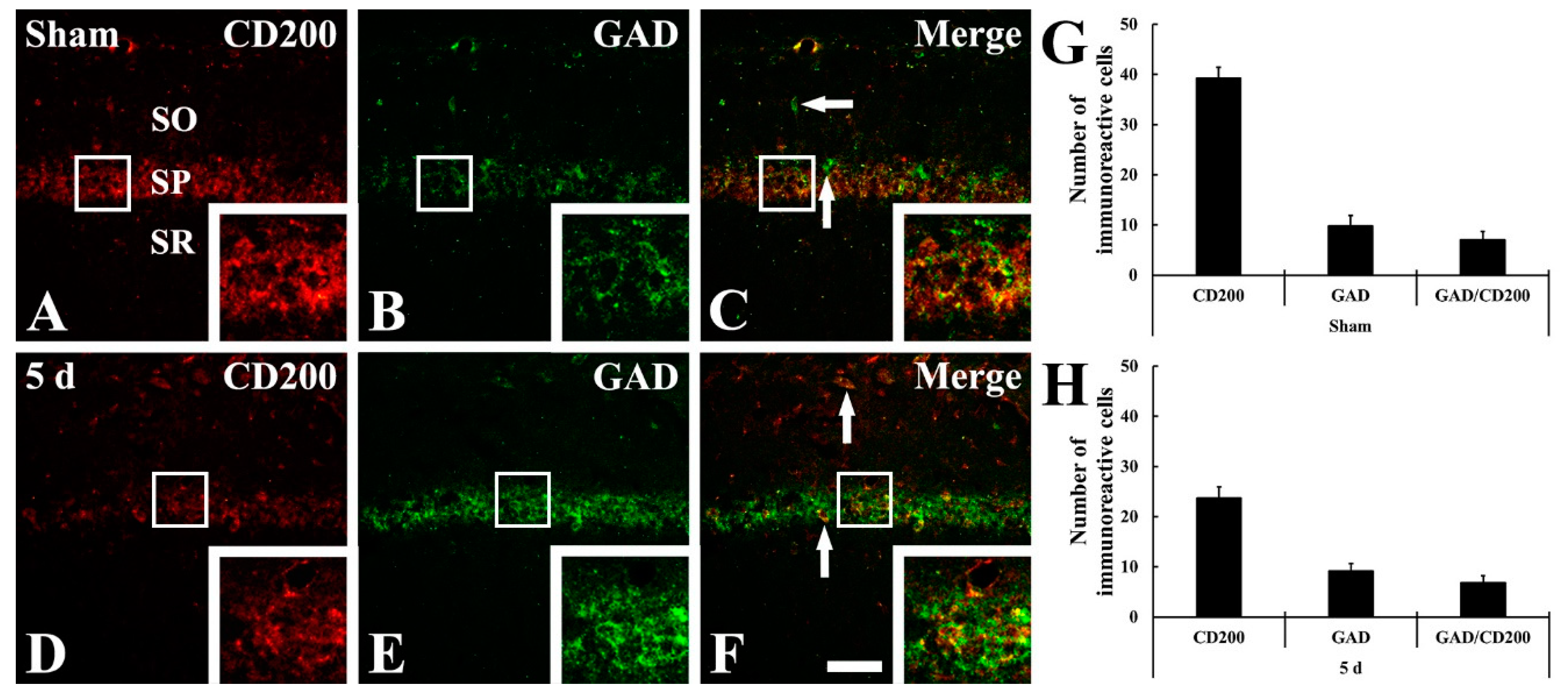
2.4. CD200 Immunoreactivity
2.5. Effects of Risperidone (RIS)
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
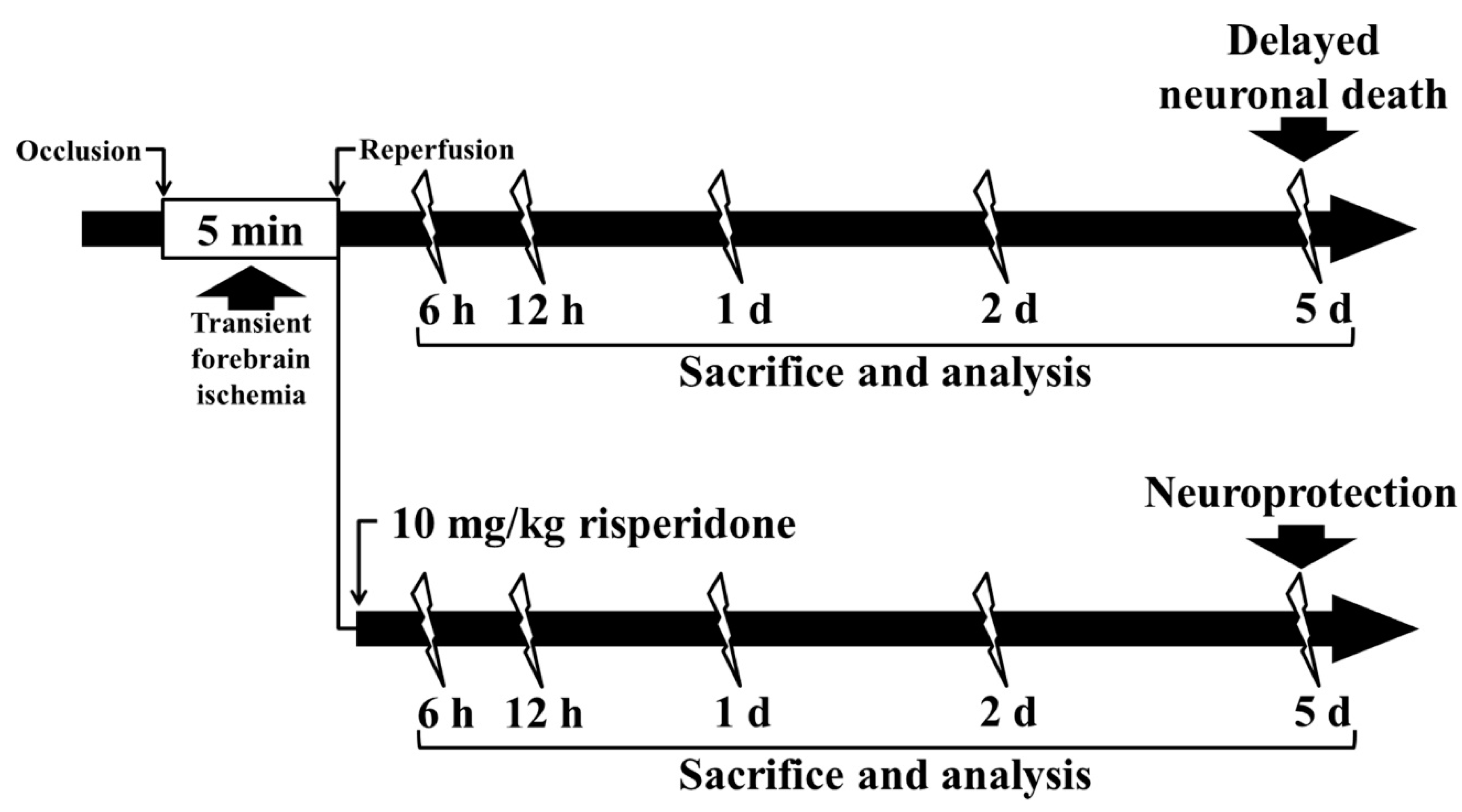
4.2. Induction of tFI
4.3. Western Blotting
4.4. Preparation of Brain Sections
4.5. Immunohistochemistry for NeuN, Iba-1, and CD200
4.6. Double Immunofluorescence Staining
4.7. Posttreatment with RIS
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, B.N.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Kim, D.W.; Won, M.H.; Hong, S.; Cho, J.H.; Lee, C.H. Ischemia-induced changes of pras40 and p-pras40 immunoreactivities in the gerbil hippocampal ca1 region after transient cerebral ischemia. Cell. Mol. Neurobiol. 2016, 36, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, J.H.; Cho, J.H.; Ahn, J.H.; Yan, B.C.; Lee, J.C.; Shin, M.C.; Cheon, S.H.; Cho, Y.S.; Cho, J.H.; et al. Changes and expressions of redd1 in neurons and glial cells in the gerbil hippocampus proper following transient global cerebral ischemia. J. Neurol. Sci. 2014, 344, 43–50. [Google Scholar] [CrossRef]
- Fujiwara, N.; Som, A.T.; Pham, L.D.; Lee, B.J.; Mandeville, E.T.; Lo, E.H.; Arai, K. A free radical scavenger edaravone suppresses systemic inflammatory responses in a rat transient focal ischemia model. Neurosci. Lett. 2016, 633, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Suyama, K.; Nishida, K.; Sei, Y.; Basile, A.S. Early increases in tnf-alpha, il-6 and il-1 beta levels following transient cerebral ischemia in gerbil brain. Neurosci. Lett. 1996, 206, 149–152. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.J.; Zhang, C.; Chen, R.; Li, L.; He, J.; Xie, Y.; Chen, Y. Loss of neuronal cd200 contributed to microglial activation after acute cerebral ischemia in mice. Neurosci. Lett. 2018, 678, 48–54. [Google Scholar] [CrossRef]
- Hughes, V. Microglia: The constant gardeners. Nature 2012, 485, 570–572. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R.M.; Cardona, A.E. The myeloid cells of the central nervous system parenchyma. Nature 2010, 468, 253–262. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Liu, L.; Cardona, A.E. Chemokines and chemokine receptors: Multipurpose players in neuroinflammation. Int. Rev. Neurobiol. 2007, 82, 187–204. [Google Scholar]
- Barclay, A.N.; Wright, G.J.; Brooke, G.; Brown, M.H. Cd200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002, 23, 285–290. [Google Scholar] [CrossRef]
- Koning, N.; Swaab, D.F.; Hoek, R.M.; Huitinga, I. Distribution of the immune inhibitory molecules cd200 and cd200r in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J. Neuropathol. Exp. Neurol. 2009, 68, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; Downer, E.J.; Crotty, S.; Nolan, Y.M.; Mills, K.H.; Lynch, M.A. Cd200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for il-4. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 8309–8313. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Simon, O.J.; Grimm, A.; Melzer, N.; Herrmann, A.M.; Spitzer, P.; Landgraf, P.; Wiendl, H. Cns inflammation and neuronal degeneration is aggravated by impaired cd200-cd200r-mediated macrophage silencing. J. Neuroimmunol. 2008, 194, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.H.; Zhang, E.; Kang, J.W.; Shin, Y.N.; Byun, J.Y.; Oh, S.H.; Seo, J.H.; Lee, Y.H.; Kim, D.W. Expression of cd200 in alternative activation of microglia following an excitotoxic lesion in the mouse hippocampus. Brain Res. 2012, 1481, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hernangomez, M.; Carrillo-Salinas, F.J.; Mecha, M.; Correa, F.; Mestre, L.; Loria, F.; Feliu, A.; Docagne, F.; Guaza, C. Brain innate immunity in the regulation of neuroinflammation: Therapeutic strategies by modulating cd200-cd200r interaction involve the cannabinoid system. Curr. Pharm. Des. 2014, 20, 4707–4722. [Google Scholar] [CrossRef] [Green Version]
- Hoek, R.M.; Ruuls, S.R.; Murphy, C.A.; Wright, G.J.; Goddard, R.; Zurawski, S.M.; Blom, B.; Homola, M.E.; Streit, W.J.; Brown, M.H.; et al. Down-regulation of the macrophage lineage through interaction with ox2 (cd200). Science 2000, 290, 1768–1771. [Google Scholar] [CrossRef]
- Lyons, A.; Minogue, A.M.; Jones, R.S.; Fitzpatrick, O.; Noonan, J.; Campbell, V.A.; Lynch, M.A. Analysis of the impact of cd200 on phagocytosis. Mol. Neurobiol. 2017, 54, 5730–5739. [Google Scholar] [CrossRef]
- Yi, M.H.; Zhang, E.; Kim, J.J.; Baek, H.; Shin, N.; Kim, S.; Kim, S.R.; Kim, H.R.; Lee, S.J.; Park, J.B.; et al. Cd200r/foxp3-mediated signalling regulates microglial activation. Sci. Rep. 2016, 6, 34901. [Google Scholar] [CrossRef]
- Denes, A.; Ferenczi, S.; Halasz, J.; Kornyei, Z.; Kovacs, K.J. Role of cx3cr1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 1707–1721. [Google Scholar] [CrossRef] [Green Version]
- Gyoneva, S.; Ransohoff, R.M. Inflammatory reaction after traumatic brain injury: Therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 2015, 36, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Takahashi, H.; Imai, Y.; Tanaka, J. Expression of cd200 by macrophage-like cells in ischemic core of rat brain after transient middle cerebral artery occlusion. Neurosci. Lett. 2007, 418, 44–48. [Google Scholar] [CrossRef]
- Matsumoto, S.; Tanaka, J.; Yano, H.; Takahashi, H.; Sugimoto, K.; Ohue, S.; Inoue, A.; Aono, H.; Kusakawa, A.; Watanabe, H.; et al. Cd200+ and cd200- macrophages accumulated in ischemic lesions of rat brain: The two populations cannot be classified as either m1 or m2 macrophages. J. Neuroimmunol. 2015, 282, 7–20. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, D.W.; Park, J.H.; Lee, T.K.; Lee, H.A.; Won, M.H.; Lee, C.H. Expression changes of cx3cl1 and cx3cr1 proteins in the hippocampal ca1 field of the gerbil following transient global cerebral ischemia. Int. J. Mol. Med. 2019, 44, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, D.W.; Lee, T.K.; Park, C.W.; Park, Y.E.; Ahn, J.H.; Lee, H.A.; Won, M.H.; Lee, C.H. Improved hcn channels in pyramidal neurons and their new expression levels in pericytes and astrocytes in the gerbil hippocampal ca1 subfield following transient ischemia. Int. J. Mol. Med. 2019, 44, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mulligan, M.K.; Nowak, T.S., Jr. Substrain- and sex-dependent differences in stroke vulnerability in c57bl/6 mice. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Manwani, B.; Liu, F.; Scranton, V.; Hammond, M.D.; Sansing, L.H.; McCullough, L.D. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 2013, 249, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.Y.; Yan, B.C.; Park, J.H.; Ahn, J.H.; Lee, D.H.; Kim, I.H.; Cho, J.H.; Chen, B.H.; Lee, J.C.; Cho, Y.S.; et al. Novel antiepileptic drug lacosamide exerts neuroprotective effects by decreasing glial activation in the hippocampus of a gerbil model of ischemic stroke. Exp. Ther. Med. 2015, 10, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar]
- Park, J.H.; Kim, Y.H.; Ahn, J.H.; Choi, S.Y.; Hong, S.; Kim, S.K.; Kang, I.J.; Kim, Y.-M.; Lee, T.-K.; Won, M.-H. Atomoxetine protects against nmda receptor-mediated hippocampal neuronal death following transient global cerebral ischemia. Curr. Neurovasc. Res. 2017, 14, 158–168. [Google Scholar] [CrossRef]
- Lee, J.C.; Shin, B.N.; Cho, J.H.; Lee, T.K.; Kim, I.H.; Noh, Y.; Kim, S.S.; Lee, H.A.; Kim, Y.M.; Kim, H.; et al. Brain ischemic preconditioning protects against moderate, not severe, transient global cerebral ischemic injury. Metab. Brain Dis. 2018, 33, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Nakano, S.; Yoshiya, I.; Hashimoto, P.H. Persistent degenerative state of non-pyramidal neurons in the ca1 region of the gerbil hippocampus following transient forebrain ischemia. Neuroscience 1993, 53, 23–38. [Google Scholar] [CrossRef]
- Zhan, R.Z.; Nadler, J.V.; Schwartz-Bloom, R.D. Depressed responses to applied and synaptically-released gaba in ca1 pyramidal cells, but not in ca1 interneurons, after transient forebrain ischemia. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2006, 26, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Lindvall, O.; Kokaia, Z. Stereological assessment of vulnerability of immunocytochemically identified striatal and hippocampal neurons after global cerebral ischemia in rats. Brain Res. 2001, 913, 117–132. [Google Scholar] [CrossRef]
- Tortosa, A.; Ferrer, I. Parvalbumin immunoreactivity in the hippocampus of the gerbil after transient forebrain ischaemia: A qualitative and quantitative sequential study. Neuroscience 1993, 55, 33–43. [Google Scholar] [CrossRef]
- Yang, G.E.; Tae, H.J.; Lee, T.K.; Park, Y.E.; Cho, J.H.; Kim, D.W.; Park, J.H.; Ahn, J.H.; Ryoo, S.; Kim, Y.M.; et al. Risperidone treatment after transient ischemia induces hypothermia and provides neuroprotection in the gerbil hippocampus by decreasing oxidative stress. Int. J. Mol. Sci. 2019, 20, 4621. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.C.; Park, J.H.; Ahn, J.H.; Kim, I.H.; Park, O.K.; Lee, J.C.; Yoo, K.Y.; Choi, J.H.; Lee, C.H.; Hwang, I.K. Neuroprotection of posttreatment with risperidone, an atypical antipsychotic drug, in rat and gerbil models of ischemic stroke and the maintenance of antioxidants in a gerbil model of ischemic stroke. J. Neurosci. Res. 2014, 92, 795–807. [Google Scholar] [CrossRef]
- Engel, T.; Schindler, C.K.; Sanz-Rodriguez, A.; Conroy, R.M.; Meller, R.; Simon, R.P.; Henshall, D.C. Expression of neurogenesis genes in human temporal lobe epilepsy with hippocampal sclerosis. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 38–47. [Google Scholar]
- Zhao, H.; Li, Z.; Wang, Y.; Zhang, Q. Hippocampal expression of synaptic structural proteins and phosphorylated camp response element-binding protein in a rat model of vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen. Res. 2012, 7, 821–826. [Google Scholar]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental pretreatment with chlorogenic acid prevents transient ischemia-induced cognitive decline and neuronal damage in the hippocampus through anti-oxidative and anti-inflammatory effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Paizs, M.; Engelhardt, J.I.; Siklos, L. Quantitative assessment of relative changes of immunohistochemical staining by light microscopy in specified anatomical regions. J. Microsc. 2009, 234, 103–112. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-K.; Shin, M.C.; Ahn, J.H.; Kim, D.W.; Kim, B.; Sim, H.; Lee, J.-C.; Cho, J.H.; Park, J.H.; Kim, Y.-M.; et al. CD200 Change Is Involved in Neuronal Death in Gerbil Hippocampal CA1 Field Following Transient Forebrain Ischemia and Postischemic Treatment with Risperidone Displays Neuroprotection without CD200 Change. Int. J. Mol. Sci. 2021, 22, 1116. https://doi.org/10.3390/ijms22031116
Lee T-K, Shin MC, Ahn JH, Kim DW, Kim B, Sim H, Lee J-C, Cho JH, Park JH, Kim Y-M, et al. CD200 Change Is Involved in Neuronal Death in Gerbil Hippocampal CA1 Field Following Transient Forebrain Ischemia and Postischemic Treatment with Risperidone Displays Neuroprotection without CD200 Change. International Journal of Molecular Sciences. 2021; 22(3):1116. https://doi.org/10.3390/ijms22031116
Chicago/Turabian StyleLee, Tae-Kyeong, Myoung Cheol Shin, Ji Hyeon Ahn, Dae Won Kim, Bora Kim, Hyejin Sim, Jae-Chul Lee, Jun Hwi Cho, Joon Ha Park, Young-Myeong Kim, and et al. 2021. "CD200 Change Is Involved in Neuronal Death in Gerbil Hippocampal CA1 Field Following Transient Forebrain Ischemia and Postischemic Treatment with Risperidone Displays Neuroprotection without CD200 Change" International Journal of Molecular Sciences 22, no. 3: 1116. https://doi.org/10.3390/ijms22031116





